Abstract
Aims: To describe the clinical features of two patients with paraproteinaemia and necrobiotic xanthogranulomatosis together with detailed immunohistochemistry of the lesions in one.
Methods: The clinical history and results of biochemical investigations of the patients were retrieved from the files. Immunohistochemistry was used to investigate the expression of macrophage and mast cell markers, amyloid A and P, S-100 protein, and apolipoprotein AI and B in xanthogranulomatous skin lesions from patient 2. In addition, protein A–sepharose chromatography was used to separate serum from patient 2 and apolipoprotein B and the IgG paraprotein were measured in the fractions eluted.
Results: Monocytes/macrophages comprised the major cellular component of the lesion, and unusually for xanthomata, areas of collagen necrosis were also seen. Activated mast cells were present at the margins of macrophage clusters and adjacent to areas of collagen necrosis. Serum paraprotein was bound to low density lipoproteins as judged by protein A–sepharose chromatography, and was also located within macrophagic foam cells of the lesion on immunohistochemistry.
Conclusions: These observations demonstrate many features similar to atherosclerosis including collagen necrosis and mast cell activation.
Keywords: necrobiotic xanthogranulomatosis, paraprotein, low density lipoprotein, collagen necrosis
Xanthomata have many features in common with atheromatous lesions, in particular the presence of macrophages with foam laden cytoplasm and the elaboration of collagen by fibroblasts. One feature of atheroma, collagen fissuring, is absent except in one of the rarest types of xanthomata, necrobiotic xanthogranulomatosis (NXG), which occurs in association with paraproteinaemia. The fissuring or dissolution of the collagen in the lesions of xanthogranulomatosis has been described as collagen necrosis.
“Necrobiotic xanthogranulomatosis consists of pronounced subcutaneous xanthogranulomatous plaques around the orbital margins, which have a tendency to become inflamed, and is often associated with hard nodules in the subcutaneous fascia elsewhere in the body”
The xanthomata most often associated with paraproteinaemia are orange planar xanthomata, located symmetrically on the neck or thorax. This type of xanthomata can occur in myeloma even when it is associated with hypolipoproteinaemia. Hypolipoproteinaemia is the most common lipoprotein disturbance associated with neoplasia, but myeloma and benign paraproteinaemia can also cause hyperlipoproteinaemia. In extreme cases, this can mimic type III hyperlipoproteinaemia with extensive xanthomatosis. This has been reported to result from physical association of the paraprotein with chylomicron remnants and intermediate density lipoprotein, which interferes with their catabolism.1 In 1980, Kossard and Winkelmann described an even more unusual form of xanthomatosis associated with paraproteinaemia, which is now termed NXG.2 This consisted of pronounced subcutaneous xanthogranulomatous plaques around the orbital margins, which had a tendency to become inflamed, and were often associated with hard nodules in the subcutaneous fascia elsewhere in the body. In many of the cases subsequently reported the paraproteinaemia often behaved as a benign paraproteinaemia and the serum lipid values were normal or occasionally low.3 Histologically, these xanthomatous lesions were different from the xanthomata typically associated with primary hyperlipoproteinaemia. The anticipated Touton-type foamy macrophages were present, but in addition there was collagen necrosis, which is not generally seen in other xanthomata. The two other skin lesions in which collagen necrosis occurs are granuloma annulare and necrobiosis lipoidica diabeticorum.
NXG is a rare disorder. Twelve years after the original description, Mehregan and Winkelmann3 gathered 48 cases (32 of their own and 16 from the literature). Since then, other examples have occasionally been reported.4–7 However, in only one case was detailed biochemical analysis reported7 and immunohistochemistry is largely lacking from reports. In part, this is probably because of the tendency of the lesions to ulcerate and heal slowly after biopsy, making repeat tissue sampling unwelcome after the initial diagnostic biopsy. Here, we report two further cases. Both have particularly florid features and in one a detailed biochemical and immunohistochemical investigation has been undertaken.
