Abstract
Aims: To investigate the prognostic relevance of vascular endothelial growth factor (VEGF) and its receptor Flt-1 in nephroblastoma and whether tumour microvessel density (MVD) immunoreactivity, determined by the CD31 antigen, is related to the expression of VEGF and Flt-1.
Methods: The expression of VEGF and Flt-1 and MVD were investigated by means of immunohistochemical analysis in 62 Wilms’s tumours. Patients were treated preoperatively with chemotherapy and had a mean follow up of 5.7 years.
Results: In general, VEGF and Flt-1 were expressed in normal kidney parenchyma and to a variable extent in the three main components of Wilms’s tumour, namely: the blastemal, epithelial, and stromal cells. In tumour tissue, 52% and 47% of blastemal cells were positive for VEGF and Flt-1, respectively. A non-significant correlation was found between the expression of VEGF and Flt-1 in blastemal and epithelial cells and the clinicopathological stage. MVD was significantly higher in VEGF and Flt-1 positive tumours than in VEGF and Flt-1 negative tumours. Univariate analysis showed that the expression of VEGF and Flt-1 in blastemal cells was indicative of clinical progression and tumour specific survival. In addition, MVD expression was indicative of clinical progression. Epithelial staining was of no prognostic value. In a multivariate analysis, VEGF protein expression by blastemal cells was an independent prognostic marker for clinical progression.
Conclusions: These results indicate that VEGF and Flt-1 protein expression are closely related to MVD and seem to be an important predictor for poor prognosis in treated patients with Wilms’s tumour. Therefore, the expression of these molecules in primary Wilms’s tumour may be useful in identifying those patients at high risk of tumour recurrence and in guiding antiangiogenic treatment.
Keywords: Wilms’s tumour, vascular endothelial growth factor, Flt-1, microvessel density, prognosis, immunohistochemistry
Wilms’s tumour is a paediatric malignancy of the kidney and one of the most common solid tumours in children.1 Despite the remarkable response to chemotherapy, 5–10% of tumours are fatal because of the occurrence of metastases and drug resistance.2 Therefore, it is important to find markers that can predict recurrence or the development of metastases so that we can screen for high risk, early stage patients, who may need preventive chemotherapy or other adjuvant treatment. In solid tumours, it has been suggested that angiogenesis plays an important role in tumour progression and the spread of metastases through the bloodstream.3 In fact, tumour growth beyond 1–2 mm is strictly dependent on angiogenesis.4
“Previous studies indicated that vascular endothelial growth factor and its receptor Flt-1 play an important role in tumour metastasis, and are associated with poor prognosis in clinical human tumours”
It has been reported that several growth factors with angiogenic activity are produced by solid tumours.5 Vascular endothelial growth factor (VEGF) is an angiogenic factor that is highly specific for endothelium, and also functions as a vascular permeability factor.6 VEGF has been identified during normal growth and development in the kidney, and it has been found in genitourinary neoplasms.7,8 The fms-like tyrosine kinase (Flt) is a transmembrane receptor of the tyrosine kinase family, which has been identified as a receptor for VEGF.9,10 Recently, the expression of VEGF receptors has been detected in several types of non-endothelial cells, such as melanoma cell lines, some leukaemic cell lines, and retinal progenitor cells, suggesting that VEGF receptors are not restricted to endothelial cells.11–13 Previous studies indicated that VEGF and its receptor Flt-1 play an important role in tumour metastasis, and are associated with poor prognosis in clinical human tumours.14–16 It has also been reported that tumour angiogenesis, as reflected by microvessel density (MVD), may provide prognostic information in carcinomas of the breast, the uterine cervix, and the ovary, in addition to a variety of other malignancies.17–21
With regard to Wilms’s tumour, one study using a murine model reported the overexpression of VEGF in a highly metastatic Wilms’s tumour line.22 However, the clinical relevance of this animal study has not yet been demonstrated. In our present study, the prognostic relevance of VEGF and its receptor Flt-1 and the association of their expression with MVD were determined in patients with Wilms’s tumour who were treated by preoperative chemotherapy and radical nephrectomy.
MATERIALS AND METHODS
Patients
During the period 1987 to 1999, 62 patients with nephroblastoma were treated by neoadjuvant chemotherapy and subsequent tumour nephrectomy. After treatment, the patients were followed regularly and all data concerning diagnosis, treatment, and follow up were stored in a database. Clinical progression was defined as histologically or cytologically confirmed local recurrence or the appearance of distant metastases. Tumour death was defined as death resulting from the direct effect of metastases.
