Abstract
Aims: To review 25 cases of breast hamartoma and discuss the pathological criteria, and the usefulness of imaging modalities, fine needle aspiration cytology (FNAC), and needle core biopsy in the diagnosis.
Methods: The hamartomas were assessed for interlobular fibrotic stroma, stromal adipose tissue content, pseudo-angiomatous stromal hyperplasia, and epithelial changes (hyperplasia, adenosis or apocrine metaplasia, and cyst formation). All imagings, previous FNACs, and biopsies were also reviewed.
Results: Imaging (mammography, ultrasound, and magnetic resonance imaging) was performed in 18 cases, and mostly showed encapsulated masses with a heterogeneous appearance. Microscopically, all hamartomas demonstrated good demarcation with fibrous tissue condensation. Adipose tissue was noted in all cases (5–90%; mean, 31%), and interlobular fibrosis in 21 cases. Benign epithelial hyperplasia occurred in 10 cases, and pseudo-angiomatous stromal hyperplasia or cystic ducts in eight cases each. Apocrine metaplasia, calcification, stromal giant cells, and adenosis occurred in four cases or less. Two cases showed coexisting ductal carcinoma in situ limited to within the hamartoma. Needle core biopsies (four cases) and FNAC (14 cases) were largely insufficient, inconclusive, or non-specific.
Conclusions: Hamartomas do not possess specific diagnostic histological features. The role of FNAC and needle core biopsy in making the diagnosis is limited, and requires clinical and radiological correlation to avoid underdiagnosis.
Keywords: breast, pathology, hamartoma
The term hamartoma was first coined by Arrigoni et al in 1971,1 as a well circumscribed breast lesion with varying amounts of benign epithelial elements, fibrous tissue, and fat. Many authors consider this entity to be underdiagnosed.2–4 Pathologically, a distinctive appearance is lacking. Several little used morphological patterns have been described.5 With the increasing use of diagnostic procedures on breast lumps, including mammography, ultrasound, fine needle aspiration cytology (FNAC), and needle core biopsy, it is expected that more hamartomas will be picked up. In contradistinction to many other benign or malignant breast lesions, the diagnosis of hamartoma can easily be missed if the clinical impression of a distinct lump or breast asymmetry and the imaging features are not taken into consideration when the biopsy is examined. For breast lumps, FNAC has become a commonly performed and reliable diagnostic test. The value of FNAC in diagnosing mammary hamartoma has not been evaluated. We report a series of 25 cases of hamartoma, and discuss the usefulness of these various increasingly popular diagnostic modalities in the diagnosis of mammary hamartoma.
“Many authors consider hamartoma to be underdiagnosed”
MATERIALS AND METHODS
The histopathology files of the authors (GMKT, TKFM) were searched for mammary hamartomas from 1984 to 2001. Clinical follow up data were obtained. All previous imagings were reviewed by the radiologists. The specimens had been formalin fixed, routinely processed, and 4 μm sections had been haematoxylin and eosin stained and examined for several morphological features considered to be characteristic of hamartoma.2,3,6 These included fibrotic stroma extending between individual breast lobules, adipose tissue in the stroma, pseudo-angiomatous stromal hyperplasia, with a network of inter-anastomising vascular-like channels lined by flattened cells,7 and epithelial changes including epithelial hyperplasia, apocrine metaplasia, adenosis, and cyst formation. The adipose tissue content of the stroma was determined by estimating the percentage area of adipose tissue after reviewing all the slides for each case. For those cases with previous biopsies or FNAC, the respective slides were also retrieved and reviewed. The original diagnoses and adequacy of the specimen were assessed.
RESULTS
In total, 25 cases of hamartoma were retrieved from 1984 to 2001. These 25 hamartomas were obtained from 24 patients, with one patient having two hamartomas in the same breast with an interval of 10 months. Two cases had coexisting ductal carcinoma in situ (DCIS; one micropapillary type, another solid and cribriform type with early focal invasion). These had been reported previously.8 All the patients were women, and the age range was 24 to 76 years (mean, 38).
Clinically, most (23) patients presented with painless, soft to firm palpable breast lumps, and the clinical impression was fibroadenoma. One patient presented with gross breast asymmetry. Twelve hamartomas were on the left and 13 were on the right.