PATIENTS
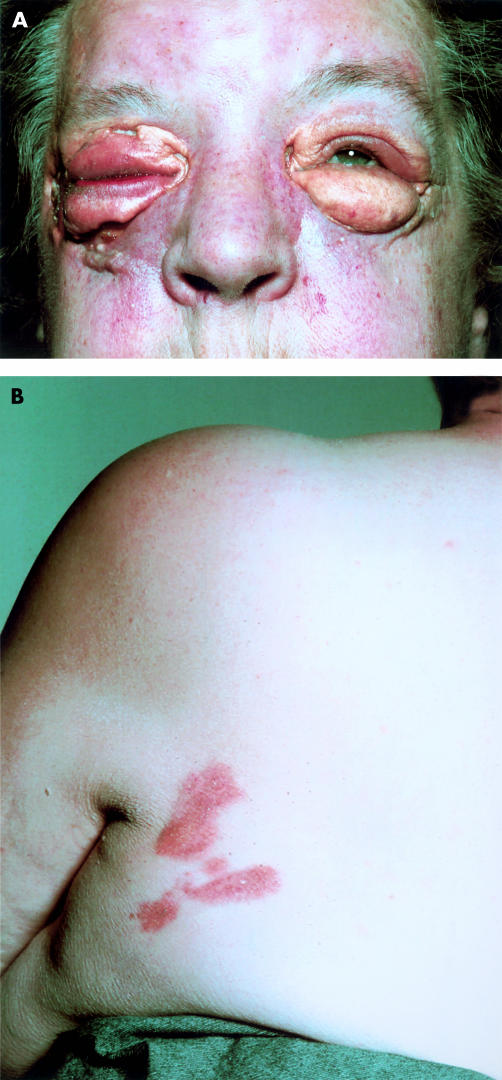
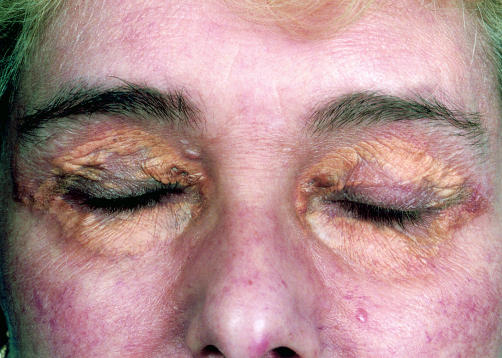
Patient 1 MP, a 65 year old woman, presented in 1973 with a history of progressive swelling of the upper and lower eyelids, which were yellow in colour. Initially, a diagnosis of hypothyroidism was considered, but this was not supported by thyroid function tests. Surgical removal was attempted in 1975, but resulted in failure of healing, recurrence of the lesions, and periorbital oedema. The oedema subsided after antibiotic treatment, but left the patient with pronounced xanthogranulomatous swellings of the eyelids (fig 1A). Histology in 1975 revealed the histological features of NXG with fibroblasts, foamy macrophages (Touton giant cells), a supporting vascular stroma, and areas of necrosis frequently containing cholesterol crystals. These histological features were not recognised as those of NXG at the time, because this syndrome was not described until 1980.2 Planar xanthomata developed dorsally, lateral to the left scapular region (fig 1B). Serum cholesterol was 6.49 mmol/litre with normal fasting and serum light scattering indices indicated normal triglyceride values.8 Serum biochemical, haematological, and immunological tests were all within normal limits with the exception of a monoclonal IgG κ protein present in serum, but not urine. There was no depression of IgA or IgM and bone marrow aspirate was normal in appearance. Patient 2 AG, a 55 year old woman, presented in 1992. She had been well until the age of 51 years apart from hypertension treated for three years. Then she began to develop raised orange/yellow, rather vascular lesions with an erythematous base around the orbits. These progressed during the next four years (fig 2) and a swelling deep in the tissues overlying the angle of her left jaw also formed. Additional findings on examination were corneal arcus and moderate emphysema (she smoked 20 cigarettes each day). Biopsy of one of the orbital lesions revealed histological features of NXG, and IgG κ paraproteinaemia (14 g/litre) was discovered in her plasma. Bone marrow showed normal cellularity with no excess of plasma cells or lymphocytes. The erythrocyte sedimentation rate was in the range 49–100 mm in the first hour, full blood count was normal, and serum IgG was 24.7 g/litre (reference range, 5.9–15.6 g/litre), IgA was 1.10 g/litre (reference range, 0.6–5.0 g/litre), and IgM was 0.94 g/litre (reference range, 0.6–2.9 g/litre). Serum cholesterol was 6.68 mmol/litre and fasting serum triglyceride was 1.95 mmol/litre. As assessed by ultracentrifugation, serum very low density lipoprotein cholesterol was 0.44 mmol/litre, serum low density lipoprotein (LDL) cholesterol was 4.87 mmol/litre, and serum high density lipoprotein (HDL) cholesterol was 1.37 mmol/litre. Serum apolipoprotein B (apo B) was 1220 mg/litre and serum apolipoprotein AI (apo AI) was 1590 mg/litre. The apolipoprotein genotype was E3/3. Fasting plasma glucose was 5.3 mmol/litre and serum thyroid function tests indicated that she was euthyroid. Serum albumin, liver enzymes, creatinine, electrolytes, and calcium were within the normal ranges.
