Abstract
Aims:Clostridium difficile is a common nosocomial pathogen and as such diagnostic and research methods may necessitate storage of faecal specimens for long periods, followed by subsequent re-examination. This study investigated the effects of storage conditions upon the viability of this organism and its toxin.
Methods: Three genotypically distinct strains of C difficile (two clinical isolates including the UK epidemic strain, and an environmental isolate) were grown anaerobically at 37°C for 72 hours in a pool of five faecal emulsions. Aliquots of each emulsion were stored at either −20°C (frozen) or 4°C (refrigerated). Emulsions were assayed for viable cells, spores, and cytotoxin titre before storage and at days 1, 3, 5, 7, 14, 28, and 56. An aliquot of each emulsion was also removed, assayed, and replaced in storage at each time point to investigate the effects of multiple freezing/refrigeration/thawing.
Results: Neither storage temperature nor multiple cycles of refrigeration/freezing and thawing adversely affected the viability of C difficile vegetative cells or spores. Single and multiple exposures of samples to 4°C had little effect upon the C difficile toxin titre. Toxin titres of multiply frozen and thawed faeces became significantly lower than for refrigerated faeces (p < 0.01) by day 5 of the experiment in two of the three strains, and in all strains by day 28. Toxin titres of singly frozen faeces became significantly lower than for refrigerated faeces (p < 0.01) by day 56 of the experiment in two of the three strains.
Conclusion: Storage temperature and multiple cycles of freezing (refrigeration)/thawing had minimal effects upon the viability of C difficile or its spores. Storage at 4°C has no discernible effect on C difficile cytotoxin. However, storage at −20°C has a detrimental effect upon C difficile cytotoxin, and multiple cycles of freezing and thawing may further adversely effect toxin titres.
Keywords: Clostridium difficile, storage temperature, viability, toxin degradation
Clostridium difficile is widely recognised as an important nosocomial pathogen, its toxin(s) being demonstrable in 90% of cases of pseudomembranous colitis. Some studies have reported C difficile toxin degradation after storage at ambient temperature.1,2 Borriello et al periodically tested a single toxin positive faecal sample stored at 4°C for toxin A and cytotoxin. Toxin A was not demonstrable after 52 days of storage.3 However, there are few data available on the viability of the organism itself during storage. Weese et al recently reported a significant qualitative decrease in the recovery of C difficile from inoculated equine faecal samples after aerobic storage at 4°C compared with anaerobic storage at the same temperature. In contrast, the authors reported that C difficile toxin was stable at 4°C, and was still detectable after storage for 30 days.4 Diagnostic and research methods may necessitate the storage of faecal specimens for long periods of time followed by subsequent re-examination. The effects of storage at 4°C or −20°C on C difficile cytotoxin, viable counts, and spore counts in faecal emulsions were investigated over eight weeks. Single and multiple exposures of C difficile faecal emulsions to storage temperatures were also investigated.
“Diagnostic and research methods may necessitate the storage of faecal specimens for long periods of time followed by subsequent re-examination”
METHODS
Faecal emulsions were prepared by pooling five faecal samples from healthy elderly individuals and diluting 1/20 (wt/vol) in pre-reduced phosphate buffered saline (PBS; pH 7.4). Emulsions were pre-reduced in an anaerobic cabinet (Don Whitely Scientific, Bradford, UK) for 24 hours before inoculation.
Pre-reduced faecal emulsions were each inoculated with 50 μl of a 24 hour Schaedlers anaerobic broth culture containing either C difficile strains p24, B32 (clinical toxigenic isolates, polymerase chain reaction (PCR) ribotype 1 and 78, respectively), or E16 (environmental toxigenic isolate, PCR ribotype 44). Faecal emulsions were incubated in an anaerobic environment for 72 hours, after which they were removed. Each faecal emulsion was divided into 16 aliquots of 200 μl in Eppendorf tubes and two aliquots of 700 μl. Eight aliquots were placed in a −20°C freezer, along with a 700 μl aliquot. The remaining aliquots were placed in a 4°C refrigerator. On days 0, 1, 3, 5, 7, 14, 28, and 56 a single 200 μl aliquot was removed from each storage condition and assayed for vegetative cells, spores, and cytotoxin (using the Vero cell culture assay), as described below. These aliquots were then discarded. At the same time points, the 700 μl aliquots were removed from storage and assayed in the same way. These aliquots were then replaced in their respective storage conditions.