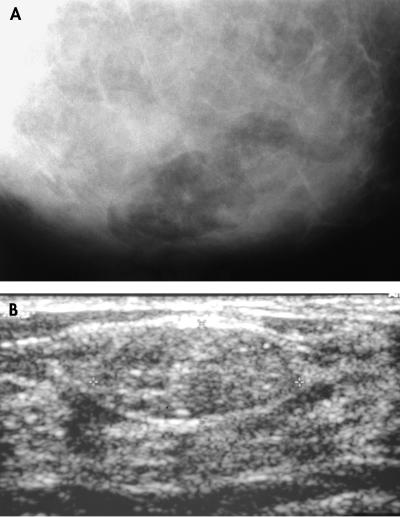
Imaging was performed in 18 patients. In four patients, ultrasound, mammography, and magnetic resonance imaging (MRI) were done, with all cases showing features characteristic of “lipofibroadenoma” or hamartoma. For the remaining 14 patients, all had ultrasound and seven also had mammography. The mammograms were diagnosed descriptively as mixed fibrous and adipose tissue without suspicion of malignancy. In mammography, the hamartomas appeared as ovoid to rounded, well circumscribed masses of mixed heterogenous density with a mottled centre. Thin smooth capsules with peripheral radiolucent zones were seen (fig 1A). In sonography, they were also well encapsulated with echogenic rims and internal inhomogenicity. They displaced the adjacent normal breast tissue both mammographically and sonographically (fig 1B). In MRI, all four lesions were well encapsulated with a dark smooth thin rim, ovoid in shape with internal heterogenicity, and showed heterogenous gadolinum enhancement. Internal fat intensity was demonstrated in all lesions.
Figure 1.
(A) Mammography showing an ovoid, well circumscribed mass of mixed density with fat, a mottled centre, and a thin smooth capsule. (B) Sonography showing well encapsulated mass with an echogenic rim and internal inhomogenicity, displacing the adjacent normal breast tissue.
Twenty four lesions were resected (lumpectomy); the single remaining case was diagnosed by biopsy and the patient was followed only because she refused surgery. All patients were followed for two months to four years before the cases were closed. All patients were well, with only one patient developing a recurrence after an interval of 10 months. For this patient, the histological features of the original and recurrent hamartomas were similar, and the initial resection was incomplete, with the presence of lesion at the margin. This patient was well without further complications after another one year follow up period. The lesion sizes ranged from 1.2 to 14 cm (mean, 3.8). Gross examination revealed nodular masses that were rounded and possessed smooth outlines (fig 2A). They were soft to firm, and some appeared to be shelled out at the time of surgery. The cut surface showed variable fatty areas within a fibrotic stroma. Clefts and fronds were not prominent.
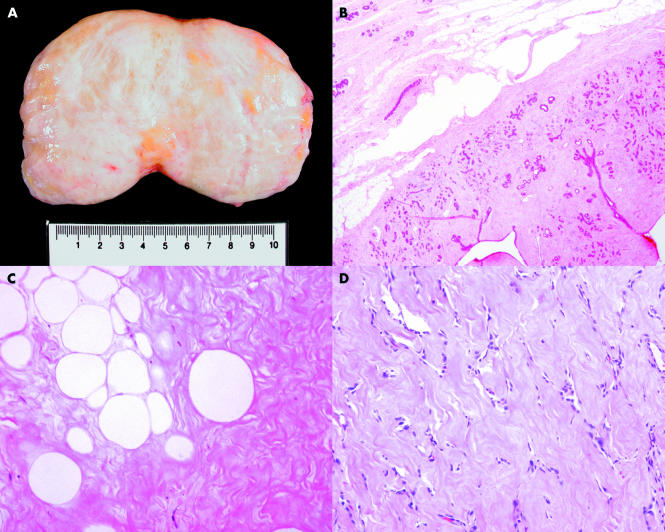
Figure 2.
(A) Specimen picture of a breast hamartoma. (B) Photomicrograph showing the smooth border of a hamartoma (haematoxylin and eosin stained; original magnification, ×20). (C) Photomicrograph showing single and small clusters of adipocytes within a densely fibrotic stroma (haematoxylin and eosin stained; original magnification, ×200). (D) Photomicrograph showing pseudo-angiomatous changes of the stroma (haematoxylin and eosin stained; original magnification, ×100).