Figure 1.
(A) Periorbital xanthogranulomata in patient 1 several weeks after surgery; (B) subcutaneous planar xanthomata over left scapular region in patient 1.
Figure 2.
Periorbital xanthogranulomata in patient 2.
After the removal of a tooth, the deposit in the jaw was also found to be NXG. Since then, other nodular deposits deep to the subcutaneous fascia have developed in the lumbosacral region and in the mons pubis. In general, the discovery of one of these lesions has been preceded by localised burning pain. Mammography showed a benign nodule in the lower half of the left breast. Marrow biopsy on three further occasions remained normal. Paraprotein values have also remained stable, in the range 13.0–19.0 g/litre, with IgG, IgA, and IgM within normal limits. Because of the progression of the lesions in the early phase of her illness, treatment was attempted with cyclical melphalan (10 mg daily) and prednisolone (40 mg daily) for seven days each month. However, this caused thrombocytopenia but the patient recovered on discontinuing the regimen. After this, plasmapheresis was carried out every 10–14 days on five occasions, but was unacceptable to the patient thereafter. Simvastatin 10 mg each evening (which decreased the serum total cholesterol to 4.0 mmol/litre), probucol 500 mg twice daily, and tocopherol 200 mg daily were all tried for one year, but did not result in resolution of the lesions, which have not, however, progressed for the past five years despite no treatment.
METHODS
Immunohistochemical methods
Formalin fixed, paraffin wax embedded xanthogranulomatous skin lesions from patient 2 were immunostained for macrophage markers (CD68 KP1, CD68 PG-M1, and HAM56), amyloid A and P, and S-100 protein with antibodies obtained from Dako (Glostrup, Denmark). Mast cells were identified with antibody to mast cell tryptase (Biogenesis, Poole, UK) and antibodies to apo AI and apo B were obtained from Immuno AG (Vienna, Austria) and Biogenesis, respectively. Primary antibodies were detected using secondary biotinylated antibodies followed by StreptABComplex conjugated to alkaline phosphatase or horseradish peroxidase (all from Dako).
Protein A–sepharose chromatography
Serum samples (1 ml) from patient 2 and from a 35 year old, healthy, female control were diluted with an equal volume of phosphate buffered saline pH 7.4 (PBS) and applied to a chromatography column containing 5 ml of protein A–sepharose (Pharmacia, Uppsala, Sweden), pre-equilibrated with PBS, at a flow rate of 20 ml/hour. The column was eluted with PBS and 5 ml fractions collected until the absorbance at 280 nm was < 0.05 absorbance units. At this point, the column was eluted with 0.1M glycine (pH 2.8) and 2 ml fractions were collected. The apo B concentration in the fractions was analysed by our in house immunoradiometric assay.9 The concentration of IgG paraprotein in the fractions was measured by electrophoresis and immunoblotting.