Clostridium difficile viable count
Each faecal emulsion was vortexed and serially diluted 10 fold in PBS. Three Brazier’s cycloserine-cefoxitin egg yolk agar plates containing 5 mg/litre lysozyme without egg yolk (CCEYL; Bioconnections, Leeds, UK)5 were inoculated, in triplicate, with 20 μl of each dilution. Plates were dried and incubated anaerobically at 37°C, for 48 hours. Dilutions yielding the growth of 30–100 colonies for each drop were counted, and colony forming units/ml determined.
Clostridium difficile spore assay
Each faecal emulsion was vortexed and a 200 μl aliquot removed to an Eppendorf tube. The bench alcohol shock procedure of Borriello et al was used to kill the vegetative cells.6 Briefly, an equal volume of absolute ethanol was added to the tube and the mixture was vortexed. The faecal emulsion/ethanol mix was left at room temperature for one hour.6 The mixture was then serially diluted (1/10) in PBS and 20 μl inoculated on to CCEYL plates, which were anaerobically incubated and counted as described above.
Clostridium difficile toxin assay
A 100 μl aliquot of each faecal emulsion was centrifuged at 16 000 ×g for 10 minutes. After centrifugation, the supernatant was removed and serially diluted (1/10) to a dilution of 10−8 in PBS. Vero cell monolayers were inoculated with these serial 10 fold dilutions. Specific confirmation of the presence of C difficile toxin was achieved by inoculating a duplicate well with 20 μl neat supernatant and 20 μl Clostridium sordellii antitoxin (prepared according to the manufacturer’s instructions (Pro-lab Diagnostics, South Wirral, Cheshire, UK)). Cell culture toxin assays were incubated at 37°C, in the presence of 5% CO2 and examined at 24 and 48 hours under an inverted microscope. A positive reaction was indicated by cell rounding. The toxin titre was defined as the dilution adhering to the following criteria:
Cell rounding of approximately ≥ 50% of the monolayer.
Cell rounding of approximately all of the monolayer in the preceding well (containing less dilute sample).
No evidence of cell rounding in the subsequent well (containing more dilute sample).
The assay was considered valid if cell rounding was completely prevented by the presence of C sordellii antitoxin.
RESULTS
Viable counts and spore counts of all strains of C difficile (p24, B32, and E16) remained approximately constant throughout the course of the experiment (56 days). Neither storage temperature nor multiple cycles of refrigeration/freezing and thawing adversely affected the viability of C difficile vegetative cells or its spores.
Single and multiple exposures of samples to 4°C had little effect upon C difficile toxin titre. Toxin titres, although fluctuating slightly during the course of the experiment, generally remained within one or two logarithmic concentrations of the initial titre.
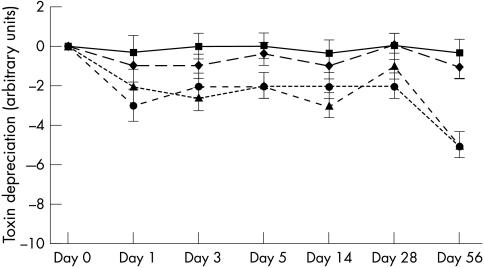
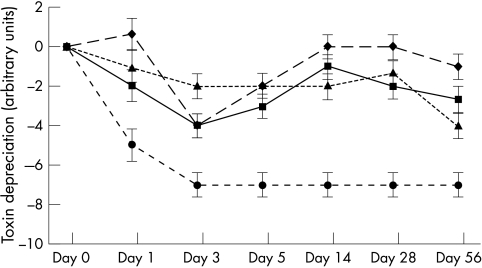
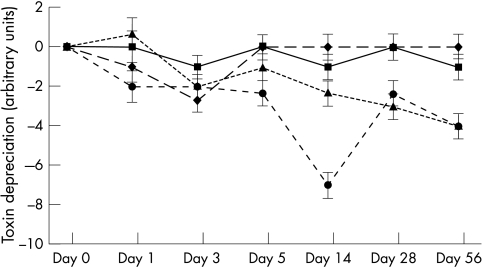
A detrimental effect was seen upon toxin titre at −20°C, in both singly and multiply frozen and thawed samples. Strain p24 decreased from an initial titre of 106 toxin units to 102 toxin units (single freeze/thaw cycle) and 101 toxin units (multiple freeze/thaw cycle) by day 56 of the study. Similar decreases were seen in strains B32 and E16. The most dramatic decrease was seen when strain B32 was exposed to multiple freeze/thaw cycles. Toxin titres decreased from 107 toxin units to undetectable values by day 3 of the experiments. Figures 1 –3 show the decreases in toxin titres relative to the initial titre. Analysis of variance was performed upon each time point. Error bars represent the minimum significant difference relating to data obtained for that time point; comparisons are not made between time points. Toxin titres of multiply frozen and thawed faeces became significantly lower than those stored at 4°C (p < 0.01) by day 5 of the experiment in two of the three strains, and in all strains by day 28. Toxin titres of singly frozen and thawed faeces became significantly lower than those stored at 4°C (p < 0.01) by day 56 of the experiment in two of the three strains.
