Abstract
Aim: To test the prognostic value of the 1998 WHO/ISUP (World Health Organisation/International Society of Urologic Pathology) consensus classification system in Ta papillary urothelial neoplasms of the bladder.
Methods: The histological slides of 322 patients with a primary Ta tumour were classified according to the consensus classification system, and recurrence free survival (RFS) and progression free survival (PFS) were assessed for a mean follow up period of 79 months. In the same patient group, the RFS and PFS rates for the 1973 WHO grading system and a low grade/high grade system were analysed.
Results: Recurrent tumours were seen in all categories of the 1998 WHO/ISUP classification system and five year RFS was not significantly different between the groups (p = 0.12). The five year PFS showed a small but significant difference (p = 0.04) between papillary neoplasms of low malignant potential (PNLMP) and high grade papillary urothelial carcinomas (HGPUCs). In the 1973 WHO classification, no significant difference was found in RFS and PFS between the different grades. In the low grade/high grade classification PFS was significantly better for low grade tumours (p = 0.01).
Conclusion: The prognostic value of the 1998 WHO/ISUP classification system is limited to predicting PFS, especially between PNLMP and HGPUC. The prognostic value of this system over other grading systems is questionable.
Keywords: bladder neoplasms, World Health Organisation/International Society of Urologic Pathology grading system, prognosis, progression, recurrence
The most common type of bladder tumour in the Western world is a non-invasive, papillary tumour, which accounts for approximately 45% of all primary bladder tumours.1 Prognosis in these Ta tumours is determined by histological grade, size, and multiplicity of tumours, early recurrence, and concomitant carcinoma in situ.2,3 Several systems have been proposed to grade urothelial carcinoma of the bladder (table 1).4–9 In the most commonly used three tier World Health Organisation (WHO) grading system,6 most tumours are graded as intermediate (grade 2). A subdivision into grade 2a and 2b has been proposed by several authors.5,8 Others have suggested a two tier system with high grade and low grade tumours.7,9 In 1998, the WHO/ISUP (International Society of Urologic Pathology) consensus classification was published in an effort to reach a universally acceptable system for the classification and grading of urothelial tumours.10 The consensus was that on the basis of eight histological features of architecture and cytology, non-invasive papillary tumours should be divided into four categories:
papillomas with a benign behaviour
papillary neoplasms of low malignant potential (PNLMP), with a low risk of recurrence and progression
low grade papillary urothelial carcinoma (LGPUC)
high grade papillary urothelial carcinoma (HGPUC).
Table 1.
Overview of grading criteria for urothelial carcinoma used in different systems
| Author/grading system | |||||
| Malmström5 | Pauwels8 | Murphy7 | Schapers9 | WHO/ISUP10 | |
| Grades | 1 | 1 | Low grade | Low grade | PNLMP |
| 2a | 2a | High grade | High grade | LGPUC | |
| 2b | 2b | HGPUC | |||
| 3 | 3 | ||||
| 4 | |||||
| Grading criteria | |||||
| Cellular atypia | + | + | + | + | + |
| Cellular polarity | + | + | + | + | + |
| Mitoses | + | + | |||
| Epithelial thickness | + | + | + | + | |
HGPUC, high grade papillary urothelial carcinoma; ISUP, International Society of Urologic Pathology; LGPUC, low grade papillary urothelial carcinoma; PNLMP, papillary neoplasms of low malignant potential; WHO, World Health Organisation.
The consensus text did not give a validation of the prognostic value of the proposed grading system. Several reports have since been published on the prognostic value of the 1998 WHO/ISUP classification, but these were often limited to selected groups of patients with Ta tumours.11–13 In our study, we evaluate the prognostic value of the 1998 WHO/ISUP consensus classification in a large group of patients with non-invasive urothelial neoplasms of the bladder treated at a single institution with a long follow up period.
