Abstract
This report describes two patients who developed leiomyosarcomas, one involving the subcutaneous tissue of the thigh and the pelvic soft tissues and the other the urinary bladder, following hereditary retinoblastoma 36 and 38 years earlier, respectively. There is an increased risk of the development of sarcoma, most commonly osteosarcoma, as a second malignancy following hereditary retinoblastoma. Leiomyosarcoma developing as a second malignancy has rarely been reported and most have occurred in the field of previous radiotherapy. The literature on leiomyosarcoma occurring as a second neoplasm following retinoblastoma is reviewed.
Keywords: retinoblastoma, leiomyosarcoma, pelvis, urinary bladder
There is an increased incidence of second primary tumours in survivors of hereditary or childhood retinoblastoma. The cumulative incidence varies widely in different long term follow up series, from 8.4% at 18 years to 90% at 30 years.1–3 Second malignancies may arise at a site of previous radiotherapy or distantly. Histological subtypes of second malignancies include osteosarcoma, pinealoblastoma (these are now considered trilateral retinoblastomas), malignant melanoma, a variety of soft tissue sarcomas, and epithelial malignancies. Most soft tissue tumours are osteosarcomas. Leiomyosarcomas are comparatively rare with, as far as we are aware, only 18 reported cases.4–14 Some of these tumours have occurred in soft tissues in the field of previous radiotherapy. However, there are case reports of leiomyosarcoma developing in sites outside the head and neck, including the femur, liver, and urinary bladder.5–8,12 The interval to the development of the second primary tumour is variable and with leiomyosarcoma has ranged from 12 to 53 years. We report two patients who developed leiomyosarcomas, one involving the subcutaneous tissues of the thigh and the pelvic soft tissues and the other the urinary bladder. Both patients had been treated successfully for retinoblastoma 36 and 38 years earlier, respectively.
“There is an increased incidence of second primary tumours in survivors of hereditary or childhood retinoblastoma”
CASE REPORTS
Case 1
A 36 year old woman underwent wide local excision of a mass on the left outer thigh. The pathology of this is described below. A subsequent computed tomography scan of the pelvis and abdomen revealed probable uterine fibroids, and myomectomy was performed one month later. Histology confirmed benign uterine leiomyomas with no features to suggest malignancy. She underwent a six week course of radiotherapy to the region of the left thigh. After this, she had persistent right iliac fossa pain and a magnetic resonance imaging scan of the pelvis and abdomen was performed 18 months after the initial surgery. This revealed a retroperitoneal mass adherent to the pelvic sidewall. Laparotomy and excision of the mass was carried out. The pathology of this is described below. She underwent postoperative radiotherapy and is currently well with no clinical or radiological evidence of further tumour.
As an infant she had developed bilateral retinoblastomas requiring enucleation of the right eye and radiotherapy to the left eye. No chemotherapy was given. Two male siblings also had bilateral childhood retinoblastomas. These siblings did not develop subsequent malignancies.
Case 2
A 38 year old man presented with haematuria. At cystoscopy there was a 5 cm mass arising from a narrow pedicle attached to the base of the bladder near the trigone. This was resected and the pathology is described below. He proceeded to cystectomy. Examination of the cystectomy specimen revealed a solitary microscopic focus of residual submucosal tumour. No further treatment was given and there is no clinical or radiological evidence of tumour recurrence or metastasis four months later.
As an infant he had been successfully treated with radiotherapy for bilateral familial retinoblastoma. No chemotherapy was given. At age 31 he had surgical removal of a temporal lobe meningioma.
PATHOLOGY
Case 1
The resection specimen from the thigh was a 4 × 1 cm skin ellipse with an underlying 4.5 × 2.5 × 2 cm lobulated firm white tumour nodule. The retroperitoneal mass was dumb bell shaped and measured 6 × 4 × 3.5 cm. On sectioning, it was firm and white with areas of necrosis.
