Abstract
Aims: Gulf War veterans report a high prevalence of musculoskeletal symptoms. The aim of this study was to establish whether there were abnormalities in bone turnover and remodelling in a group of symptomatic subjects who had served in the Gulf War.
Methods: Iliac crest bone biopsies were obtained from 17 Gulf War veterans who were seeking litigation and compared with those of 13 age and sex matched healthy controls. Bone histomorphometry was performed using image analysis.
Results: Cancellous bone area was significantly lower in Gulf War veterans than in control subjects (p = 0.027) and this was associated with a significantly reduced mineral apposition rate (p = 0.002), mean wall width (p < 0.0001), and bone formation rate at the tissue level (p < 0.0001).
Conclusions: These results demonstrate that in this group of Gulf War veterans there was a significant reduction in bone formation at both the cellular and tissue level and this was associated with a reduction in cancellous bone area. The cause of these abnormalities is unknown but might be related to potentially harmful exposures during service in the Gulf War or to changes in life style as a result of chronic ill health. The clinical relevance of the observed reduction in bone formation remains to be established.
Keywords: Gulf War syndrome, Gulf War veterans, bone histomorphometry, organophosphates, reduced bone formation
A n increased prevalence of symptoms and disorders has been reported by veterans of the Gulf War including chronic fatigue, headache, irritability, musculoskeletal symptoms, and depression.1,2 The existence of a specific Gulf War syndrome has been widely debated,3–8 but there is little evidence to support this concept, similar health problems being reported by veterans from other wars.9 Nevertheless, active military service in the Gulf War was associated with several hazardous exposures; some of these, such as exposure to the smoke of burning oil, vaccinations against biological warfare, organophosphates, and measures to protect against chemical warfare were more prominent in Gulf War veterans than in other military cohorts whereas others, such as multiple vaccinations, were used with similar frequency.2
“The existence of a specific Gulf War syndrome has been widely debated, but there is little evidence to support this concept, similar health problems being reported by veterans from other wars”
Musculoskeletal symptoms were reported with significantly greater frequency by Gulf War veterans than UK servicemen from the Bosnia conflict or those serving during the Gulf War but not deployed there.2 In our study, we report results from a histomorphometric study of bone biopsies that were obtained as part of a more extensive study in which Gulf War veterans seeking litigation underwent a variety of investigations on a volunteer basis.
SUBJECTS AND METHODS
Seventeen Gulf War veterans, aged 27–51 years (mean, 34.9) who were seeking litigation for a range of health problems volunteered to undergo bone biopsy. The study was approved by the Queen’s Medical Centre local ethics committee and written informed consent was obtained from all subjects before biopsy. Bone mineral density was measured in the proximal femur and lumbar spine using a Lunar DPX dual energy x ray absorptiometer.
Trans-iliac bone biopsies, 8 mm internal diameter, were obtained using a modified Bordier trephine 1 inch below and behind the anterior superior iliac spine. None of the subjects was receiving medication known to affect bone metabolism. All subjects received double tetracycline labelling before biopsy.10 After the biopsy procedure, biopsies were halved longitudinally, one half being snap frozen, and the other embedded in LR White medium resin (London Resin Co, London, UK). Non-decalcified sections (8 μm thick) were stained using the von Kossa and toluidine blue techniques. Histomorphometric analysis of bone remodelling and structure was performed using an in house image analysis system as described previously.11
Bone area/tissue area (B.Ar/T.Ar) and osteoid perimeter/bone perimeter (O.Pm/B.Pm) were measured on von Kossa stained sections on a minimum of 25 fields from three to six sections. Tetracycline labelling was viewed by fluorescence microscopy on a minimum of six 15 μm unstained sections at ×156 magnification. The mean width of completed bone remodelling units (wall width; W.Wi) was measured on toluidine blue stained sections viewed under polarised light at ×156 magnification. The eroded perimeter was measured on toluidine blue stained sections, resorption cavities being identified under polarised light.
The mineralising perimeter (Md.Pm) was calculated as follows:
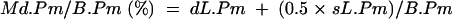 |
where dL.Pm is the double labelled perimeter and sL.Pm is the single labelled perimeter.
The mean distance between double labels was measured directly at ×312 magnification. Measurements were made at approximately four equidistant points along each double label. A minimum of 20 labels was measured for each biopsy on a minimum of six sections.