MATERIALS AND METHODS
A total of 359 consecutive patients, 295 men (82%) and 64 women (18%) with a diagnosis of a primary non-invasive (Ta) transitional cell carcinoma of the bladder were seen at Stichting Ziekenhuizen Noord-Limburg, Venlo, The Netherlands between 1 January 1979 and 1 June 2000. In all patients, after informed consent was obtained, the tumour was resected by transurethral resection (TUR) and the patient data were prospectively entered into a database. Solitary carcinoma in situ lesions and other bladder malignancies, such as squamous cell carcinoma, adenocarcinoma, and undifferentiated carcinoma, were not included in our study. Seven patients who underwent a cystectomy within one year of diagnosis of the primary Ta tumour and 23 patients who were lost to follow up within one month of diagnosis were excluded from the study. In addition, all patients diagnosed with a recurrent bladder carcinoma within three months of the primary tumour (n = 9) were excluded from our study because of the possibility that the recurrence resulted from residual tumour.14,15 Thus, a final study group of 320 patients remained, with a mean age of 66.6 years (SD, 11.4; range, 22.1–92.6). Pathological staging was done according to the TNM system.16 Initially, grading was carried out according to a modification of the standard WHO grading system with a simplified low grade/high grade system by one of the authors (RFMS).8,9 All histological slides were reviewed and classified by the same pathologist, without knowledge of previous classification results or clinical outcome, according to the 1998 WHO/ISUP consensus classification of urothelial neoplasms.10 Treatment of the primary tumour consisted of TUR in all patients, except for a group of 43 patients (13%) in whom intravesical treatment was also given, mainly because of multifocal tumours. The drugs used were mitomycin C in 27 patients, BCG in eight, epirubicine in one, and a combination of drugs in eight.
Follow up
Follow up examinations were carried out at least six monthly and included urine cytology and cystoscopy. All follow up data were prospectively collected and entered into a database throughout the study period. Study endpoints were recurrence, progression, and death. The cause of death was recorded as exactly as possible, using postmortem reports, hospital files, and information from the family practitioner. The last clinical assessment was used for evaluating the follow up period in patients without recurrence, progression, or death. Recurrence was defined as the reappearance of histopathologically confirmed urothelial neoplasm in the bladder of any stage or grade. Progression was defined as an increase in stage, the diagnosis of metastasis, or death caused by tumour. If a patient was lost to follow up, the family practitioner or municipal registry was consulted to assess survival.
Statistical methods
Survival analysis was performed for each grading system using the LIFETEST procedure and differences in survival data between different grades were compared by means of the log rank test. Recurrence free survival (RFS) was taken as the time from diagnosis of the primary tumour until recurrence or, in patients without recurrence, the date of death or last follow up. Progression free survival (PFS) was taken as the time from diagnosis of the primary tumour until progression or, in patients without progression, the date of death or last follow up. Patients who died (from any cause) were censored on the date of death. Patients alive without recurrence or progression and those lost to follow up were censored on the date of last follow up. The statistical analyses were performed with the statistical analytic system (SAS) package (SAS Institute Inc, Cary, North Carolina, USA).
RESULTS
Tables 2 and 3 summarise the results from the classification with the different grading systems. The mean ages (SD) in the respective groups were: papilloma, 62.4 (12.7) years; PNLMP, 62.9 (13.6) years; LGPUC, 64.6 (11.2) years; and HGPUC, 68.0 (11.0) years. The difference in mean age between patients with PNLMP and HGPUC was significant (p = 0.025), but the mean ages in the other groups did not differ significantly.
Table 2.
| 1998 WHO/ISUP | |||||
| Modified WHO 1973 | Papilloma | PNLMP | LCPUC | HGPUC | N (%) |
| Papilloma | 2 | 0 | 0 | 0 | 2 (1%) |
| 1 | 8 | 18 | 5 | 0 | 31 (9%) |
| 2a | 8 | 94 | 104 | 8 | 214 (66%) |
| 2b | 0 | 4 | 32 | 36 | 72 (22%) |
| 3 | 0 | 0 | 0 | 1 | 1 (1%) |
| N (%) | 18 (5%) | 116 (36%) | 141 (44%) | 45 (14%) | 320 (100%) |
HGPUC, high grade papillary urothelial carcinoma; ISUP, International Society of Urologic Pathology; LGPUC, low grade papillary urothelial carcinoma; PNLMP, papillary neoplasms of low malignant potential; WHO, World Health Organisation.
