Abstract
Background: Two cell specific neutral proteases, tryptase and chymase, are produced by human mast cells (MC). Tryptase is constitutively expressed by all MC, whereas chymase is found only in an MC subset. Very little is known about chymase expression in MC proliferative disorders (mastocytosis).
Aims and Methods: Routinely processed, formalin fixed, and paraffin wax embedded bone marrow trephine biopsy specimens obtained from patients with various subtypes of mastocytosis (n = 47) and myelodysplastic syndromes (MDS; n = 28) were immunostained with antibodies against chymase and tryptase. Normal/reactive bone marrow specimens with intact haemopoiesis (n = 31) served as controls. The numbers of chymase expressing (C+) and of tryptase expressing (T+) MC were assessed morphometrically using a computer assisted video camera system.
Results: In normal/reactive bone marrow, the numbers of C+ MC (median, 8/mm2; maximum, 159/mm2) were in the same range as those of T+ MC (median, 4/mm2; maximum, 167/mm2). Because normal MC express both chymase and tryptase, these findings indicate that the common phenotype of bone marrow MC in normal/reactive states is MCTC (MC expressing both tryptase and chymase). In contrast, in MDS and mastocytosis, the bone marrow exhibited far more T+ MC than C+ MC in almost all cases.
Conclusions: According to these findings, the predominant MC type in the bone marrow in neoplastic states such as MDS and mastocytosis is MCT (MC expressing only tryptase). Although the pathophysiological basis of this apparent lack of chymase expression in most neoplastic MC in mastocytosis and MC involved in MDS remains unknown, this study has produced further evidence of the superior value of antitryptase antibodies in the diagnosis of mastocytosis.
Keywords: bone marrow, chymase, mast cell, mastocytosis, tryptase
Human tissue mast cells (MC) produce and store two almost cell specific neutral serine proteases: tryptase and chymase.1 The functional properties of these enzymes have not yet been fully elucidated. Although tryptase has been found to activate fibroblasts, chymase exhibits a relatively broad array of biological functions, including activation of angiotensin, cleavage of basement membrane through the lamina lucida, and potentiation of the effects of histamine.2 In humans, two major subpopulations of MC can be discriminated on the basis of their enzyme content. MC expressing both tryptase and chymase (MCTC) are found mainly in the skin, lymph nodes, and submucosal layers of the gastrointestinal tract, whereas MC that express only tryptase (MCT) are the predominant cell type in the mucosa of the gut and lung.3 Whether MC that express only chymase (MCC) exist, either in physiological or neoplastic states, remains a matter of discussion.4 The essential role of antitryptase antibodies in the immunohistochemical diagnosis of MC proliferative disorders/mastocytosis has been demonstrated.5,6 Because very little is known about the tissue distribution of chymase expressing (C+) MC, our study was designed to assess the frequency and distribution of C+ MC in normal/reactive bone marrow and in marrow involved by myelodysplasia (MDS) or mastocytosis. MDS was included in our study because an increase in MC numbers is seen in a large proportion of cases.7,8
“In humans, two major subpopulations of mast cells can be discriminated on the basis of their enzyme content”
MATERIAL AND METHODS
Our study was performed on bone marrow trephine biopsies taken from the iliac crest. There were 47 patients with various subtypes of mastocytosis, 28 patients with myelodysplastic syndromes (MDS), and 31 patients with normal/reactive bone marrow (controls), most of whom had undergone bone marrow biopsy during staging of a lymphoma of high grade malignancy. A diagnosis of mastocytosis was established only when at least one dense MC infiltrate comprising a minimum of 15 cells, irrespective of their shape, was detected.9 The diagnoses were based on current systems of classification of haematological malignancies, especially the French–American–British criteria.10 The biopsies were routinely processed, fixed in 5% buffered formalin, subject to mild decalcification overnight in adetic acid/EDTA, and embedded in paraffin wax. Serial sections were cut and immunostained by the avidin–biotin–peroxidase complex method of Hsu et al,11 using antibodies against tryptase (AA1; DakoDiagnostika, Hamburg, Germany) and chymase (B7; Chemicon, Temecula, California, USA). A detailed description of the technical procedures has already been published.6 The numbers of tryptase positive (T+) and C+ cells were assessed with a computer assisted video camera system. The two tailed Fisher’s exact test, the Kruskal-Wallis test, and the Wilcoxon rank sum test were used for statistical analysis.