Mineral apposition rate (MAR) was calculated as:
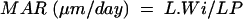 |
where L.Wi is the interlabel width and LP is the labelling period (12 days).
The tissue based bone formation rate (BFR/B.Pm) was calculated as follows:
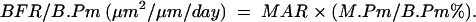 |
Activation frequency was calculated as:
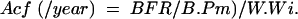 |
Strut analysis, trabecular bone pattern factor, and marrow star volume were assessed as reported previously.12
Control data were obtained from 13 healthy men who formed part of an earlier study of normal bone,13,14 in which iliac crest biopsies were obtained from normal subjects during general anaesthesia for a minor surgical procedure. The mean age of these controls was 36.6 years (range, 19–51). Results of histomorphometric analysis performed previously using an eye piece graticule and micrometre were used because sections were no longer available from this cohort. The measurements in biopsies from the controls and Gulf War veterans were made by the same observer (SV). Statistical analysis was performed using an unpaired Student’s t test after log transformation of the data. Results are expressed as the mean (SD).
RESULTS
Demographic details and bone mineral density
The age of the veterans at the time of the study ranged between 27 and 51 years (mean, 34.9). They had spent between two and four months in the Gulf during the war and most had spent time in a blackadder camp where there was obvious spraying of organophosphates. At least four were aware of exposure to sarin but it was not possible to obtain an accurate vaccination history. All but one complained of musculoskeletal symptoms (most commonly arthralgia) but none had a history of low trauma fracture. Seven of the men were regular cigarette smokers, six of whom smoked more than 15 daily, and four had an alcohol intake in excess of 10 units weekly (16, 40, 40, and 50 units, respectively).
The mean bone mineral density in the lumbar spine (L2–4), expressed as a Z score, was +0.55 (range, −1.6 to +2.3) and in the proximal femur (femoral neck) was +0.45 (range, −0.7 to +1.9). None of the men had osteoporosis as defined by a T score below −2.5.15 Body weight ranged between 64 and 120 kg (mean, 85.2).
Bone histology and histomorphometry
Qualitative examination of the biopsies from the Gulf War veterans revealed considerable heterogeneity, with some biopsies appearing normal and others showing reduced cancellous bone area, trabecular thinning, and reduced connectedness of the cancellous bone structure. Very few osteoclasts were seen; in addition, very few active osteoblasts were evident.
Table 1 shows the histomorphometric data. Cancellous bone area was significantly lower in the Gulf War veterans than in the controls (p = 0.027). Mineral apposition rate and mean wall width, both indices of bone formation at the cellular level, were also significantly lower in the Gulf War veterans (p = 0.002 and p < 0.0001, respectively), as were measurements that reflect bone formation at the tissue level, namely bone formation rate (p < 0.0001) and activation frequency (p = 0.0006). The eroded perimeter was significantly higher in the Gulf War veterans (p < 0.0001).
Table 1.
Gulf war group v controls
| Indices | Gulf war group | Control group | p Value (log transformed independent t test) |
| Number | 17 | 13 | |
| Age (years) | 34.9 (6.4) | 36.6 (10.7) | <0.599 |
| Trabecular bone area (%) | 20.5 (5.3) | 25.3 (6.1) | <0.027 |
| Osteoid perimeter (%) | 8.5 (4.5) | 23.7 (10.3) | <0.0001 |
| Mineralising perimeter (%) | 4.5 (3.1) | 11.3 (5.8) | <0.0003 |
| Mineral apposition rate (μ/day) | 0.58 (0.19) | 0.77 (0.12) | <0.002 |
| Bone formation rate (μm2/μm/day) | 0.028 (0.021) | 0.087 (0.044) | <0.0001 |
| Activation frequency (/year) | 0.24 (0.16) | 0.54 (0.25) | <0.0006 |
| Eroded perimeter (%) | 5.98 (2.64) | 1.87 (0.85) | <0.0001 |
| Wall width (μm) | 42.5 (4.3) | 57.8 (7.9) | <0.0001 |
Values are mean (SD).
No significant differences between the groups were found in cancellous bone structure, assessed by strut analysis, marrow star volume, and trabecular bone pattern factor (data not shown).
DISCUSSION
Our results demonstrate that in this group of Gulf War veterans there was a significant reduction in bone formation, both at the cellular and tissue level, in association with a reduction in cancellous bone area. Although the eroded perimeter was increased compared with the controls, this was generally of a small magnitude and the scarcity of osteoclasts in association with resorption cavities indicates that reduced bone formation rather than increased resorption was the most likely cause of the observed increase.