Table 3.
| 1998 WHO/ISUP | |||||
| Low grade/high grade | Papilloma | PNLMP | LCPUC | HGPUC | N (%) |
| Papilloma | 2 | 0 | 0 | 0 | 2 (1%) |
| Low grade | 16 | 112 | 109 | 8 | 245 (76%) |
| High grade | 0 | 4 | 32 | 37 | 73 (22%) |
| N (%) | 18 (5%) | 116 (36%) | 141 (44%) | 45 (14%) | 320 (100%) |
HGPUC, high grade papillary urothelial carcinoma; ISUP, International Society of Urologic Pathology; LGPUC, low grade papillary urothelial carcinoma; PNLMP, papillary neoplasms of low malignant potential; WHO, World Health Organisation.
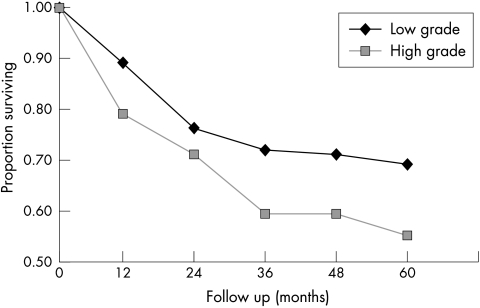
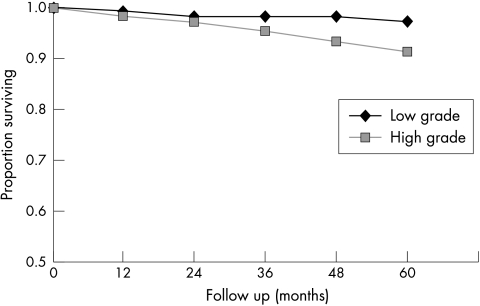
The mean follow up period was 79 months (range, 1–244; median, 63). In total, 110 first recurrences were found during the follow up period after a median interval of 13 months in the study group as a whole. The patterns of recurrences and progression for the 1998 WHO/ISUP classification system are summarised in table 4, and the RFS and PFS rates are shown in figs 1 and 2. The log rank test for five year PFS (p = 0.06) and five year RFS (p = 0.13) were not significantly different between the groups as a whole. The only significant difference between two subgroups was for PFS between the PNLMP and HGPUC groups (log rank test, p = 0.04). No differences could be shown between the HGPUC and LGPUC groups in RFS (log rank test, p = 0.42) or in PFS (log rank test, p = 0.16). In all groups, except for the papillomas, progression occurred during follow up, mainly to Tis and T1. The grade of recurrent turmour was most often intermediate, but high grade recurrent tumours were also seen, even in PNLMP (table 4). The RFS and PFS rates for the modified 1973 WHO classification system6,8 are shown in figs 3 and 4. The log rank test for five year RFS (p = 0.27) and the five year PFS (p = 0.07) was not significantly different between the groups. Figures 5 and 6 show the outcome of grading according to the two tier low grade/high grade system,9 in which the five year RFS did not differ significantly (p = 0.1), but the five year PFS did (p = 0.01). The two patients with a papilloma according to the strict criteria of the 1973 WHO classification6 did not show recurrence or progression. In the group of 22 patients with a papilloma (1998 WHO/ISUP), three patients had a single recurrent papillary Ta tumour, detected after seven, 25, and 152 months, which were graded as grade 2b, grade 2a, and grade 1, respectively, using the modified 1973 WHO grading system.6,8 The five year RFS was 85% and no patient with a papilloma showed progression or death as a result of tumour. In the group of patients with PNLMP, 30 patients (25%) had a recurrent tumour after a mean interval of 30 months, with a mean number of recurrences of 2.1 (range, 2–9). The five year RFS in PNLMP was 67%. Progression in PNLMP was seen in three patients (2%) after a mean interval of 61 months, with a five year PFS of 97%. One of the patients with a PNLMP who showed progression died as a result of bladder carcinoma 44 months after diagnosis of the primary tumour. Patients with LGPUC had recurrence in 43 cases (30%) after a mean period of 20 months and a mean number of recurrences of 1.9 (range, 1–6). Survival analysis in LGPUC showed a five year RFS of 65%, which is not significantly different from PNLMP (log rank test, p = 0.35). The PFS was 96% in LGPUC, which was also not significantly different from PNLMP (log rank test, p = 0.36). In the LGPUC group, five patients (3.5%) died as a result of bladder carcinoma after a mean of 79 months. Patients with HGPUC had recurrence in 18 cases (40%) after a mean interval of 20 months. The RFS was 56% and the PFS for HGPUC was 92%. Two patients with HGPUC died after a mean period of 166 months as a result of bladder carcinoma. There was a difference between HGPUC and PNLMP in RFS (p = 0.1), but only PFS between these two groups was significantly different (p = 0.04). No difference could be shown between HGPUC and LGPUC in RFS (p = 0.42) or in PFS (p = 0.16). In all groups, except for papillomas, progression occurred during follow up, mainly to Tis and T1. The grade of the recurrent tumour was most often intermediate, but high grade recurrent tumours were also seen, even in PNLMP (table 4).
Table 4.
1998 WHO/ISUP grading system. Characteristics and outcome of different grades: grading of recurrent tumours by modified 1973 WHO6,8 and staging by TNM16
| First recurrence | First progression | |||||||
| Grade of primary tumour (1998 WHO/ISUP)10 | N | Mean time to recurrence (months) | Grade | N | Stage | N | Stage | N |
| Papilloma | 18 | 61 | 1 | 1 | Ta | 3 | Tis | 0 |
| 2a | 1 | T1 | 0 | T1 | 0 | |||
| 2b | 1 | T2 | 0 | T2 | 0 | |||
| 3 | 0 | Tis | 0 | |||||
| Low malignant potential | 116 | 30 | 1 | 5 | Ta | 28 | Tis | 0 |
| 2a | 22 | T1 | 1 | T1 | 2 | |||
| 2b | 1 | T2 | 1 | T2 | 1 | |||
| 3 | 2 | Tis | 0 | |||||
| Low grade carcinoma | 141 | 20 | 1 | 8 | Ta | 39 | Tis | 1 |
| 2a | 26 | T1 | 2 | T1 | 4 | |||
| 2b | 8 | T2 | 1 | |||||
| 3 | 1 | Tis | 1 | |||||
| High grade carcinoma | 45 | 20 | 1 | 1 | Ta | 16 | Tis | 0 |
| 2a | 4 | T1 | 1 | T1 | 1 | |||
| 2b | 11 | T2 | 1 | T2 | 1 | |||
| 3 | 2 | Tis | 0 | |||||
ISUP, International Society of Urologic Pathology; WHO, World Health Organisation.
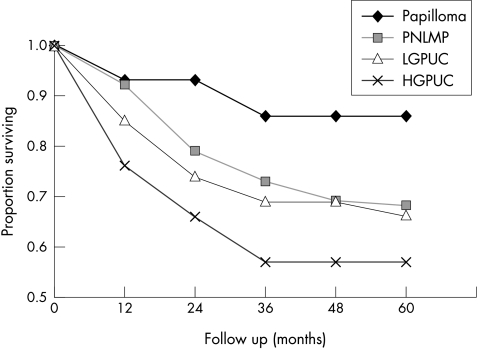
Figure 1.
Recurrence free survival (RFS) in pTa tumours for different categories of the WHO/ISUP 1998 classification. Papilloma: n = 18; five year RFS = 86%; low malignant potential (PNLMP): n = 116; five year RFS = 68%; low grade carcinoma (LGPUC) : n = 141; five year RFS = 66%; high grade carcinoma (HGPUC): n = 45; five year RFS = 57%. Log rank test for all groups, p = 0.13.