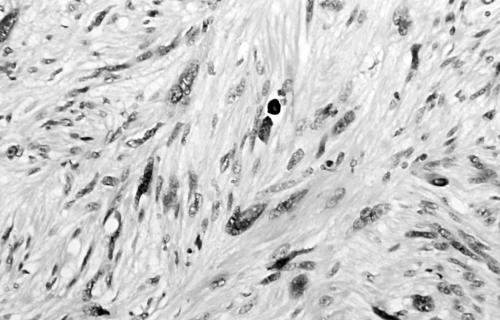
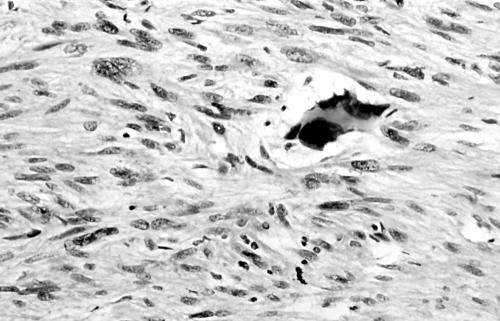
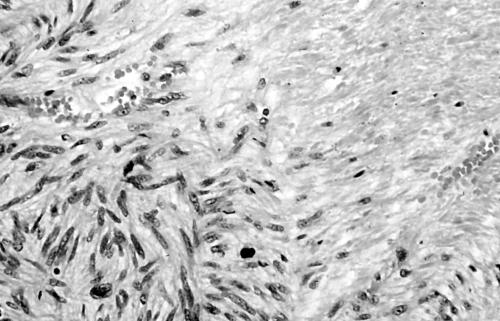
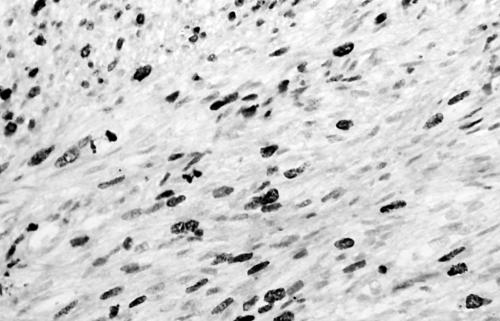
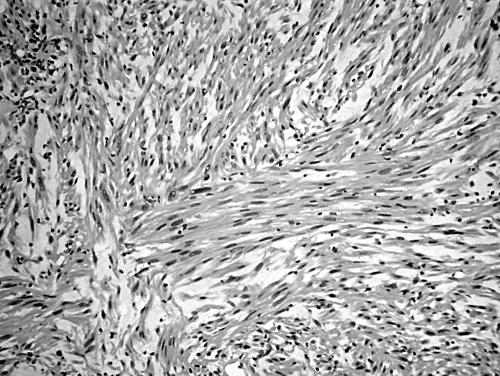
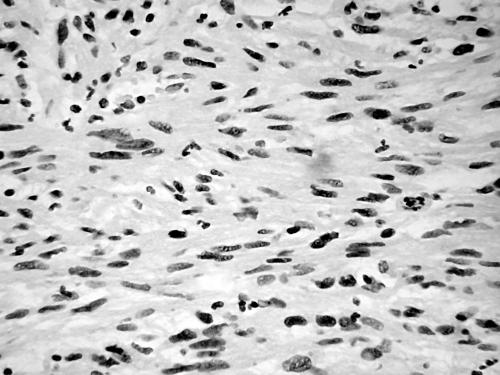
Histology of the thigh lesion showed a cellular spindle cell neoplasm located within the dermis and subcutaneous tissue. Tumour cells had vesicular nuclei and eosinophilic cytoplasm. In areas, the nuclear features were bland but focally there was moderate nuclear pleomorphism (fig 1). The mitotic count was 10 mitoses/10 high power fields. There were no areas of necrosis or vascular invasion. The pelvic tumour mass had a similar histological appearance. Focally the tumour was bland. However, there were areas of pronounced nuclear pleomorphism (fig 2) and many foci of coagulative tumour necrosis were present (fig 3). Up to 35 mitoses/10 high power fields were present. Smooth muscle differentiation in both neoplasms was confirmed immunohistochemically by widespread positive staining for α smooth muscle actin (Sigma, Poole, Dorset, UK) and desmin (Dako, Ely, Cambridgeshire, UK). MIB1 (Immunotech, Marseilles, France) staining revealed a proliferation index of 40% (fig 4). There was no staining for p53 (D07; Novocastra, Newcastle upon Tyne, UK) or cyclin D1 (Dako). Both tumours were interpreted as leiomyosarcomas.
Figure 1.
Area of moderate nuclear pleomorphism in thigh leiomyosarcoma (case 1).
Figure 2.
Area of pronounced nuclear pleomorphism in pelvic leiomyosarcoma (case 1).
Figure 3.
Foci of coagulative tumour cell necrosis in pelvic leiomyosarcoma (case 1).
Figure 4.
MIB1 staining showing a proliferative index of 35–40% (case 1).
Case 2
The surgical specimen from the mass in the urinary bladder comprised 10 g of tumour fragments.
Histology showed a cellular lesion composed of plump spindle shaped cells with abundant eosinophilic cytoplasm (fig 5). Tumour cell nuclei were moderately pleomorphic and the mitotic count was 8/10 high power fields. Foci of necrosis were present. The tumour exhibited widespread positivity for α smooth muscle actin and desmin. MIB1 staining showed a proliferation index of 40%. Staining for cyclin D1 was negative, whereas most tumour cell nuclei were positive for p53 (fig 6). The tumour was interpreted as an intermediate grade leiomyosarcoma.
Figure 5.