Microscopy showed that the nodules were well demarcated from the adjacent breast tissue (fig 2B). Condensation of fibrous tissue around the mass was seen. In 21 cases, there was dense hyalinised fibrotic tissue between the individual lobules. In all cases, adipose tissue was noted within the stroma in variable amounts, ranging from 5% to 90% (mean, 31%) in area (fig 2C). In eight cases, the stroma showed areas of pseudo-angiomatous changes (fig 2D). The following epithelial changes were seen: mild epithelial hyperplasia without atypia in 10 cases, cystic changes in eight cases, apocrine metaplasia in four cases, and adenosis in one case. The following stromal changes were seen: dense stromal hyalinisation in three cases, and bizarre stromal cells and microcalcification in two cases each. One case each showed myoid changes in the stroma and focal ossification. Two cases showed small foci of DCIS (one micropapillary and one cribriform with focal invasion).
In this series, four needle core biopsies were done. Three were done before excision, and in the other, excision was refused by the patient. In all cases, the biopsy showed benign breast tissue components and a diagnosis of hamartoma was made in the one case without excision, after correlation with the clinical and radiological impressions.
A total of 14 FNACs had been performed on 12 hamartomas from 11 patients. Of the 14 aspirates, three were almost acellular and were diagnosed as insufficient in quantity. For the other 11 cases, seven showed scanty groups of benign ductal epithelium, one of which also exhibited tiny stromal fragments, and these cases were diagnosed as benign, not otherwise specified. The other four aspirates were more cellular, including the two cases with DCIS. Of these, three showed clusters and sheets of ductal epithelium, two of which also contained stromal fragments. One case was diagnosed as fibroadenoma, and the other two (including one of the cases with DCIS) were diagnosed as benign, not otherwise specified. The remaining case (the other case of DCIS within hamartoma) showed epithelial fragments with some showing atypical features. It was diagnosed as atypical cells present. None of these cases was diagnosed as hamartoma because the clinical and/or radiological information was not made known.
DISCUSSION
Mammary hamartoma has a reported incidence of 0.1% to 0.7%.2,6,9 The true incidence is probably higher, as pointed out by Daya et al,3 Fechner,10 and substantiated by our own experience that with the increasing use of breast imaging more hamartomas are likely to be identified.
Hamartoma has the typical mammographic appearance of lucent lesions containing fat, varying radio dense fibrous and adenomatous elements, a sharp margin, and sometimes a thin capsule. Lobulated densities are dispersed within the encapsulated fat, described as a “slice of salami”. The ultrasound shows sharp definition and displacement of surrounding structures. It contains sonolucent fat and echogenic fibrous components with a heterogeneous internal echo pattern.11 The MRI shows the presence of internal fat density in addition to the smooth well defined hypointense rim and internal heterogeneous enhancement, which are characteristic of breast hamartoma.
The pathology of hamartoma remains poorly defined; the original definition1 was used for a clinically discrete nodule composed of a variable amount of epithelial elements in a fibrofatty stroma. Early attempts to subclassify hamartomas according to the histological parameters resulted in a confusing state of affairs. A three category classification of the “fibrous”, “fatty”, and “fibro fatty” hamartoma has been put forward by McGuire and Cohn,12 and Jones et al suggested a four category classification of “encapsulated fibrocystic changes”, “fibroadenoma with fibrous stroma”, “fibroadenoma- like”, and “circumscribed adenolipoma”.5 Neither of these descriptive classification systems has been widely adopted. The current criteria used by practising pathologists have not been described in a detailed manner.13 Fechner has described the difference in lobular distribution and the presence of fat in hamartomas as the differentiating features against the more common fibroadenomas.10 In the literature, several good reviews have been published encompassing moderate numbers of cases.2,3,5,6,14 In most of these series, efforts were devoted to the identification of definitive histological criteria. Different authors had used different criteria, but overlap exists.
The presence of lobules within a fibrotic stroma, which surrounds and extends to between individual lobules and obliterates the usual interlobular specialised loose stroma, is most characteristic. Most authors2,3,5,6 have alluded to this interlobular fibrosis. The same is seen in our series of 25 cases, in which most showed such a feature. However, this feature is not unique to hamartomas. In sclerosing lobular hyperplasia (fibroadenomatoid mastopathy), which is a well defined, rounded mass with enlarged lobules showing increased numbers of intralobular glands, the presence of interlobular stromal sclerosis mimics hamartoma. Its frequent association with fibroadenoma, and the absence of fat in the stroma, may help to distinguish sclerosing lobular hyperplasia from hamartoma.