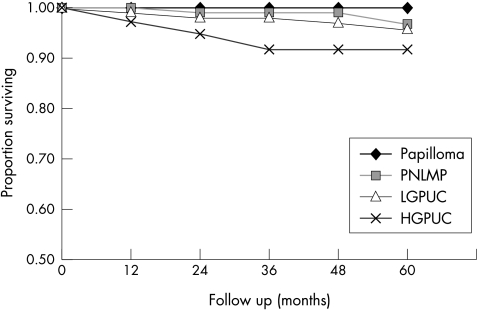
Figure 2.
Progression free survival (PFS) in pTa tumours for the different categories of the WHO/ISUP 1998 classification. Papilloma: n = 18; five year PFS = 100%; papillary neoplasm of low malignant potential (PNLMP): n = 116; five year PFS = 97%; low grade papillary urothelial carcinoma (LGPUC): n = 141 five year PFS = 96%; high grade papillary urothelial carcinoma (HGPUC): n = 45; five year PFS = 92%. Log rank test for all groups, p = 0.06.
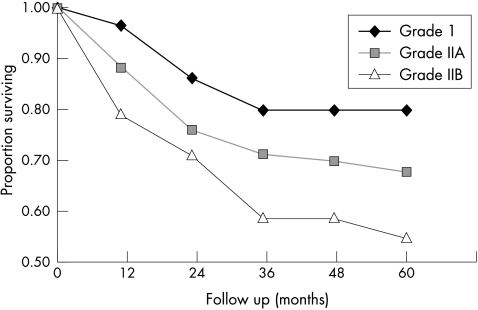
Figure 3.
Recurrence free survival (RFS) in pTa tumours for the different groups according to the modified 1973 WHO grading scheme. Grade 1: n = 31; five year RFS = 80%; grade 2a: n = 214; five year RFS = 68%; grade 2b: n = 72; five year RFS = 55%; grade 3: n = 1 not included. Log rank test for all groups p=0.27.
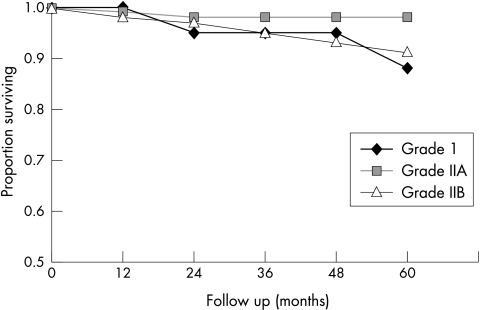
Figure 4.
Progression free survival (PFS) in pTa tumours for the different groups according to the modified 1973 WHO grading scheme. Grade 1: n = 31; five year PFS = 88%; grade 2a: n = 214; five year PFS = 99%; grade 2b: n = 72; five year PFS = 92%; grade 3: n = 1 not included. Log rank test for all groups, p = 0.07.
Figure 5.
Recurrence free survival (RFS) in pTa tumours according to the low grade/high grade system. Low grade carcinoma: n = 245; five year RFS = 70%; high grade carcinoma: n = 73, five year RFS = 55%. Log rank, p = 0.1.
Figure 6.
Progression free survival (PFS) in pTa tumours according to the low grade/high grade system. Low grade carcinoma: n = 245, five year PFS = 98%; high grade carcinoma: n = 73; five year PFS = 92%. Log rank, p = 0.01.
DISCUSSION
We studied the pattern of recurrence and progression in a group of 320 patients with a non-invasive papillary urothelial neoplasm of the bladder and evaluated the prognostic value of the 1998 WHO/ISUP consensus classification system. Papillomas did not show progression, although recurrences were seen in three patients. All tumour grades showed recurrences, but the RFS was not significantly different between the grades of the 1998 WHO/ISUP classification system. Progression was seen in all groups, except for papillomas. A significant difference was seen in PFS, particularly between PNLMP and HGPUC.
Because several of our patients were given adjuvant intravesical treatment, the true natural history of tumours graded according to the 1998 WHO/ISUP consensus may be underestimated. However, because intravesical treatment was only used in 13% of patients, its influence on the outcome of our study is unlikely to be of major importance.