RESULTS
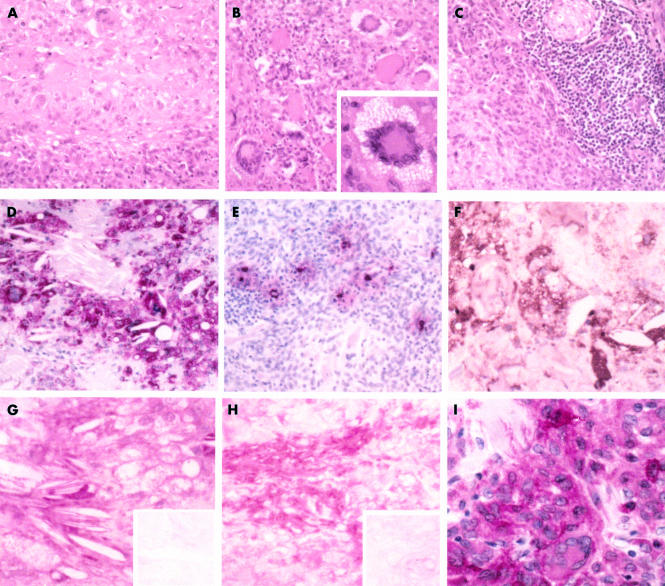
Biopsy specimens were formalin fixed, paraffin wax embedded, and sectioned, and the initial diagnosis of NXG was reached from haematoxylin and eosin staining. The lesion contained amorphous, acellular regions of collagenous matrix, staining deeply eosinophilic (fig 3A). Macrophages, foam cells, giant cells, and lymphocytes were recognised; most of the giant cells were of the Touton type with nuclei grouped around eosinophilic cytoplasm (fig 3B). T cell infiltrations were seen frequently (fig 3C).
Figure 3.
Histological features and immunoreactivity patterns of necrobiotic xanthogranulomatosis (patient 2). Part of the lesion showing (A) fields of necrobiotic collagen and (B) numerous giant cells, mostly of the Touton type (insert: high magnification of a typical example). (C) The accumulation of lymphocytes and a cross section of a nerve bundle in the top right corner of the microphotograph. (D) Staining for CD68 (KP1) revealed that most of the cells surrounding the cholesterol clefts are of monocyte/macrophage lineage. (E) Mast cells scattered between macrophages and lymphocytes showed signs of activation/degranulation as judged by extracellular staining for mast cell tryptase. (F) Staining for IgG κ light chains was seen extracellularly, in plasma cells, and in macrophagic foam cells. (G) Apolipoprotein AI and (H) apolipoprotein B were similarly located (inserts: negative controls). (I) Some macrophages stained for amyloid A. Original magnifications: (A–F), ×140; (G–I) and insert in (B), ×350. Staining: (A–C), haematoxylin and eosin; (A–G) counterstained with Mayer’s haematoxylin; (H, I), no counterstaining.
Monocytes/macrophages comprised the major cellular component of the lesion, as demonstrated by the macrophage markers CD68 (KP1), CD68 (PG-M1), and HAM56 (fig 3D). Foam cells and giant cells were all stained with CD68 (KP1), whereas HAM56 staining was most pronounced at the periphery of the lesion, demonstrating smaller macrophages and the absence of giant cells. Mast cells, identified by tryptase immunostaining (fig 3E), were seen mainly in perivascular locations and at the margins of macrophage accumulations, often close to necrobiotic collagen and lymphocytes. Mast cell activation, as judged by extracellular tryptase staining, was seen frequently in the NXG lesion, in contrast to mast cells of the adjacent normal skin, which were sparse and showed no evidence of activation/degranulation.
Immunostaining for IgG κ chains was mainly located in and around foam cells and some plasma cells, with limited extracellular staining on the perivascular matrix (fig 3F). Immunostaining for IgG λ chains was restricted to plasma cells (not shown). Cholesterol clefts were seen in association with foam cells and some giant cells, these sites also showing extracellular and intracellular lipoproteins indicated by immunostaining for apo AI (fig 3G) and apo B (fig 3H).
Although amyloid was not detectable with conventional Congo red staining, amyloid A immunostaining was demonstrated both in and around some medium sized macrophagic foam cells (CD68/KP1+ on adjacent sections), using an antigen retrieval method recommended by Dako (fig 3I). In contrast, the immunolocalisation of amyloid P was restricted to elastic fibres of the skin surrounding the NXG lesion. Small nerve bundles in the adjacent skin tissue and trapped in the inflammatory infiltrate surrounding the lesion, in addition to Langerhans cells of the epidermis, were stained with antibody to the S-100 protein (not shown). However, this was absent from the lesion itself.