RESULTS
General aspects
MC almost always exhibited intense staining of the intracytoplasmic granules for tryptase and/or chymase and could therefore be easily identified (fig 1). In a considerable proportion of the cases of mastocytosis some of the cytomorphologically atypical, often non-metachromatic MC exhibited weaker immunoreactivity because of their reduced granule content. However, all the immunoreactive cells could be easily identified, irrespective of the intensity of staining. In reactive non-mastocytotic bone marrow and in many cases of MDS, MC were loosely distributed throughout the marrow and exhibited a round to oval shape, a low nuclear–cytoplasmic ratio, and inconspicuous nucleoli. In cases of mastocytosis, the MC shape showed greater variability: spindle shaped cells were frequent and were the predominant cell type in most cases. Except in a few cases of MDS, in which a minority of blast cells were labelled by antitryptase, no haemopoietic cells other than MC expressed appreciable amounts of chymase or tryptase.
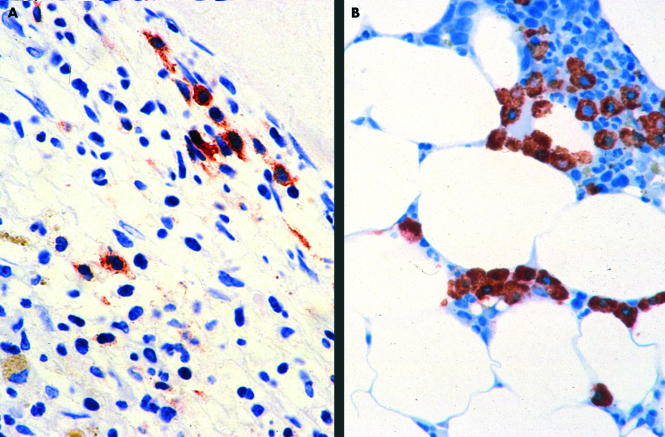
Figure 1.
Bone marrow in systemic mastocytosis. (A) An extremely hypercellular bone marrow in a case of aggressive systemic mastocytosis with an increase in spindle shaped mast cells. Very few of the mast cells are immunoreactive for chymase. (B) A hypocellular bone marrow with a focal increase in mast cells in a case of indolent systemic mastocytosis. Here, all the mast cells strongly express chymase. Avidin–biotin–peroxidase complex method, antichymase (B7) antibody.
Morphometric and statistical analysis
Table 1 summarises the morphometric findings concerning the numbers of C+ and T+ cells in mastocytosis, normal/reactive bone marrow, and MDS. The main finding was a highly significant difference (p < 0.001) between the very high numbers of T+ cells in mastocytosis (median, 446/mm2) and the relatively low numbers in normal/reactive marrow (median, 4/mm2) and MDS (median, 32/mm2). However, the difference in numbers of T+ cells between MDS and normal bone marrow was not significant. In contrast, the numbers of C+ MC in MDS (maximum, 46/mm2; median, 0/mm2) and mastocytosis (maximum, 993/mm2; median, 0/mm2) were lower in most cases, and only rarely exceeded 30/mm2. Altogether, the number of T+ cells far exceeded that of C+ cells in most cases of mastocytosis and MDS. No significant differences in the numbers of C+ MC could be found among the diagnostic groups (median for mastocytosis, 0/mm2; median for non-mastocytotic states, 4/mm2). In normal/reactive bone marrow the number of C+ MC (median, 8/mm2) was similar to, or at least in the same range as that of T+ cells, reaching a maximum of 159/mm2. In mastocytosis, the number of T+ cells always exceeded that of C+ cells. About 30% of the mastocytosis cases exhibited more than 1000 T cells/mm2, with a maximum of 5343/mm2.
Table 1.
Numbers of tryptase expressing (T+) and chymase expressing (C+) bone marrow (BM) mast cells/mm2 in mastocytosis, myelodysplastic syndromes (MDS), and normal/reactive BM
| T+ | C+ | |||||||||
| Max | 75th | Median | 25th | Min | Max | 75th | Median | 25th | Min | |
| Mastocytosis (n=47) | 5343 | 1113 | 446 | 254 | 106 | 993 | 38 | 0 | 0 | 0 |
| MDS (n=28) | 493 | 53 | 32 | 7 | 2 | 46 | 4 | 0 | 0 | 0 |
| Normal BM (n=31) | 167 | 61 | 4 | 1 | 0 | 159 | 27 | 8 | 4 | 0 |
25th and 75th refer to the 25th and 75th centiles, respectively.