Several studies, focusing on specific patient groups, have evaluated the prognostic value of the 1998 WHO/ISUP consensus classification system. Using a two year, multicentre registration of bladder carcinoma and follow up data in a region of Sweden, Holmäng et al studied the difference in outcome between tumours classified as PNLMP and LGPUC and found significantly more recurrences in LGPUC (71%) compared with PNLMP (35%) after five years of follow up.13 The difference was more striking at the first cystoscopy at four months. Progression was not seen in PNLMP, and only rarely in LGPUC (2.4%). In our study, we did not find a significant difference between these two groups in RFS and PFS and conclude that differentiation between PNLMP and LGPUC in terms of prognosis is not useful. The recurrence rates in the study by Holmäng et al are much higher than we have found, perhaps partly because of a slightly different survival analysis, but more probably because small lesions that were coagulated at cystoscopy without histological confirmation were also counted as recurrent tumours. In addition, very early recurrences at four month cystoscopy were included in the analysis, whereas we excluded these from our analysis for the reasons stated.14,15 Another difficulty when comparing this study with ours is that it included only Malmström grade 1 and grade 2a tumours.5,13 Because the 1998 WHO/ISUP classification system uses different criteria to other grading systems, we chose to review all histological slides, regardless of the outcome of any previous grading. In a more recent report from the same group, the prognostic value of the 1998 WHO/ISUP system was evaluated in a group of 363 Ta tumours, comprising the previously studied group of 255 patients with PNLMP and LGPUC, extended with a group of 108 patients with HGPUC. As in our study, no differences were found in recurrence between LGPUC and HGPUC, but significantly more HGPUC tumours progressed during follow up.17 The large number of patients with HGPUC in this study (28%) is striking when compared with our study (14%).
In a group of 112 Ta tumours with a PNLMP, diagnosed at the Mayo Clinic and followed up for more than 12 years, Cheng et al found a recurrence rate of 29% and a progression rate of 4%.11 Although this study did not assess other tumour grades, the findings are in accordance with our findings for PNLMP. In another study of 164 Ta tumours with a median survival of 9.2 years, focusing on tumour heterogeneity and PFS, Cheng et al found PFS rates, based on worst grade, of 93% for PNLMP, 82% for LGPUC, and 61% for HGPUC.12 As in our study, they found a significant difference in PFS between the different groups in the total study group, but the authors did not state whether a significant difference was also found between the subgroups—for example, PNLMP and LGPUC.
“Progression occurred in all patients, except those with papillomas”
Evaluation of the most commonly used 1973 WHO grading system for urothelial tumours has shown significant interobserver variation with varying prognostic implications.18–20 Interobserver variation with the described simplified two tier grading method has been found to be very low.9 All tumours were classified in our study by the same pathologist to avoid interobserver variation.
Because grading of papillary urothelial neoplasms in the 1998 WHO/ISUP consensus classification has been partly based on previous classification systems, some authors have translated these into the 1998 WHO/ISUP grades—for example, Malmström grade 1 is the same as PNLMP and grade 2a is LGPUC.13,17 However, this procedure is not correct because the grading criteria differ. For example, mitoses in LGPUC may be present at any level, whereas they should be found in Malmström grade 2a tumours only in the lower half of the epithelium.5,10 In addition, PNLMP should have a normal cellular organisation, whereas Malmström grade 1 tumours can have a tendency towards deviation.5,10 The 1998 WHO/ISUP classification criteria for papilloma are not identical to the 1973 WHO criteria because the number of cell layers need not be counted as was previously required.6,10 This explains the higher number of papillomas in our study when tumours were reclassified according to the 1998 WHO/ISUP criteria. The prognosis of patients with a papilloma remains favourable after reclassification, because progression was not seen and the mean time to recurrence was over five years in our study.
Take home messages.