Figure 1.
Toxin titres of UK epidemic Clostridium difficile strain (p24) stored in faecal emulsion. Error bars represent the minimum significant difference. Squares, multiple refrigeration/thaw at 4°C; diamonds, single refrigeration/thaw at 4°C; triangles, single freeze/thaw at −20°C; circles, multiple freeze/thaw at −20°C.
Figure 2.
Toxin titres of non-epidemic Clostridium difficile strain (B32) stored in faecal emulsion. Error bars represent the minimum significant difference. Squares, multiple refrigeration/thaw at 4°C; diamonds, single refrigeration/thaw at 4°C; triangles, single freeze/thaw at −20°C; circles, multiple freeze/thaw at −20°C.
Figure 1.
Toxin titres of environmentally isolated Clostridium difficile strain (E16) stored in faecal emulsion. Error bars represent the minimum significant difference. Squares, multiple refrigeration/thaw at 4°C; diamonds, single refrigeration/thaw at 4°C; triangles, single freeze/thaw at −20°C; circles, multiple freeze/thaw at −20°C.
DISCUSSION
Our study showed that C difficile remains viable both as vegetative cells and spores, with little quantitative variation, after single or multiple exposure to either 4°C or −20°C for at least 56 days. Bowman and Riley reported recovery of C difficile in faecal specimens for up to 10 days, depending on the isolation medium used,2 and Weese et al reported recovery of C difficile in 25 of 26 C difficile positive equine faecal samples after anaerobic storage at 4°C for 30 days.4 The above studies described the survival of C difficile in specimens as either “positive” or “negative” with no reference to numbers or the presence or absence of C difficile spores. Our present study used lysozyme supplemented media to optimise C difficile recovery.5 Culture showed little fluctuation in the numbers of either total viable counts or spore counts, indicating that the organism does not sporulate in response to storage at either 4°C or −20°C. It is interesting to note that Weese et al found that samples stored aerobically at 4°C yielded decreasing counts with time. This was not seen in our study, although all specimens were stored aerobically, and some were periodically exposed to air during multiple freezing/refrigeration and thawing processes. This discrepancy in observations may have arisen as a result of differences in methodology. In our study, faeces were emulsified in PBS, providing a buffered environment for the organism after its removal from an anaerobic environment. It is possible that exposure to air may influence the pH of faeces and this may affect C difficile. Continued fermentation of unabsorbed sugars to acids by bacteria outside the buffered environment of the large bowel may lower faecal pH. Some studies have reported a decrease in faecal pH associated with decreased numbers of C difficile.7,8 In addition, Futter and Richardson investigated the effect of the pH value of solid medium on the recovery of spores of C histolyticum, C septicum, C sporogenes, and C welchii (perfringens), and found that the optimum pH for spore recovery lay between 7.5 and 8.8.9 It is possible that the buffering of faecal specimens would be a feasible method of preserving C difficile viability during transport or storage. This requires further investigation.
Although the viability of C difficile is unaffected by storage at either 4°C or −20°C, the activity of cytotoxin was considerably reduced when samples were stored at −20°C. This is in agreement with previous observations that cytotoxin degrades at −20°C.3 Conversely, Weese et al reported detectable toxin in broth cultures stored at −70°C, −20°C, and 4°C, but no titrations were performed to detect reductions in toxin titre.4 In addition, toxin was detected using an enzyme linked immunosorbent assay system, whereas our study used a cell culture cytotoxicity assay. Therefore, it is possible that storage at −20°C results in the loss of cytotoxic, but not immunological, activity. Bowman and Riley found a 1.7 log reduction in average toxin titre after two days of storage at 25°C and a similar, although less pronounced reduction (1.4 log), after two days of storage at 5°C.2 However, we found no such decrease after storage at 4°C, when samples were subjected to either single or multiple cycles of freeze/refrigeration and thawing. We cannot account for this discrepancy.