Sample selection
All nephrectomy specimens were fixed in 10% buffered formalin and sections routinely obtained from the most vital appearing tumour areas were embedded in paraffin wax. The haematoxylin and eosin stained slides were reviewed by an experienced paediatric pathologist. The tumour stage was assessed according to the SIOP trial protocol established in the SIOP meeting in Stockholm in 1994.23 Among the tissue blocks available from individual patients, tumour samples containing the three different cell types of Wilms’s tumour were selected. In addition, adjacent normal kidney tissue was taken from each patient.
Antibodies
The following primary antibodies were used: rabbit antihuman VEGF and Flt-1 polyclonal antibodies (A-20 and C-17, respectively) from Santa Cruz, Santa Cruz, California, USA and mouse monoclonal antibody against CD31 (clone JC/70A; Dako A/S, Glostrup, Denmark). The specificity and characteristics of these antibodies have been published elsewhere.3,11,12
Immunohistochemical staining procedures
Rabbit polyclonal antibodies against VEGF and Flt-1
The PAP (peroxidase–antiperoxidase) immunohistochemistry technique was used and applied to serial sections (5 μm) from all samples, which were mounted on 3-aminopropyltrietoxysilane (Sigma Co, St Louis, Missouri, USA) coated glass slides, and which were subsequently incubated overnight at 60°C. For antigen retrieval, the slides were microwaved at 700 W in 0.1M citrate buffer at pH 6.0 for 15 minutes. Sections were incubated with 10% normal goat serum (Dako A/S) in phosphate buffered saline (PBS)/5% bovine serum albumin (BSA) for 15 minutes. Subsequently, the sections were incubated with the primary antibody for one hour at 37°C in a humid chamber. The antibody was diluted in PBS/5% BSA at a 1/75 dilution for VEGF. After being incubated with goat antirabbit antiserum, the PAP complex (Dako) was diluted in PBS/5% BSA and incubated for 30 minutes, after which antigen antibody binding was visualised with diaminobenzidine tetrahydrochloride dihydrate (DAB; Fluka, Neu-Ulm, Germany). Negative controls were included by replacing the primary antibody with the IgG fraction of normal rabbit serum. Normal kidney tissue, which was present in most tissue specimens, served as a positive control.
Immunohistochemical staining with rabbit polyclonal antibody to Flt-1 was performed in the same way as that for VEGF, except that the microwave treatment was omitted.15 The dilution of antibody for Flt-1 was 1/30.
Monoclonal antibody to CD31
Serial sections of the same specimens immunostained for VEGF and Flt-1 were used. The immunostaining procedure was similar to that of VEGF, with the exception of microwave treatment, which was performed by digestion with 0.1% trypsin for 10 minutes at 37°C. After rinsing with cooled PBS, slides were incubated with 10% normal rabbit serum for 15 minutes (Dako A/S). Subsequently, slides were incubated with the primary antibody to CD31 (Dako A/S) for one hour at 37°C in a humid chamber. The antibody was diluted 1/10 in PBS/5% BSA. After being incubated with rabbit antimouse immunoglobulin (Dako A/S), the PAP complex was diluted in PBS/5% BSA and incubated for 30 minutes, after which antigen antibody binding was visualised with DAB (Fluka).
Immunostaining analysis (quantification)
The slides were examined at ×25 magnification without knowledge of the clinical outcome of the patients. The proportion of tumour cells staining for VEGF and Flt-1 was graded on an arbitrary scale of 0–4, namely: 0, no positive tumour cells; 1+, < 10% positive; 2+, 10–25% positive; 3+, 25–50% positive; and 4+, > 50% positive. The specimens were regarded as positive when the percentage of positive cells was > 10%.
Microvessel staining and counting
Immunohistochemical staining for the endothelial marker CD31 was carried out to evaluate MVD in tumour tissues. The number of CD31 positive vessels was recorded by counting any positively stained endothelial cells or endothelial cell clusters as a single, countable microvessel in a ×250 field (×25 objective and ×10 ocular; equivalent to 0.64 mm2/×250 field) in the five areas with the highest vascular density. The mean of five counts was used as the MVD for each case. MVD was performed by two investigators (GM and MRB), neither of whom had knowledge of the clinical outcomes, clinicopathological features, or expression of VEGF. MVD was not only analysed as a continuous variable, but also as a dichotomous variable, defining < 15 microvessels/field (0.64 mm2) as less vascularised and > 15/field as highly vascularised. The results of the CD31 counts were expressed as a mean (SD).
Statistical analysis
Statistical analysis was performed using the SPSS 9.0 software package. The association between VEGF and Flt-1 expression and clinicopathological features was analysed using Pearson’s χ2 test. VEGF and Flt-1 immunostaining and its association with CD31 counts were analysed by the paired sample t test and the ANOVA test. For the analysis of survival data, Kaplan Meier curves were constructed and the logrank test for trend was performed. Multivariate analysis was performed using Cox’s proportional hazards model, with p < 0.05 being considered significant.