The 1998 World Health Organisation/International Society of Urologic Pathology (WHO/ISUP) classification system subdivides papillary urothelial neoplasms into categories with a different progression free survival (PFS) and recurrence free survival (RFS), but these differences are not significant
This system is most useful for predicting PFS, particularly between papillary neoplasms of low malignant potential and high grade papillary urothelial carcinomas
The prognostic value of this system over other grading systems is questionable
In the 1973 WHO classification, no significant difference was found in RFS and PFS between the different grades
In the low grade/high grade classification PFS was significantly better for low grade tumours
In general, tumour grade is considered an important prognostic factor for most patient outcome variables in urothelial tumours.21 However, stage and grade are strongly related and most studies have evaluated the prognostic value of tumour grade for mixed groups of non-invasive, papillary (Ta) and invasive (T1 and higher) tumours. Several studies that have used a multivariate analysis have failed to show that grade is a significant prognostic parameter.9,22 In addition, grade is not a prognostic factor in muscle invasive carcinoma.23 Studies that have concentrated on Ta tumours could not find a relation between tumour grade and recurrence or progression.3,24,25 Our study shows that, like previous systems, the classification of papillary urothelial neoplasms according to the 1998 WHO/ISUP system does not divide patients into categories with a clearly different prognosis, because progression occurred in all patients, except those with papillomas. The five year PFS rates in the different grades varied between 92% (HGPUC) and 97% (PNLMP). In absolute terms this is a small difference, which probably does not justify a different clinical approach or the introduction of a new pathological category like PNLMP.26 Rather, any primary Ta tumour should be regarded as a risk factor for developing an invasive urothelial carcinoma in the future. Because the classic grading criteria may not simply be translated into the 1998 WHO/ISUP system, studies using the different systems cannot be compared without the reclassification of all tumours.
Abbreviations
HGPUC, high grade papillary urothelial carcinoma
ISUP, International Society of Urologic Pathology
LGPUC, low grade papillary urothelial carcinoma
PFS, progression free survival
PNLMP, papillary neoplasm of low malignant potential
RFS, recurrence free survival
TUR, transurethral resection
WHO, World Health Organisation
REFERENCES
- 1.Haukaas S, Daehlin L, Maartmann-Moe H, et al. The long-term outcome in patients with superficial transitional cell carcinoma of the bladder: a single-institutional experience. BJU Int 1999;83:957–63. [DOI] [PubMed] [Google Scholar]
- 2.Reuter VE. Bladder: risk and prognostic factors—a pathologist's perspective. Urol Clin North Am 1999;26:481–92. [DOI] [PubMed] [Google Scholar]
- 3.Zieger K, Wolf H, Olsen PR, et al. Long-term follow-up of noninvasive bladder tumours (stage Ta): recurrence and progression. BJU Int 2000;85:824–8. [DOI] [PubMed] [Google Scholar]
- 4.Bergkvist A, Ljungqvist A, Moberger G. Classification of bladder tumours based on the cellular pattern. Preliminary report of a clinical-pathological study of 300 cases with a minimum follow-up of eight years. Acta Chir Scand 1965;130:371–8. [PubMed] [Google Scholar]
- 5.Malmström PU, Busch C, Norlen BJ. Recurrence, progression and survival in bladder cancer. A retrospective analysis of 232 patients with greater than or equal to 5-year follow-up. Scand J Urol Nephrol 1987;21:185–95. [DOI] [PubMed] [Google Scholar]
- 6.Mostofi FK, Sobin LH, Tosoni I. Histological typing of urinary bladder tumours. International classification of tumours, No. 19. Geneva: World Health Organisation, 1973.
- 7.Murphy WM, Beckwith JB, Farrow GM. Tumours of the urinary bladder. In: Tumours of the kidney, bladder, and related urinary structures. Washington DC: Armed Forces Institute of Pathology, 1994:211–19.