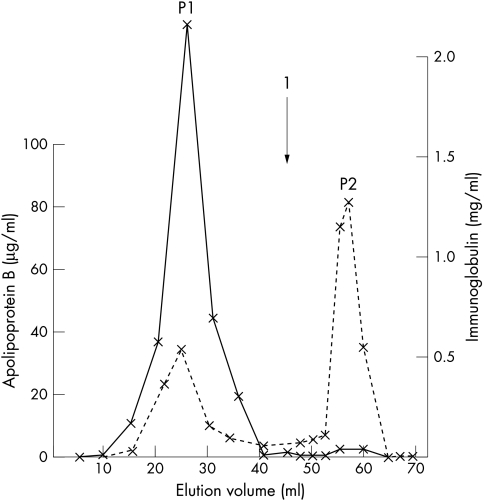
Protein A–sepharose chromatography of serum from patient 2 (fig 4) indicated that a substantial proportion of apo B was associated with the paraprotein.
Figure 4.
Column chromatography of serum from patient 2 on a protein A–sepharose gel. The solid line represents the concentration of IgG paraprotein in the eluant and the broken line that of apolipoprotein B. The arrow (1) indicates the start of the glycine elution. Apolipoprotein B co-elutes with the paraprotein in peak P1 and separately in peak P2.
DISCUSSION
NXG is a rare disorder for which there is no satisfactory treatment. Melphalan did not shrink the lesions in patient 2 and neither did the reduction of LDL cholesterol with Simvastatin or antioxidant treatment. Surgery has nothing to offer, as was dramatically illustrated by patient 1, who underwent surgery in 1975 before NXG had been described in the literature.2 After surgery there was failure of healing, periorbital oedema, and recurrence of the lesions (fig 1Af1). In our present study, a substantial proportion of the serum paraprotein co-eluted with the apo B containing lipoproteins, on protein A–sepharose chromatography, indicating that they are physically associated, because otherwise they would elute separately on the basis of their different molecular masses. This confirms a previous report that paraprotein in a patient with xanthomatosis, who had a cholesterol level that was around average for the healthy population, can be physically associated with lipoproteins.10 This is the first occasion that an immunohistochemical study of the NXG lesion has been undertaken and this revealed that macrophages in the lesion contain both paraprotein and apo B, suggesting the possibility that the apo B–paraprotein complex is taken up by them in vivo. The finding that apo AI and serum amyloid A are also present suggests either that HDL can complex with the paraprotein or that it is recruited into the lesion in response to it: similar apo AI findings have been reported for atheromatous lesions.11
Although uncommon, NXG has attracted renewed interest because some of the events contributing to the lesion may resemble those that occur in atherogenesis.12 The presence of macrophage foam cells is common to all types of xanthomata and to atheroma,13 but the collagen necrosis occurring in NXG appears to be unique among xanthomata. However, it does resemble the collagen dissolution that occurs in the fibrous cap of atheromatous lesions in areas of macrophage and lymphocyte derived foam cell activity, which has been suggested to be a feature of cholesterol rich atheromata, making them liable to rupture.14,15 Metalloproteinases secreted by foam cells or fibroblasts have been proposed as the mechanism responsible for the disappearance of collagen at these sites.16 The release of the enzymes, tryptase and chymase, by the activated mast cells that are frequently seen in NXG lesions is well known to activate metalloproteinase precursors secreted by macrophages.17 However, LDL uptake via the scavenger receptor pathway of macrophages (at least in vitro) does not lead to metalloproteinase secretion by these cells. In contrast, the uptake of LDL containing immune complexes by macrophagic U937 cells has been shown to induce matrix metalloproteinase 1 expression.18 Macrophage uptake of LDL aggregated with immunoglobulins has been reported to occur by phagocytosis rather than by receptor mediated mechanisms.19 Such LDL–immune complex aggregates were shown in our patient 2, suggesting a potential interaction with the monocytes/macrophages of the xanthomatosis lesions, and thereby promoting collagenolytic activity. There have been two in vitro studies of macrophage uptake of LDL from patients with xanthomatosis and paraproteinaemia. These show that LDL uptake can occur both as a phagocytic response7 and as the result of scavenger receptor activity.10
“A substantial proportion of the serum paraprotein co-eluted with the apolipoprotein B containing lipoproteins, on protein A–sepharose chromatography, indicating that they are physically associated”
The presence of strongly bound LDL–immunoglobulin complexes in NXG leading to phagocytic uptake by macrophages may explain why collagen necrosis is more evident than in other xanthomata. Indeed, a physical association between the paraprotein and lipoproteins was not as readily demonstrable as in our case 2 in a patient with the more common disease, planar xanthomata.20 Presumably, the foam cells in most xanthomata are the result of LDL uptake via the scavenger receptors of macrophages, and one rare family has been described in which xanthomatosis occurred as a result of the overexpression of scavenger receptors.21
Our in situ observations of mast cells intermixed with macrophages and foam cells suggest a further mechanism by which macrophage foam cells could be formed, in addition to LDL uptake via scavenger receptors or as LDL–immunoglobulin complexes. Macrophage foam cells can be formed in vitro as a result of their phagocytic uptake of LDL aggregates induced by partial proteolysis by chymase and carboxypeptidase and by heparin released from mast cell granules.22 Furthermore, activated mast cells are also reported to reduce cholesterol efflux from foam cells, a process probably reflecting the action of chymase on HDL.23 These observations have previously been applied to mast cell/foam cell associations in atherosclerotic lesions,22,23 but may be equally relevant to the NXG histology reported here.