RESULTS
Clinicopathological findings
Twenty six of the patients were girls and 36 were boys. The stage distribution was pT1 in 22, pT2 in 19, and pT3 in 21 patients. All the tumours studied were of the classic triphasic type. Three of the patients had low risk tumours, whereas 59 had an intermediate risk tumour. The mean overall follow up period was 5.7 years, and the mean age at surgery was 4.7 years. Clinical progression occurred in 14 patients and seven patients died from their tumour. At the end of the follow up period, 55 patients were alive.
Expression of VEGF and its receptor Flt-1 in Wilms’s tumour tissues
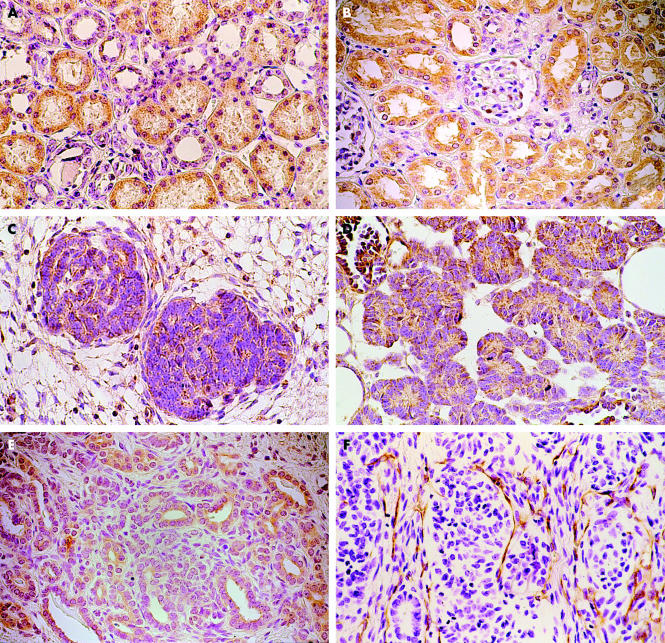
Immunoreactivity for VEGF and Flt-1 was found in most renal tubular structures, whereas staining was faint or absent in glomeruli (fig 1A, B). VEGF and Flt-1 immunoreactive blastemal cells were found in 32 and 29 of the patients with Wilms’s tumour, respectively. VEGF and Flt-1 immunoreactive tumour epithelial cells were found in 38 and 35 of the patients, respectively. Immunostaining was mainly localised to the cytoplasm of both the blastemal and epithelial cells (fig 1C–E). Most sections stained more intensely for Flt-1 than for VEGF. In addition, VEGF and Flt-1 staining was seen in the stromal component of all the tumours studied. Tumour cells with relatively strong staining for Flt-1 were seen more often in the peripheral than in the central zone of the tumours (results not shown). Absent or faint VEGF staining was seen on some endothelial cells. Although increased expression of VEGF and Flt-1 was seen in both the blastemal and epithelial component of the tumours with increasing pathological stages (T1 to T3), this difference was not significant (table 1). Intertumoral variation was limited. Epithelial differentiation in tumours was accompanied by diffuse expression (fig 1D, E).
Figure 1.
Immunohistochemical staining for vascular endothelial growth factor (VEGF) and its receptor (Flt-1) in (A, B) normal renal tissue and (C–E) nephroblastoma tissue. (A) VEGF and (B) Flt-1 were detected in the tubular structures of normal renal tissue. VEGF and Flt-1 were mainly identified in the cytoplasm of the blastemal component (C and E, respectively) and in the epithelium of nephroblastoma tissue (D and E, respectively). (F) Nephroblastoma tissue stained with CD31 detected microvessels, which are seen in the stromal tissue. Original magnification, ×400.
Table 1.
Relation between pT stage and blastemal and epithelial cell expression
| VEGF | Flt-1 | |||
| Stage | Blastemal | Epithelial | Blastemal | Epithelial |
| T1 | 9 (15) | 10 (16) | 8 (13) | 8 (13) |
| T2 | 9 (15) | 13 (21) | 8 (13) | 12 (19) |
| T3 | 14 (23) | 15 (24) | 13 (21) | 15 (24) |
| Total | 32 (52) | 38 (61) | 29 (47) | 35 (57) |
| p Value | >0.05 | >0.05 | ||
Data are presented as numbers, with percentages in parenthesis.
VEGF, vascular endothelial growth factor.