Adipose tissue within the stroma is also commonly reported in hamartomas. In most series,2,3,5,6 adipose tissue is present in more than 90% of the cases, although the volume of adipose tissue generally accounts for 10–20% of the lesion volume. Charpin et al reported cases consisting of up to 70% fat.6 We report similar findings, with fat being present in all cases, and ranging from 5% to 90%, with a mean volume of 31% of the hamartoma.
The presence of pseudo-angiomatous stroma within the hamartoma has been reviewed and described in detail by Fisher et al.2 Subsequent experience by other authors3,15 found this to be a constant observation, even though the incidence varies from a high 71%2 to a low 16–20%.3,15 In our series, pseudo-angiomatous stroma was found in 32% of the hamartomas.
“The correct identification of hamartoma is important because there are the problems of recurrence and coincidental epithelial malignancy”
Epithelial changes were also seen in our series. Simple epithelial hyperplasia without atypia occurred in 40%, whereas cystic changes occurred in 32%, apocrine metaplasia was present in 16%, overlapping with cystic changes, and adenosis occurred in 4%. Cystic changes with or without concomitant apocrine metaplasia was also reported as a common feature of mammary hamartoma,2,3,6 even though in another series15 cystic changes were present in 20% of the cases reported. In our series, 32% showed significant cyst formation.
Other rare features that have been described in the literature, but occurring in a smaller proportion of cases include microcalcification,3 myoid (smooth muscle) differentiation,3,5,6,16 stromal oedema,6 and stromal giant cells.6 To add to the list is one case with focal ossification within the stroma of the hamartoma seen in our series.
Take home messages.
Hamartomas do not possess specific diagnostic histological features, and diagnosis is therefore difficult
The presence of fibrous tissue within the lobules, or fibrous tissue and fat in the stroma, with or without pseudo-angiomatous changes, should alert the pathologist to the possibility of a hamartoma
The role of fine needle aspiration cytology and needle core biopsy in making the diagnosis is limited, and requires clinical and radiological correlation to avoid underdiagnosis
Although hamartomas are benign, coincidental malignancy can occur, and the issue of potential recurrences has not been resolved
For all the features that have been evaluated, none is unique to hamartoma. The presence of fibrosis or hyaline fibrosis within the stroma can be seen in a variety of breast lesions, including fibroadenoma, in which it constitutes the major differential diagnosis, as well as sclerosing lobular hyperplasia. Within the spectrum of fibrocystic changes, sclerosis, stromal fibrosis, apocrine metaplasia, and cystic changes of the ducts are common; pseudo-angiomatous changes in the stroma have also been reported in many types of breast lesions.17
The correct identification of hamartoma is important because there are the problems of recurrence and coincidental epithelial malignancy. In the series of Daya et al,3 two of 25 cases recurred after local excision, after an interval of seven and 18 months, and in our series, one lesion recurred after 10 months. This is different from the experience of other authors, who reported no recurrences.2,5,6,14,15 More experience is clearly needed. Furthermore, there are occasional case reports8,18–20 of coincidental in situ or invasive ductal or lobular carcinoma occurring in hamartomas. Although such cases are rare, it is imperative for the pathologist to be diligent in sampling hamartomas for suspicious areas.
The use of FNAC in diagnosing breast lesions has now been well established and proved to be accurate. The role of FNAC in diagnosing hamartoma remains limited. In the literature, only one report has commented on its lack of usefulness in this respect.14 In another cytology report of a single case of FNAC of a breast hamartoma,21 a diagnostic label could not be assigned to the FNAC specimen, although the hamartoma was confirmed by the histology of the excision specimen. Similar findings occurred in our series, in which none of the hamartomas was diagnosed correctly at FNAC. The two main contributing factors are the scanty materials obtained during the aspiration and the lack of specific cytological or architectural features in hamartoma. For the rare cases in which the FNACs were of moderate to high cellularity, differentiation from fibroadenoma was not possible. Even core biopsy is of limited value, especially when the imaging findings or the clinical impressions were not provided.
Diagnosing hamartoma of the breast is difficult, especially in biopsy or FNAC. The pathologist who sees fibrous tissue within the lobules, or fibrous tissue and fat in the stroma with or without pseudo-angiomatous changes, should be alerted to the possibility of a hamartoma. Correlation with the imaging findings and clinical impression may avoid the embarrassing situation of diagnosing “no significant pathology” in a palpable, radiologically distinct lesion. The radiologist who performs FNAC or needle core biopsy should remember that FNAC can rarely yield sufficient sample for diagnosis, and that both FNAC and needle biopsy are unlikely to provide enough information for the pathologist. Good communication of imaging findings is essential. The surgeon should also realise that although hamartomas are benign, coincidental malignancy may occur, and the issue of potential recurrences has not been resolved.