“It is of interest that the bone abnormalities observed in these Gulf War veterans were similar to those recently reported in agricultural workers with chronic organophosphate exposure as a result of sheep dipping”
The control values used in our study were obtained from healthy men in whom bone biopsies had been obtained previously.13,14 Because of the time that had elapsed between that study and our present one, it was not possible to obtain and quantify fresh sections so that the results of an earlier histomorphometric analysis made by the same observer (SV) were used. Although some differences may arise as a result of inter-method variations,16,17 these are generally small and cannot explain the observed differences between the Gulf War veterans and controls, particularly with respect to indices such as wall width, mineral apposition rate, and mineralising surface.
The cause of the reduction in bone formation seen in these biopsies cannot be ascertained from our study. One possibility is that changes in the life style of these subjects may have been responsible—for example, tobacco and excessive alcohol consumption and reduced levels of physical activity. However, in this cohort there was no clear relation between any of these variables and indices of bone remodelling and turnover. Alternatively, it is possible that the abnormalities may have arisen as a result of one or more of the chemical exposures experienced by the veterans. It was not possible to ascertain precisely either the nature or degree of these in this cohort, but nearly all have a history of definite or probable organophosphate exposure and, in addition, pyridostigmine was widely used by servicemen during the Gulf War.2 It has recently been reported that acetylcholinesterase is expressed by osteoblasts; its presence in bone matrix along cement lines and in osteoid indicates that it may have an extracellular role in bone.18 Furthermore, the demonstration of Cbfa-1 and other osteogenic factor binding motifs on the acetylcholinesterase gene promoter19 also indicates a role for acetylcholinesterase in bone formation and provides a potential mechanism for reduction in bone formation in individuals exposed to organophosphates. In this context, it is of interest that the bone abnormalities observed in these Gulf War veterans were similar to those recently reported in agricultural workers with chronic organophosphate exposure as a result of sheep dipping.20
Take home messages.
In this group of Gulf War veterans, cancellous bone area was significantly reduced and this was associated with a significant reduction in mineral apposition rate, mean wall width, and bone formation rate at the tissue level
The cause of these abnormalities is unknown but might be related to potentially harmful exposures during service in the Gulf War (such as exposure to organophosphates or pyridostigmine) or to changes in life style as a result of chronic ill health
The clinical relevance of the observed reduction in bone formation remains to be established
The clinical relevance of the observed changes in bone formation is unclear. Reduced bone formation at the cellular level is associated with trabecular thinning which, although initially associated with the preservation of cancellous bone architecture, will eventually progress to trabecular penetration with loss of connectivity.21 In addition, low bone turnover results in an increase in bone age and increased secondary mineralisation, changes that may lead to increased microdamage and reduced mechanical strength.22 None of the subjects gave a history of low trauma fracture and bone density values were within the normal range in all subjects, none having osteoporosis as defined by the World Health Organisation classification. There was also considerable heterogeneity in the bone histology and in histomorphometric indices; in six of the individuals the bone histology and histomorphometric indices were judged to be within normal limits.
In conclusion, our results indicate that in this group of war veterans bone formation was significantly reduced. The cause of the observed abnormalities has not been identified in our study and, in particular, it is not possible to conclude whether they arose as a direct result of chemical exposures or were an indirect consequence resulting from changes in life style. Finally, the long term effects of these changes on bone fragility and fracture risk are currently unknown.
Acknowledgments
JEC, SV, and SB are supported by the Wellcome Trust. Support for the study was received from the Legal Aid Board and the Royal College of Surgeons of England.
Abbreviations
Acf, activation frequency
B.Ar, bone area
BFR/B.Pm, tissue based bone formation rate
B.Pm, bone perimeter
dL.Pm, double labelled perimeter
LP, labelling period
L.Wi, interlabel width
MAR, mineral apposition rate
Md.Pm, mineralising perimeter
O.Pm, osteoid perimeter
sL.Pm, single labelled perimeter
T.Ar, tissue area
W.Wi, wall width
Footnotes
A R Lyons died July 1998.