MVD and its correlation with VEGF and Flt-1 expression
Microvessels were defined by the presence of CD31 stained capillaries or small clusters of CD31 positive cells (fig 1F). The microvessel count ranged from 0 to 50 with a mean of 20.5 (SD, 14.8). The number of vessels counted in tumours with VEGF and Flt-1 blastemal and epithelial positive cells was significantly greater than that found in the negative tumours (table 2). The high vascularisation foci occurred most frequently within the tumour stroma. An increase in the microvessel count was seen in both the blastemal and epithelial component of tumours with increasing pathological stages (data not shown). However, some patients had a high MVD despite being VEGF and Flt-1 negative and some patients had a low MVD although they were VEGF and Flt-1 positive.
Table 2.
Correlation between MVD and the expression of VEGF and Flt-1
| MVD | ||||
| Cell component | Expression | Mean (SD) | p Value | |
| VEGF | Blastema | Negative | 11.5 (10.1) | <0.0005* |
| Positive | 29.5 (12.9) | |||
| Epithelial | Negative | 11.5 (2.9) | <0.0005* | |
| Positive | 26.7 (13.2) | |||
| Flt-1 | Blastema | Negative | 13.8 (12.2) | <0.0005* |
| Positive | 28.7 (13.3) | |||
| Epithelial | Negative | 14.2 (12.4) | <0.003* | |
| Positive | 25.9 (14.3) | |||
| VEGF and Flt-1 | Blastema | Both negative | 10.3 (9.6) | <0.0005† |
| Both positive | 31.1 (12.6) | |||
| Either VEGF or Flt-1 positive | 22.1 (12.6) | |||
| Epithelial | Both negative | 9.8 (9.5) | <0.0005† | |
| Both positive | 27.3 (13.4) | |||
| Either VEGF or Flt-1 positive | 21.9 (15.2) | |||
*Paired sample t test; †ANOVA test.
MVD, microvessel density; VEGF, vascular endothelial growth factor.
Prognostic value of the VEGF and Flt-1 molecules
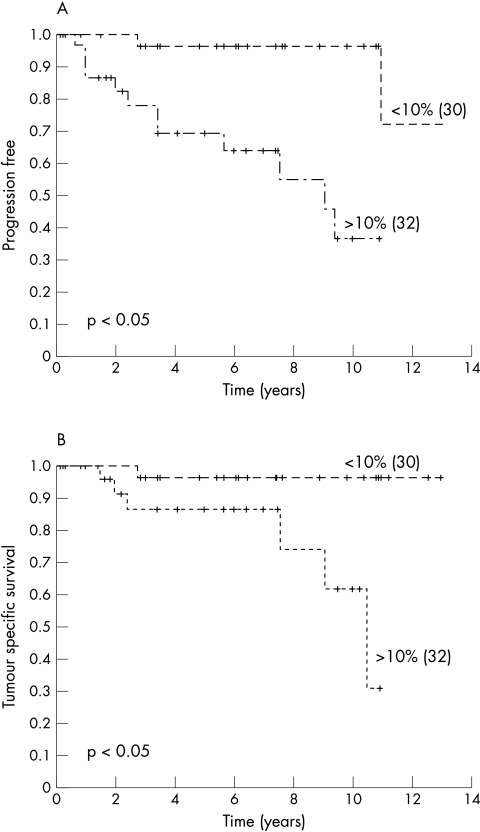
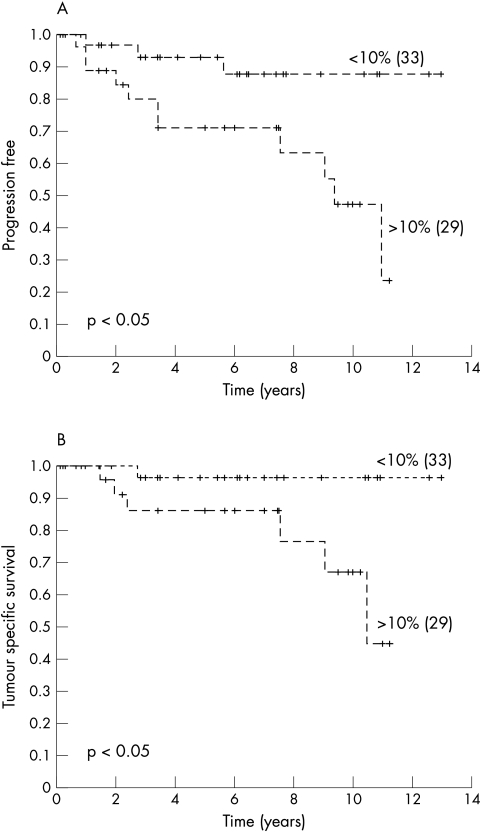
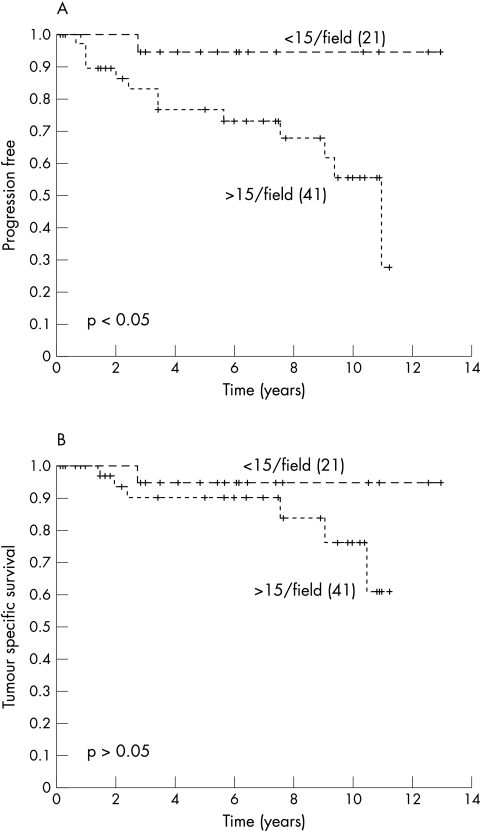
Univariate analysis using the logrank test for trend showed a prognostic value of blastemal cell VEGF and Flt-1 expression for clinical progression and tumour related death (table 3; figs 2, 3). However, the expression of VEGF and Flt-1 by epithelial cells had no prognostic value (table 3), whereas MVD had prognostic value for clinical progression but not for tumour related death (fig 4). To test whether VEGF, Flt-1, and MVD had prognostic impact, a multivariate Cox’s regression analysis was performed using the parameters pT stage, and VEGF, Flt-1, and MVD expression. The