Quite why different types of xanthomata have predilections for different parts of the body is poorly understood. The vulnerability of periorbital subcutaneous tissues to the more common, less florid xanthelasmata may explain the particularly aggressive nature of NXG in this area. The burning pain experienced in the lesions may be the result of neural involvement, which can be a feature of other florid xanthomata, occurring in—for example, type V hyperlipoproteinaemia24 and primary biliary cirrhosis.25
Take home messages
Necrobiotic xanthogranulomatosis (NXG) has many features in common with atherosclerosis including collagen necrosis and mast cell activation
The presence of strongly bound low density lipoprotein–immunoglobulin complexes in NXG leading to phagocytic uptake by macrophages may explain why collagen necrosis is more evident than in other xanthomata
As new cases of NXG arise, immunohistochemistry of fresh biopsy material for metalloproteinases may provide further insight into its pathogenesis and may also contribute to our understanding of atheromatous plaque rupture
As new cases of NXG arise, immunohistochemistry of fresh biopsy material for metalloproteinases may provide further insight into its pathogenesis and may also contribute to our understanding of atheromatous plaque rupture.
Acknowledgments
We are grateful to the late Professor S W Stanbury for drawing our attention to the earlier patient who underwent surgery. We thank Ms C Price for expertly preparing this manuscript and the University of Manchester Department of Medical Illustration for the photographs of patients. Support for the study was gratefully received from the NHS Research and Development Levy.
Abbreviations
apo, apolipoprotein
LDL, low density lipoprotein
HDL, high density lipoprotein
NXG, necrobiotic xanthogranulomatosis
PBS, phosphate buffered saline
REFERENCES
- 1.Roberts-Thompson PJ, Venables GS, Onitiri AC, et al. Polygenic 1gA myeloma, hyperlipidaemia and xanthomatosis: a further case and review. Postgrad Med J 1975;51:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kossard S, Winkelmann RK. Necrobiotic xanthogranuloma. Australas J Dermatol 1980;21:85–8. [DOI] [PubMed] [Google Scholar]
- 3.Mehregan DA, Winkelmann RK. Necrobiotic granuloma. Arch Dermatol 1992;128:94–100. [PubMed] [Google Scholar]
- 4.Rose GE, Patel BC, Garner A, et al. Orbital xanthogranuloma in adults. Br J Ophthalmol 1991;75:680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornblath WT, Dotan SA, Trobe JD, et al. Varied clinical spectrum of necrobiotic xanthogranuloma. Ophthalmology 1992;99:103–7. [DOI] [PubMed] [Google Scholar]
- 6.Winkelmann RK, Litzow MR, Umbert IJ, et al. Giant cell granulomatous pulmonary and myocardial lesions in necrobiotic xanthogranuloma with paraproteinemia. Mayo Clin Proc 1997;72:1028–33. [DOI] [PubMed] [Google Scholar]
- 7.Matsuura F,Yamashita S, Hirano K, et al. Activation of monocytes in vivo causes intracellular accumulation of lipoprotein-derived lipids and marked hypocholesterolaemia—a possible pathogenesis of necrobiotic xanthogranuloma. Atherosclerosis 1999;142:355–65. [DOI] [PubMed] [Google Scholar]
- 8.Stone MC, Thorpe JM. A new technique for the investigation of the low density lipoproteins in health and disease. Clin Chim Acta 1966;14:812–30. [Google Scholar]