“It is possible that the buffering of faecal specimens would be a feasible method of preserving C difficile viability during transport or storage”
The differences in the reduction of toxin titres between C difficile strain p24 (PCR ribotype 1) and strain B32 (PCR ribotype 78) were interesting. Clostridium difficile PCR ribotype 1 is the UK epidemic strain, and accounts for 60% of UK C difficile isolates.10 Virulence assays performed in our laboratory have shown several differences between C difficile strain p24 and other strains, namely: (1) C difficile strain p24 produced significantly more spores than other non-prevalent strains11; C difficile strain p24 produced significantly more spores than other strains in response to ampicillin treatment (including C difficile strains B32 and E16) (Freeman J, Wilcox MH. Does antibiotic exposure affect sporulation in an epidemic Clostridium difficile strain? Poster presented at 40th Interscience Conference on Antimicrobial Agents and Chemotherapy); and C difficile strain p24 germinated to a significantly greater degree than strains B32 and E16 (Freeman and Wilcox, unpublished data, 2000). Thus, the cytotoxin of the UK epidemic strain may be more robust than those of other C difficile strains, a hypothesis that warrants further study.
Take home messages.
Storage temperature and multiple cycles of freezing (refrigeration)/thawing had little effect upon the viability of Clostridium difficile or its spores
Storage at 4°C had no discernible effect on C difficile cytotoxin
Storage at −20°C had a detrimental effect upon C difficile cytotoxin, and multiple cycles of freezing and thawing may further adversely effect toxin titres
We recommend that specimens should be stored at 4°C instead of −20°C to minimise toxin degradation
The buffering of faeces with phosphate buffered saline may allow C difficile to survive for prolonged periods of time
It is also possible that the structure of the toxin is damaged by ice crystals, formed during freezing, which may disrupt protein structure. This may explain why repeated freezing and thawing caused the greatest reductions in toxin titres. If this is the case, the fact that we emulsified the faecal samples in PBS may have contributed to toxin degradation because more water molecules would have been present in samples. This could be alleviated by the use of a cytoprotective storage medium. Samples stored as solid faeces may provide a more protective environment for the toxin.
On the basis of our results, we recommend that specimens should be stored at 4°C instead of −20°C to minimise toxin degradation. The high recovery rates of C difficile in PBS emulsified faeces stored at either 4°C or −20°C throughout the duration of the experiment may indicate that buffering of faeces allows C difficile to survive for prolonged periods of time. This warrants further investigation, but may provide a suitable means of longterm storage of faecal specimens containing C difficile.
Abbreviations
CCEYL, cycloserine-cefoxitin egg yolk agar plates with lysozyme
PBS, phosphate buffered saline
PCR, polymerase chain reaction
REFERENCES
- 1.Brazier JS. Role of the laboratory in investigations of Clostridium difficile diarrhoea. Clin Infect Dis 1993;4(suppl):S228–33. [DOI] [PubMed] [Google Scholar]
- 2.Bowman RA, Riley TV. Isolation of Clostridium difficile from stored specimens and comparative susceptibility of various tissue culture cell lines to cytotoxin. FEMS Microbiol Lett 1986;34:31–5. [Google Scholar]
- 3.Borriello SP, Vale T, Brazier JS, et al. Evaluation of a commercial enzyme immunoassay for the detection of Clostridium difficile toxin A. Eur J Clin Microbiol Infect Dis 1988;7:476–84. [DOI] [PubMed] [Google Scholar]
- 4.Weese JS, Staempfli HR, Prescott JF. Survival of Clostridium difficile and its toxins in equine feces: implications for diagnostic test selection and interpretation. J Vet Diagn Invest 2000;12:332–6. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox MH, Fawley WN, Parnell P. Value of lysozyme agar incorporation and alkaline thioglycollate exposure for the environmental recovery of Clostridium difficile. J Hosp Infect 2000;44:65–9. [DOI] [PubMed] [Google Scholar]
- 6.Borriello SP, Honour P. Simplified procedure for the routine isolation of Clostridium difficile from faeces. J Clin Pathol 1981;34:1124–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May T, Mackie RI, Fahey GC, Jr, et al. Effect of fiber source on short-chain fatty acid production and on the growth and toxin production by Clostridium difficile. Scand J Gastroenterol 1994;29:916–22. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Moriwaki H, Muto Y, Kato N, et al. Effect of lactulose on short-chain fatty acids and lactate production and on the growth of faecal flora, with special reference to Clostridium difficile. J Med Microbiol 1997;46:80–4. [DOI] [PubMed] [Google Scholar]
- 9.Futter BV, Richardson G. Viability of Clostridial spores and requirements of damaged organisms. I Method of colony count, period and temperature of incubation and pH value of the medium. J Appl Bacteriol 1970;33:321–30. [DOI] [PubMed] [Google Scholar]
- 10.Brazier JS. The epidemiology and typing of Clostridium difficile. J Antimicrob Chemother 1998;41(suppl 4):C47–57. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox MH, Fawley WN. Hospital disinfectants and spore formation by Clostridium difficile. Lancet 2000;356:1324. [DOI] [PubMed] [Google Scholar]