parameters that were not dichotomic were dichotomised as follows: pT1–2 versus pT3; immunoreactive score for VEGF and Flt-1< 10% versus > 10%, and for MVD < 15 microvessels/field (0.64 mm2 ) versus > 15/field. In that analysis, the expression of VEGF by blastemal cells had a hazard ratio of 7.1, indicating that it was a predictor of poor prognosis for clinical progression. There was no independent marker to predict tumour specific survival.
Table 3.
Univariate analysis of prognostic markers
| Blastemal cells | Epithelial cells | |||||||
| Clinical progression | Tumour specific survival | Clinical progression | Tumour specific survival | |||||
| Variable | χ2 | p Value | χ2 | p Value | χ2 | p Value | χ2 | p Value |
| VEGF | 12.8 | 0.0003 | 6.7 | 0.001 | 0.01 | 0.91 | 0.58 | 0.45 |
| Flt-1 | 6.25 | 0.01 | 5.2 | 0.02 | 0.18 | 0.68 | 0.07 | 0.79 |
p Values were estimated using the logrank test.
VEGF, vascular endothelial growth factor.
Figure 2.
Kaplan Meier curves showing a relation between blastemal cell vascular endothelial growth factor expression and (A) clinical progression and (B) survival. Censored patients are indicated by vertical marks along the line. The numbers of patients in each group are shown in parenthesis.
Figure 3.
Kaplan Meier curves showing a relation between blastemal cell Flt-1 expression and (A) clinical progression and (B) survival. Censored patients are indicated by vertical marks along the line. The numbers of patients in each group are shown in parenthesis.
Figure 4.
Kaplan Meier curves showing a relation between microvessel density and (A) clinical progression and (B) survival. Censored patients are indicated by vertical marks along the line. The numbers of patients in each group are shown in parenthesis.
DISCUSSION
Angiogenesis is essential for tumour growth and metastatic activity and is regulated by a variety of angiogenic molecules. Recently, several angiogenic factors have been identified, and VEGF is thought to be one such factor.7 Our present study was carried out to investigate whether the expression of the VEGF protein and its receptor (Flt-1) has prognostic value in specimens of nephroblastoma, using paraffin wax embedded tissue sections. All the patients received chemotherapy before nephrectomy. The expression of VEGF and its receptor (Flt-1) in normal kidney, which was present in almost all stained tissue sections, served as an internal control for the immunostaining procedure.