Table 2 summarises the numbers of C+ and T+ bone marrow MC in the main subtypes of mastocytosis—namely, indolent and aggressive systemic mastocytosis—compared with normal/reactive bone marrow and MDS. No significant difference was detected between the indolent and aggressive subtypes of mastocytosis and non-mastocytotic conditions. The numbers of T+ MC also did not differ significantly between the indolent (median, 427/mm2; maximum, 5343/mm2) and aggressive (median, 808/mm2; and maximum, 2835/mm2) subtypes of mastocytosis, although the median was considerably higher in aggressive mastocytosis.
Table 2.
Numbers of tryptase expressing (T+) and chymase expressing (C+) bone marrow (BM) mast cells (MC)/mm2 in the two main subtypes of mastocytosis and non-mastocytotic states (myelodysplasia/MDS and normal/reactive BM)
| T+ | C+ | |||||||||
| Max | 75th | Median | 25th | Min | Max | 75th | Median | 25th | Min | |
| ISM (n=29) | 5343 | 728 | 427 | 168 | 106 | 864 | 38 | 0 | 0 | 0 |
| ASM (n=18) | 2835 | 1321 | 808 | 304 | 160 | 993 | 36 | 4 | 0 | 0 |
| Normal BM (n=31) | 167 | 61 | 4 | 1 | 0 | 159 | 27 | 8 | 4 | 0 |
| MDS (n=28) | 493 | 53 | 32 | 7 | 2 | 46 | 4 | 0 | 0 | 0 |
25th and 75th refer to the 25th and 75th centile, respectively.
ASM, aggressive systemic mastocytosis; ISM, indolent systemic mastocytosis.
DISCUSSION
Our study focuses on the immunohistochemical identification and morphometric enumeration of C+ bone marrow MC in reactive and neoplastic states, with special reference to MC proliferative disorders/mastocytosis and MDS. MDS exhibits increased MC numbers in most cases,7,8 but it is not known whether these MC are reactive, thus representing a hyperplastic state, or belong to the malignant cell clone. In each of our cases, the numbers of C+ MC were compared with those of T+ cells in serial sections.
The main findings were the following:
When the numbers of C+ MC in mastocytosis, irrespective of the subtype, were compared with that seen in the non-mastocytotic states (MDS and normal/reactive bone marrow) no significant differences could be found, with the median value even being lower in mastocytosis (0/mm2) than in non-mastocytotic states (4/mm2). The numbers of C+ MC were in the same range as those of T+ MC in most (67%) normal/reactive bone marrow specimens.
However, the number of T+ cells proved to be significantly higher (p < 0.001) in mastocytosis (median, 532/mm2) than in MDS (median, 32/mm2) and normal/reactive bone marrow (median, 4/mm2).
From these findings it can be concluded that the principal phenotype of neoplastic bone marrow MC in most cases of mastocytosis, especially the aggressive variant, is that of an MCT. However, the major phenotype of MC in normal/reactive states and in many cases of indolent systemic mastocytosis is that of an MCTC. These findings also confirm the superior value of antitryptase antibodies for the recognition of atypical, especially non-metachromatic MC, and thus for the diagnosis of the aggressive and leukaemic subtypes of mastocytosis.6
Only a few quantitative immunohistochemical analyses of the tissue distribution of T+ and C+ MC have been published. Kankkunen and colleagues12 reported a preponderance of T+ MC over C+ MC in malignant breast tumours, particularly at the invasion front. In contrast, benign breast lesions contained almost equal numbers of C+ and T+ MC. Jeziorska and colleagues13 found a predominance of MCTC in atherosclerotic lesions in human carotid arteries at all stages of development. Jarvikallio and colleagues14 investigated T+ and C+ MC in non-lesional and lesional skin of patients with atopic dermatitis and nummular eczema. They found a concentration of MC in the upper dermis, with a predominance of MCT. Mori and colleagues15 investigated the frequency distribution of T+ and C+ MC in the human uterus. They found MC to be concentrated in the inner (luminal) half of the myometrium, with almost equal numbers of MCT and MCTC, whereas the numbers of MCTC were significantly lower than those of MCT in the outer half of the myometrium and cervix.
Take home messages.