- 8.Pauwels RP, Schapers RF, Smeets AW, et al. Grading in superficial bladder cancer. (1). Morphological criteria. Br J Urol 1988;61:129–34. [DOI] [PubMed] [Google Scholar]
- 9.Schapers RF, Pauwels RP, Wijnen JT, et al. A simplified grading method of transitional cell carcinoma of the urinary bladder: reproducibility, clinical significance and comparison with other prognostic parameters. Br J Urol 1994;73:625–31. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JI, Amin MB, Reuter VR, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 1998;22:1435–48. [DOI] [PubMed] [Google Scholar]
- 11.Cheng L, Neumann RM, Bostwick DG. Papillary urothelial neoplasms of low malignant potential. Clinical and biologic implications. Cancer 1999;86:2102–8. [PubMed] [Google Scholar]
- 12.Cheng L, Neumann RM, Nehra A, et al. Cancer heterogeneity and its biologic implications in the grading of urothelial carcinoma. Cancer 2000;88:1663–70. [DOI] [PubMed] [Google Scholar]
- 13.Holmäng S, Hedelin H, Anderström C, et al. Recurrence and progression in low grade papillary urothelial tumours. J Urol 1999;162:702–7. [DOI] [PubMed] [Google Scholar]
- 14.Brausi M, Kurth K, Meijden AP vd, et al. Variability in the three-month recurrence rate after TUR in superficial transitional cell carcinoma (TCC) of the bladder: a combined analysis of 8 EORTC studies. J Urol 1998;159(suppl):541. [Google Scholar]
- 15.Kurth KH, Bouffioux C, Sylvester R, et al. Treatment of superficial bladder tumours: achievements and needs. The EORTC genitourinary group. Eur Urol 2000;37(suppl 3):1–9. [DOI] [PubMed] [Google Scholar]
- 16.American Joint Committee on Cancer: AJCC cancer staging manual. Philadelphia: Lippincott-Raven, 1997:241–3.
- 17.Holmäng S, Andius P, Hedelin H, et al. Stage progression in Ta papillary urothelial tumours: relationship to grade, immunohistochemical expression of tumour markers, mitotic frequency and DNA ploidy. J Urol 2001;165:1124–8. [PubMed] [Google Scholar]
- 18.Blomjous CE, Smeulders AW, Baak JP, et al. A comparative study in morphometric grading of transitional cell carcinoma of the urinary bladder. Anal Quant Cytol Histol 1989;11:426–32. [PubMed] [Google Scholar]
- 19.Meijden A vd, Oosterlinck W, Brausi M, et al. Significance of bladder biopsies in Ta,T1 bladder tumours: a report from the EORTC genito-urinary tract cancer cooperative group. EORTC-GU group superficial bladder committee. Eur Urol 1999;35:267–71. [DOI] [PubMed] [Google Scholar]
- 20.Tosoni I, Wagner U, Sauter G, et al. Clinical significance of interobserver differences in the staging and grading of superficial bladder cancer. BJU Int 2000;85:48–53. [DOI] [PubMed] [Google Scholar]
- 21.Bostwick DG, Ramnani D, Cheng L. Diagnosis and grading of bladder cancer and associated lesions. Urol Clin North Am 1999;26:493–507. [DOI] [PubMed] [Google Scholar]
- 22.Fujii Y, Fukui I, Kihara K, et al. Late recurrence and progression after a long tumour-free period in primary Ta and T1 bladder cancer. Eur Urol 1999;36:309–13. [DOI] [PubMed] [Google Scholar]
- 23.Jimenez RE, Gheiler E, Oskanian P, et al. Grading the invasive component of urothelial carcinoma of the bladder and its relationship with progression-free survival. Am J Surg Pathol 2000;24:980–7. [DOI] [PubMed] [Google Scholar]
- 24.Morgan JD, Bowsher W, Griffiths DF, et al. Rationalisation of follow-up in patients with non-invasive bladder tumours. A preliminary report. Br J Urol 1991;67:158–61. [DOI] [PubMed] [Google Scholar]
- 25.Prout GR, Jr, Bassil B, Griffin P. The treated histories of patients with Ta grade 1 transitional-cell carcinoma of the bladder. Arch Surg 1986;121:1463–8. [DOI] [PubMed] [Google Scholar]
- 26.Oyasu R. World Health Organization and International Society of Urological Pathology classification and two-number grading system of bladder. Cancer 2000;88:1509–12. [DOI] [PubMed] [Google Scholar]