VEGF is a multifunctional cytokine that acts in a highly specific way as a mitogen on endothelial cells.3,6 VEGF is overexpressed in several malignant tumours.14 An association between VEGF expression in the primary tumour and poor prognosis has been found in colorectal carcinoma, node negative breast cancer, and early stage ovarian cancer. An increase in the serum concentration of VEGF was also shown to be a predictor of metastasis in hepatocellular carcinoma and gastrointestinal tumours.24–28 In contrast, a recent study by Kawauchi et al could not show a correlation between VEGF expression and either MVD or prognosis in synovial sarcoma.29 With respect to VEGF expression and gastric cancer prognosis, contradictory results are reported.30,31
A few studies have reported an association between the Flt-1 receptor and poor prognosis in human tumours.15,16 In our present study, we have shown that the expression of VEGF and Flt-1 by blastemal cells in primary Wilms’s tumour correlates with poor prognosis for patients with nephroblastoma. This is in line with the view that the blastemal component of Wilms’s tumour is associated with increased aggressiveness of the tumour, with a higher tendency for clinical progression; that is, metastatic ability.23 Chemotherapy is known to affect the cellular compartments of the Wilms’s tumour—the blastemal component in particular. Having access to this type of material derived from a considerable number of patients with a good stage distribution, the aim of our study was to determine those factors that could predict the clinical outcome of patients after chemotherapy and surgery. We were able to perform a similar immunohistochemical study on material derived from a limited number of patients with Wilms’s tumour who did not receive chemotherapy before surgery. Preliminary results of VEGF and Flt-1 staining in some of these tumours showed that the overall scores of the blastemal and epithelial cells are higher, but that these patients have a similar clinical outcome to those in the pretreatment group described in here.
“The significant correlation between microvessel density and vascular endothelial growth factor (VEGF) expression seen in our study suggests that VEGF is one of the major molecules responsible for neoangiogenesis in nephroblastoma”
Our immunohistochemical study also revealed that high MVD correlated positively with the degree of VEGF expression and poor prognosis for patients with nephroblastoma. Recently, Skoldenberg et al confirmed the significant impact of high MVD in Wilms’s tumour on survival and also found that the serum concentration of VEGF in these patients was three times higher than that seen in controls.32 The significant correlation between MVD and VEGF expression seen in our study suggests that VEGF is one of the major molecules responsible for neoangiogenesis in nephroblastoma, although the angiogenic potential is believed to be regulated by a balance between various types of angiogenic molecules and angiostatic substances. An association between VEGF expression and MVD has also been reported in carcinomas of the lung, stomach, breast, and vulva.20,31–34 A high MVD may promote metastatic disease by exposing tumours to a greater endothelial surface area, thus increasing the likelihood of haematogenous dissemination.14 In addition, the production of VEGF may enhance the metastatic capability of a tumour in several other ways.35,36
Although the reciprocal role of VEGF in tumour growth and chemotherapeutic efficacy remains to be elucidated, our data support the independent prognostic value of blastemal cell VEGF expression, which has also been documented for patients with oral squamous cell carcinoma treated with chemotherapy.37 However, Albo et al have shown a possible correlation between tumour MVD and chemosensitivity—they found that tumours with a higher MVD are more sensitive to chemotherapy.38 Because the delivery of chemotherapeutic reagents to the tumour is largely dependent on the blood supply, VEGF induced neovascularisation may in turn favourably affect the response to systemic chemotherapy.39
The finding that an enhanced vascular supply stimulated by VEGF induces an increased risk of metastasis suggests that antiangiogenic treatments could be a potential adjuvant therapeutic strategy for the prevention of metastasis after the resection of the tumour. In experimental animal studies, anti-VEGF antibody (A4.6.1) successfully suppressed both primary tumour growth and metastasis in a murine model of anaplastic Wilms’s tumour.40 These results, together with the results of our clinical study, suggest that antiangiogenesis treatments, such as ligands that antagonise the interaction of VEGF with its receptors or antibodies directed against VEGF and its receptors, may prove useful in the management of Wilms’s tumour.41,42
In conclusion, the results of our present study have shown that the expression of VEGF and its receptor (Flt-1) in primary Wilms’s tumour correlates with an increase in MVD in the tumour tissue and poor prognosis for patients with nephroblastoma. These findings suggest that VEGF secreted by nephroblastoma cells elicits neoangiogenesis. If these results are confirmed in additional studies, VEGF, Flt-1, and MVD could emerge as additional parameters for predicting the outcome of patients with nephroblastoma. VEGF and its receptor Flt-1 may also be considered as potential targets of an antiangiogenic therapeutic strategy.
Take home messages.