REFERENCES
- 1.Fukuda K, Nisenbaum R, Stewart G, et al. Chronic multisystem illness affecting air force veterans of the Gulf War. JAMA 1998;280:981–8. [DOI] [PubMed] [Google Scholar]
- 2.Unwin C, Blatchley N, Coker W, et al. Health of UK servicemen who served in Persian Gulf War. Lancet 1999;353:169–78. [DOI] [PubMed] [Google Scholar]
- 3.Wegman DH, Woods NF, Bailar JC. Invited commentary: how would we know a Gulf War syndrome if we saw one? Am J Epidemiol 1997;146:704–11. [DOI] [PubMed] [Google Scholar]
- 4.Haley R, Thomas L, Hom J. Is there a Gulf War syndrome? Searching for syndromes by analysis of symptoms. JAMA 1997;277:215–22. [PubMed] [Google Scholar]
- 5.Haley R. Point: bias from the “healthy warrior effect” and unequal follow-up in three government studies of health effects of the Gulf War. Am J Epidemiol 1998;148:315–23. [DOI] [PubMed] [Google Scholar]
- 6.Kang HK, Bullman T. Counterpoint: negligible “healthy warrior effect” on Gulf War veterans’ mortality. Am J Epidemiol 1998;148:324–5. [DOI] [PubMed] [Google Scholar]
- 7.Haley RW. Counterpoint: Haley replies. Am J Epidemiol 1998;148:334–8. [Google Scholar]
- 8.Ismail K, Everitt B, Blatchley N, et al. Is there a Gulf War syndrome? Lancet 1999;353:179–82. [DOI] [PubMed] [Google Scholar]
- 9.Strauss SE. Bridging the gulf in war syndromes. Lancet 1999;353:162–3. [DOI] [PubMed] [Google Scholar]
- 10.Frost HM. Tetracycline-based analysis of bone remodelling. Calcif Tissue Int 1969;3:211–37. [DOI] [PubMed] [Google Scholar]
- 11.Compston JE. Bone histomorphometry. In: Feldman D, Glorieux FH, Pike JW, ed. Vitamin D. California USA: Academic Press Inc, 1997:576–86.
- 12.Croucher PI, Garrahan NJ, Compston JE. Assessment of cancellous bone structure: comparison of strut analysis, trabecular bone pattern factor and marrow space star volume. J Bone Miner Res 1996;11:955–61. [DOI] [PubMed] [Google Scholar]
- 13.Vedi S, Compston JE, Webb A, et al. Histomorphometric analysis of bone biopsies from the iliac crest of normal British subjects. Metab Bone Dis Relat Res 1982;4:231–6. [DOI] [PubMed] [Google Scholar]
- 14.Vedi S, Compston JE, Webb A, et al. Histomorphometric analysis of dynamic parameters of trabecular bone formation in the iliac crest of normal British subjects. Metab Bone Dis Relat Res 1984;5:69–74. [DOI] [PubMed] [Google Scholar]
- 15.WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser 1994;843. [PubMed]
- 16.Chavassieux PM, Arlot ME, Meunier PJ. Intermethod variation in bone histomorphometry; comparison between manual and computerised methods applied to iliac bone biopsies. Bone 1985;6:211–29. [DOI] [PubMed] [Google Scholar]
- 17.Wright CDP, Vedi S, Garrahan NJ, et al. Combined inter-observer and inter-method variation in bone histomorphometry. Bone 1992;13:205–8. [DOI] [PubMed] [Google Scholar]
- 18.Genever PG, Birch MA, Brown E, et al. Osteoblast-derived acetylcholinesterase: a novel mediator of cell–matrix interactions in bone? Bone 1999;24:297–304. [DOI] [PubMed] [Google Scholar]
- 19.Grisaru D, LevLehman E, Schapira M, et al. Human osteogenesis involves differentiation-dependent increases in the morphogenetically active 3′ alternative splicing variant of acethylcholinesterase. Mol Cell Biol 1999;19:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Compston JE, Vedi, S, Stephen AB, et al. Reduced bone formation after exposure to organophosphates. Lancet 1999;354:1791–2. [DOI] [PubMed] [Google Scholar]
- 21.Compston JE, Mellish RWE, Croucher P, et al. Structural mechanisms of trabecular bone loss in man. Bone Miner 1989;6:339–50. [DOI] [PubMed] [Google Scholar]
- 22.Burr DB, Martin RB, Schaffler MB, et al. Bone remodelling in response to in vivo microdamage. J Biomech 1985;18:189–200. [DOI] [PubMed] [Google Scholar]