The main phenotype of the neoplastic bone marrow mast cell in mastocytosis is that of a mast cell that only expresses tryptase
This is consistent with earlier findings concerning the high diagnostic value of antitryptase antibodies in mastocytosis
Mast cells that express only chymase may be present in normal or reactive bone marrow
“We revealed convincing light microscopic and statistical evidence that a considerable number of mast cells that express only chymase may be present in normal or reactive bone marrow”
On the basis of our morphometric findings of a slight predominance of C+ MC over T+ MC in a considerable number of cases with normal/reactive bone marrow, it could be speculated that, apart from the well recognized phenotypes of MCT and MCTC, MC that express only chymase (MCC) may also exist. Because the numbers of C+ MC never exceeded those of T+ MC in mastocytosis, this “C only” phenotype appears to be of minor importance in MC neoplasms involving the bone marrow. The existence of an “MCC” is still a matter of debate.4,16
Our study clearly shows that the main phenotype of the neoplastic bone marrow MC in mastocytosis is that of an MCT, which is consistent with our earlier findings concerning the high diagnostic value of antitryptase antibodies in mastocytosis.6 However, we also revealed convincing light microscopic and statistical evidence that a considerable number of MC that express only chymase may be present in normal or reactive bone marrow.
Abbreviations
C+, chymase expressing
MC, mast cells
MCC, mast cells expressing only chymase
MCT, mast cells expressing only tryptase
MCTC, mast cells expressing both tryptase and chymase
MDS, myelodysplastic syndromes
T+, tryptase expressing
REFERENCES
- 1.Irani AA, Schechter NM, Craig SS, et al. Two human mast cell subsets with distinct neutral protease compositions. Proc Natl Acad Sci U S A 1986;83:4464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algermissen B, Hermes B, Feldmann-Bödekker I, et al. Mast cell chymase and tryptase during tissue turnover: analysis on in vitro mitogenesis of fibroblasts and keratinocytes and alterations in cutaneous scars. Exp Dermatol 1999;8:193–8. [DOI] [PubMed] [Google Scholar]
- 3.Buckley MG, McEuen AR, Walls AF. The detection of mast cell subpopulations in formalin-fixed human tissues using a new monoclonal antibody specific for chymase. J Pathol 1999;189:138–43. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Krillis SA. Conditioned media obtained from a human mastocytosis cell strain induce mast cells expressing chymase but not tryptase from human progenitors. Int Arch Allergy Immunol 1997;113:289–90. [DOI] [PubMed] [Google Scholar]
- 5.Li WV, Kapadia SB, Sonmez-Alpan E, et al. Immunohistochemical characterization of mast cell disease in paraffin sections using tryptase, CD68, myeloperoxidase, lysozyme, and CD20 antibodies. Mod Pathol 1996;9:982–8. [PubMed] [Google Scholar]
- 6.Horny H-P, Sillaber C, Menke D, et al. Diagnostic utility of staining for tryptase in patients with mastocytosis. Am J Surg Pathol 1998;22:1132–40. [DOI] [PubMed] [Google Scholar]
- 7.Prokocimer M, Polliack A. Increased bone marrow mast cells in preleukemic syndromes, acute leukemia, and lymphoproliferative disorders. Am J Clin Pathol 1981;75:34–8. [DOI] [PubMed] [Google Scholar]
- 8.Yoo D, Lessin LS. Bone marrow mast cell content in preleukemic syndrome. Am J Med 1982;73:539–42. [DOI] [PubMed] [Google Scholar]
- 9.Horny H-P, Valent P. Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res 2001;25:543–51. [DOI] [PubMed] [Google Scholar]
- 10.Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French–American–British cooperative group. Ann Intern Med 1985;103:620–4. [DOI] [PubMed] [Google Scholar]
- 11.Hsu S, Raine L, Fanger H. Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981;29:577–80. [DOI] [PubMed] [Google Scholar]
- 12.Kankkunen JP, Harvima IT, Naukkarinen A. Quantitative analysis of tryptase and chymase containing mast cells in benign and malignant breast lesions. Int J Cancer 1997;72:385–8. [DOI] [PubMed] [Google Scholar]
- 13.Jeziorska M, McCollum C, Woolley DE. Mast cell distribution, activation, and phenotype in atherosclerotic lesions of human carotid arteries. J Pathol 1997;182:115–22. [DOI] [PubMed] [Google Scholar]
- 14.Jarvikallio A, Naukkarinen A, Harvima IT, et al. Quantitative analysis of tryptase- and chymase-containing mast cells in atopic dermatitis and nummular eczema. Br J Dermatol 1997;136:871–7. [PubMed] [Google Scholar]
- 15.Mori A, Zhai YL, Toki T, et al. Distribution and heterogeneity of mast cells in the human uterus. Hum Reprod 1997;12:368–72. [DOI] [PubMed] [Google Scholar]
- 16.Klein JA, Godthelp T, Blom HM, et al. Fixation with Carnoy’s fluid reduces the number of chymase-positive mast cells: not all chymase-positive mast cells are also positive for tryptase. Allergy 1996;51:614–20. [DOI] [PubMed] [Google Scholar]