A non-significant correlation was found between the expression of vascular endothelial growth factor (VEGF) and its receptor (Flt-1) in blastemal and epithelial cells of Wilms’s tumour and the clinicopathological stage
The expression of VEGF and Flt-1 correlated with microvessel density (MVD), in addition to clinical progression and tumour specific survival
MVD expression was indicative of clinical progression
In a multivariate analysis, VEGF protein expression by blastemal cells was an independent prognostic marker for clinical progression
Thus, VEGF and Flt-1 protein expression are closely related to MVD and seem to be an important predictor for poor prognosis in treated patients with Wilms’s tumour
The expression of these molecules in primary Wilms’s tumour may be useful in identifying those patients at high risk of tumour recurrence and in guiding antiangiogenic treatment
Abbreviations
BSA, bovine serum albumin
DAB, diaminobenzidine tetrahydrochloride dihydrate
MVD, microvessel density
PAP, peroxidase-antiperoxidase
PBS, phosphate buffered saline
VEGF, vascular endothelial growth factor
REFERENCES
- 1.Beckwith JB. Renal neoplasms of childhood. In: Sternberg SS, ed. Diagnostic surgical pathology. New York: Raven Press, 1994:1741–66.
- 2.Groot-Loonen JJ, Pinkerton CR, Morris-Jones PH, et al. How curable is relapsed Wilms’ tumour? The United Kingdom children’s cancer study group. Arch Dis Child 1990;65:968–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senger D, Van De Water L, Brown LF, et al. Vascular permeability factor (VPF, VEGF) in tumour biology. Cancer Metastasis Rev 1993;12:303–24. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990;82:4–6. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J, Klagsbrun M. Angiogenic factors. Science 1995;235:442–7. [DOI] [PubMed] [Google Scholar]
- 6.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983;219:983–5. [DOI] [PubMed] [Google Scholar]
- 7.Breier G, Albrecht U, Sterrer S, et al. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 1992;114:521–32. [DOI] [PubMed] [Google Scholar]
- 8.Sowter HM, Corps AN, Evans AL, et al. Expression and localization of the vascular endothelial growth factor family in ovarian epithelial tumors. Lab Invest 1997;77:607–14. [PubMed] [Google Scholar]
- 9.De Vries C, Escobedo JA, Ueno H, et al. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992;255:989–91. [DOI] [PubMed] [Google Scholar]
- 10.Shibuya M, Yamaguchi S, Yamane A, et al. Nucleotide sequence and expression of a novel human receptor type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 1990;5:519–24. [PubMed] [Google Scholar]
- 11.Gitay-Goren H, Halaban R, Neufeld G. Human melanoma cells but not normal melanocytes express vascular endothelial growth factor receptors. Biochem Biophys Res Commun 1993;190:702–8. [DOI] [PubMed] [Google Scholar]
- 12.Shibuya M. Role of VEGF-Flt receptor system in normal and tumor angiogenesis. Adv Cancer Res 1995;67:281–316. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Cepko CL. Flk-1, a receptor for vascular endothelial growth factor (VEGF), is expressed by retinal progenitor cells. J Neurosci 1996;16:6089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dvorak HF, Brown LF, Detmar M, et al. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995;146:1029–39. [PMC free article] [PubMed] [Google Scholar]
- 15.Xie B, Tam NN, Tsao SW, et al. Co-expression of vascular endothelial growth factor (VEGF) and its receptors (flk-1 and flt-1) in hormone-induced mammary cancer in the Noble rat. Br J Cancer 1999;81:1335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takanami I, Tanaka F, Hashizume T, et al. Vascular endothelial growth factor and its receptor correlate with angiogenesis and survival in pulmonary adenocarcinoma. Anticancer Res 1997;17:2811–14. [PubMed] [Google Scholar]
- 17.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- 18.Wiggins DL, Granai CO, Steinhoff MM, et al. Tumor angiogenesis as a prognostic factor in cervical carcinoma. Gynecol Oncol 1995;56:353–6. [DOI] [PubMed] [Google Scholar]
- 19.Hollingsworth HC, Kohn EC, Steinberg SM, et al. Tumor angiogenesis in advanced stage ovarian carcinoma. Am J Pathol 1995;147:33–41. [PMC free article] [PubMed] [Google Scholar]
- 20.Fontanini G, Bigimi D, Vignati S, et al. Microvessel count predicts metastatic disease and survival in non-small cell lung cancer. J Pathol 1995;177:57–63. [DOI] [PubMed] [Google Scholar]