- 9.Arrol S, Mackness MI, Laing I, et al. Lipoprotein secretion by the human hepatoma cell line Hep G2: differential rates of accumulation of apolipoprotein B and lipoprotein lipids in tissue culture media in response to albumin, glucose and oleate. Biochim Biophys Acta 1991;1086:72–80. [DOI] [PubMed] [Google Scholar]
- 10.Feingold KR, Castro GR, Ishikawa Y, et al. Cutaneous xanthoma in association with paraproteinaemia. J Clin Invest 1989;83:796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackness B, Hunt R, Mackness MI, et al. Increased immunolocalization of paraoxonase, clusterin and apolipoprotein AI in the human artery wall with progression of atherosclerosis. Arterioscler Thromb Vasc Biol 1997;17:1233–38. [DOI] [PubMed] [Google Scholar]
- 12.Ylä-Herttuala S, Palinski W, Butler SW, et al. Rabbit and human atherosclerotic lesions contain IgG that recognises epitopes of oxidized LDL. Arterioscler Thromb 1994;14:32–40. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi W, Naito M. Lipid storage disease: part 1. Ultrastructure of xanthoma cells in various diseases. Acta Pathol Jpn 1983;33:959–77. [DOI] [PubMed] [Google Scholar]
- 14.Lendon C, Davies MJ, Born G, et al. Atherosclerotic plaque caps are locally weakened when macrophage density is increased. Atherosclerosis 1991;87:87–90. [DOI] [PubMed] [Google Scholar]
- 15.Moreno PR, Falk E, Palacios IF, et al. Macrophage infiltration in acute coronary syndromes: implications for plaque rupture. Circulation 1994;90:775–8. [DOI] [PubMed] [Google Scholar]
- 16.Galis ZS, Sukhova GK, Lark MW, et al. Increased expression of matrix-metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994;94:2493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lees M, Taylor DJ, Woolley DE. Mast cell proteinases activate precursor forms of collagenase and stromolysin, but not of gelatinase A and B. Eur J Biochem 1994;223:171–7. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Fleming AJ, Wu S, et al. Fc-γ receptor cross-linking by immune complexes induces matrix metalloproteinase-1 in U937 cells via nitrogen-activated protein kinase. Arterioscler Thromb Vasc Biol 2000;20:2533–8. [DOI] [PubMed] [Google Scholar]
- 19.Klimov AN, Denisenko AD, Popov AN, et al. Lipoprotein–antibody immune complexes. Their catabolism and role in foam cell formation. Atherosclerosis 1985;58:1–15. [DOI] [PubMed] [Google Scholar]
- 20.Johnston JD, Lumb PJ, Wierzbicki AS. Hyperlipidaemia in association with benign paraproteinaemia. Ann Clin Biochem 1997;34:697–9. [DOI] [PubMed] [Google Scholar]
- 21.Giry C, Giroux L-M, Roy M, et al. Characterization of inherited scavenger receptor overexpression and abnormal macrophage phenotype in a normolipidemic subject with planar xanthomas. J Lipid Res 1996;37:1422–35. [PubMed] [Google Scholar]
- 22.Kovanen PT. Role of mast cells in atherosclerosis. In: Marone G, ed. Human basophils and mast cells: clinical aspects. Chemical Immunology 1995, Vol. 62. Basel: Karger, 1995:132–70. [PubMed]
- 23.Jeziorska M, McCollum C, Woolley DE. Mast cell distribution, activation and phenotype in atherosclerotic lesions of human carotid arteries. J Pathol 1997;182:115–22. [DOI] [PubMed] [Google Scholar]
- 24.Matthew NT, Meyer JS, Achori AN, et al. Hyperlipidaemic neuropathy and dementia. Eur Neurol 1976;14:370–82. [DOI] [PubMed] [Google Scholar]
- 25.Turnberg LA, Mahoney MP, Gleeson MH, et al. Plasmapheresis and plasma exchange in the treatment of hyperlipaemia and xanthomatous neuropathy in patients with primary biliary cirrhosis. Gut 1972;13:976–81. [DOI] [PMC free article] [PubMed] [Google Scholar]