Abbreviations
DCIS, ductal carcinoma in situ
FNAC, fine needle aspiration cytology
MRI, magnetic resonance imaging
REFERENCES
- 1.Arrigoni MG, Dockerty MA, Judd ES. The identification and treatment of mammary hamartomas. Surg Gynecol Obstet 1971;133:577–82. [PubMed] [Google Scholar]
- 2.Fisher CJ, Hanby AM, Robinson L, et al. Mammary hamartoma—review of 35 cases. Histopathology 1992;20:99–106. [DOI] [PubMed] [Google Scholar]
- 3.Daya D, Trus T, D'Souza TJ, et al. Hamartoma of the breast, an underrecognized breast lesion. Am J Clin Pathol 1995;103:685–9. [DOI] [PubMed] [Google Scholar]
- 4.Petrik PK. Mammary hamartoma. Am J Surg Pathol 1987;11:234–9. [DOI] [PubMed] [Google Scholar]
- 5.Jones MW, Norris HJ, Wargotz ES. Hamartomas of the breast. Surg Gynecol Obstet 1991;173:54–6. [PubMed] [Google Scholar]
- 6.Charpin C, Mathoulin MP, Andrac L, et al. Reappraisal of breast hamartomas. A morphological study of 41 cases. Pathol Res Pract 1994;190:362–71. [DOI] [PubMed] [Google Scholar]
- 7.Vuitch MF, Rosen PP, Erlandson RA. Pseudoangiomatous hyperplasia of mammary stroma. Hum Pathol 1986;17:185–91. [DOI] [PubMed] [Google Scholar]
- 8.Tse GM, Law BK, Pang LM, et al. Ductal carcinoma in situ arising in mammary hamartomas. J Clin Pathol 2002;55:541–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linell F, Ostberg G, Soderstrom J, et al. Breast hamartomas: an important entity in mammary pathology. Virchows Arch Pathol Anat Histol 1979;383:253–64. [DOI] [PubMed] [Google Scholar]
- 10.Fechner RE. Fibrodenoma and related lesions. In: Page DL, Anderson TJ, eds. Diagnostic histopathology of the breast. Edinburgh: Churchill Livingstone, 1987:72–85.
- 11.Kopans DB. Pathologic, mammographic and sonographic correlation. In: Kopans DB. Breast imaging, 2nd ed. Boston, Massachusetts: Lippincott-Raven, 1998:558–60.
- 12.McGuire LI, Cohn D. Hamartoma of the breast. Aust N Z J Surg 1991;61:713–16. [DOI] [PubMed] [Google Scholar]
- 13.Rosen PP. Benign mesenchymal neoplasms. In Rosen PP, ed. Breast pathology. Philadelphia: Lippincott-Raven, 1996:676–81.
- 14.Gogas J, Markopoulos C, Gogas H, et al. Hamartomas of the breast. Am Surg 1994;60:447–50. [PubMed] [Google Scholar]
- 15.Chiacchio R, Panica L, D'Antonia A, et al. Mammary hamartomas: an immunohistochemical study of ten cases. Pathol Res Pract 1999;195:231–6. [DOI] [PubMed] [Google Scholar]
- 16.Magro G, Bisceglia M. Muscular hamartoma of the breast. Case report and review of literature. Pathol Res Pract 1998;194:349–55. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim RE, Sciotto CG, Weidner N. Pseudoangiomatous hyperplasia of mammary stroma. Some observations regarding its clinicopathologic spectrum. Cancer 1989;63:1154–60. [DOI] [PubMed] [Google Scholar]
- 18.Coyne J, Hobbs FM, Boggis C, et al. Lobular carcinoma in a mammary hamartoma. J Clin Pathol 1992;45:936–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anani PA, Hessler CH. Breast hamartoma with invasive ductal carcinoma. Report of two cases and review of the literature. Pathol Res Pract 1996;192:1187–94. [DOI] [PubMed] [Google Scholar]
- 20.Mester J, Simmons RM, Vazquez MF, et al. In situ and infiltrating ductal carcinoma arising in a breast hamartoma. AJR Am J Roentgenol 2000;175:64–6. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, Nawaz S. Fine needle aspiration of breast hamartoma. Acta Cytol 1998;42:437–8. [PubMed] [Google Scholar]