- 21.Gasparini G, Weidner N, Maluta S. Intratumoral microvessel density and p53 protein: correlation with metastasis in head and neck squamous cell carcinoma. Int J Cancer 1993;55:739–744. [DOI] [PubMed] [Google Scholar]
- 22.Kayton M, Rowe D, O’Toole K, et al. Metastasis correlates with production of vascular endothelial growth factor in a murine model of human Wilms’ tumor. J Pediatr Surg 1999;34:743–8. [DOI] [PubMed] [Google Scholar]
- 23.Boccon-Gibod L. Pathological evaluation of renal tumors in children: international society of pediatric oncology approach. Pediatr Dev Pathol 1998;1:243–8. [DOI] [PubMed] [Google Scholar]
- 24.Ishigami SI, Arii S, Niwano M, et al. Predictive value of vascular endothelial growth factor (VEGF) in metastasis and prognosis of human colorectal cancer. Br J Cancer 1998;78:1379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasparini G, Toi M, Gion M, et al. Prognostic significance of vascular endothelial growth factor protein in node-negative breast carcinoma. J Natl Cancer Inst 1997;89:139–47. [DOI] [PubMed] [Google Scholar]
- 26.Paley PJ, Staskus KA, Gebhard K, et al. Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer 1997;80:98–106. [DOI] [PubMed] [Google Scholar]
- 27.Jinno K, Tanimizu M, Hyodo I, et al. Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroentrol 1998;33:376–82. [DOI] [PubMed] [Google Scholar]
- 28.Brown LF, Berse B, Jackmon RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinoma of gastrointestinal tract. Cancer Res 1993;53:4727–35. [PubMed] [Google Scholar]
- 29.Kawauchi S, Fukuda T, Tsuneyoshi M. Angiogenesis does not correlate with prognosis or expression of vascular endothelial growth factor in synovial sarcoma. Oncol Rep 1999;6:959–64. [DOI] [PubMed] [Google Scholar]
- 30.Tanigawa N, Amaya H, Matsumura M, et al. Correlation between expression of vascular endothelial growth factor and tumor vascularity, and patient outcome in human gastric carcinoma. J Clin Oncol 1997;15:826–32. [DOI] [PubMed] [Google Scholar]
- 31.Maeda H, Chung Y, Ogawa Y, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996;77:858–63. [DOI] [PubMed] [Google Scholar]
- 32.Skoldenberg E, Christiansson J, Sandstedt B, et al. Angiogenesis and angiogenic growth factors in Wilms’ tumor. J Urol 2001;165:2274–9. [DOI] [PubMed] [Google Scholar]
- 33.Toi M, Inada K, Suzuki H, et al. Tumor angiogenesis in breast cancer: its importance as a prognostic indicator and the association with vascular endothelial growth factor expression. Breast Cancer Res Treat 1995;36:193–204. [DOI] [PubMed] [Google Scholar]
- 34.Obermair A, Kohlberger P, Bancher-Todesca D, et al. Influence of microvessel density and vascular permeability factor/vascular endothelial growth factor expression on prognosis in vulvar cancer. Gynecol Oncol 1996;63:204–9. [DOI] [PubMed] [Google Scholar]
- 35.Pepper MS, Ferrara N, Orci L, et al. Vascular endothelial growth factor induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun 1991;181:902–6. [DOI] [PubMed] [Google Scholar]
- 36.Mandriota SJ, Seghezzi G, Vassalli JD, et al. Vascular endothelial growth factor increases urokinase receptor expression in vascular endothelial cells. J Biol Chem 1995;270:9709–16. [DOI] [PubMed] [Google Scholar]
- 37.Maeda T, Matsumura S, Hiranuma H, et al. Expression of vascular endothelial growth factor in human oral squamous cell carcinoma: its association with tumour progression and p53 gene status. J Clin Pathol 1998;51:771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albo D, Granick, MS, Jhala N, et al. The relationship of angiogenesis to biological activity in human squamous cell carcinomas of the head and neck. Ann Plast Surg 1994;32:588–94. [DOI] [PubMed] [Google Scholar]
- 39.Kaya M, Wada T, Akatsuka T, et al. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res 2000;6:572–7. [PubMed] [Google Scholar]
- 40.Rowe D, Huang J, Kayton M, et al. Anti-VEGF antibody suppresses primary tumor growth and metastasis in an experimental model of Wilms’ tumor. J Pediatr Surg 2000;35:30–3. [DOI] [PubMed] [Google Scholar]
- 41.Mori S, Ueda Y, Kuratsu S, et al. Suppression of pulmonary metastasis by angiogenesis inhibitor TNP-470 in murine osteosarcoma. Int J Cancer 1995;61:148–52. [DOI] [PubMed] [Google Scholar]
- 42.Morisita T, Mii Y, Mitauchi Y, et al. Efficiency of the angiogenesis inhibitor O-(chloroacetyl-carbamoyl) fumagillol (AGM-1470) on osteosarcoma growth and lung metastasis in rats. Jpn J Clin Oncol 1995;25:25–31. [PubMed] [Google Scholar]