Urinary bladder leiomyosarcoma exhibiting moderate nuclear pleomorphism (case 2).
Figure 6.
Urinary bladder leiomyosarcoma exhibiting strong nuclear staining for p53 (case 2).
DISCUSSION
Long term survivors of hereditary retinoblastoma are at risk of developing a variety of non-ocular second primary malignancies, the most common histological subtype of which is osteosarcoma. The reported risk varies widely, but a cumulative incidence of 1% for each year of life has been suggested as an approximate estimate. Earlier reports indicated that many of these malignancies are radiation or chemotherapy induced.4,8,15,16 However, more recent studies16–19 suggest that the development of second primary neoplasms in patients with hereditary retinoblastoma results partly from a genetic predisposition and partly from the potentiating effect of radiotherapy on tumorigenesis through mutation of the second retinoblastoma gene (RB1) allele, the first RB1 allele being mutated in all patients with hereditary retinoblastoma.20
Leiomyosarcomas are relatively rare neoplasms outside the uterus and the gastrointestinal tract. Retroperitoneal leiomyosarcomas form the largest group of soft tissue leiomyosarcomas.21 In retinoblastoma survivors, some of the reported leiomyosarcomas have been in the field of radiation,4,9–11 whereas others have been described at distant sites, such as the urinary bladder, liver, and femur.5–8,12 One urinary bladder leiomyosarcoma developed 14 years after chemotherapy with cyclophosphamide for retinoblastoma.12 There was no history of chemotherapy in the two cases we describe. In female patients, uterine leiomyosarcoma has not been reported after hereditary retinoblastoma, although simultaneously occurring benign uterine leiomyomas have been described.7 One of the patients we describe also had uterine leiomyomas. We feel this is a coincidental association, although it is possible that, because of genetic factors, there is an increased risk of the development of smooth muscle neoplasms at various sites.
“The RB gene is a tumour suppressor gene encoding a nuclear phosphoprotein that functions as a regulator of cell cycle progression”
A recent analysis of second primary neoplasms in retinoblastoma survivors revealed that 76% of the second tumours occurred in the head and neck region and that soft tissue sarcomas were the single largest category, comprising 24% of the total.19 The median age at diagnosis of second tumour for the entire series was 16.4 years. The youngest patient to develop a soft tissue tumour was 4 years old and the oldest was 41. Previously reported cases of leiomyosarcoma occurring outside the head and neck region have been in the 4th to 6th decades. The cases we report, together with others described, suggest that with increasing duration of survival after hereditary retinoblastoma there is an increased risk of developing leiomyosarcoma in areas remote from the site of irradiation. Such patients thus require appropriate follow up.
The patient in case 1 developed a leiomyosarcoma of the pelvic soft tissues 18 months after removal of the leiomyosarcoma from the thigh. It is probable that the pelvic leiomyosarcoma was a metastasis from the thigh neoplasm, although we cannot exclude the possibility of synchronous development of independent leiomyosarcomas.
We could not demonstrate the expression of cyclin D1 immunohistochemically in either of the leiomyosarcomas. One tumour was negative for p53 but the other exhibited diffuse nuclear positivity. The RB gene is a tumour suppressor gene encoding a nuclear phosphoprotein that functions as a regulator of cell cycle progression. This function is lost when the protein is phosphorylated by cyclin dependent kinases. p16, a product of the CDKN2/MTS1 gene, is known to inhibit phosphorylation by cyclin dependent kinases in a manner similar to the retinoblastoma protein. p53 and retinoblastoma protein–cyclin D pathway abnormalities have been detected in sporadic leiomyosarcomas in recent studies.22,23
In summary, we report two cases of leiomyosarcoma developing 36 and 38 years after bilateral hereditary retinoblastomas. In both cases, the leiomyosarcoma occurred outside the head and neck region. Patients with hereditary retinoblastomas are probably at increased risk of developing leiomyosarcoma and other soft tissue sarcomas with the increasing duration of survival.
Take home messages
We report two patients who developed leiomyosarcomas, outside the head and neck region, following hereditary retinoblastoma 36 and 38 years earlier
In survivors of hereditary retinoblastoma there is an increased risk of the development of sarcoma, most commonly osteosarcoma, especially with the increasing duration of survival
Leiomyosarcomas developing as a second malignancy have rarely been reported and most have occurred in the field of previous radiotherapy
Acknowledgments
We would like to thank Professor F C Hamdy (Urology, Royal Hallamshire Hospital, Sheffield, UK) for his assistance with this manuscript.
REFERENCES
- 1.Draper GJ, Sanders BM, Kingston JE. Second primary neoplasms in patients with retinoblastoma. Br J Cancer 1986;53:661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moll AC, Imhof SM, Schouten-Van Meeteren AY, et al. Second primary tumours in hereditary retinoblastoma: a register based study, 1945–1997: is there an age effect on radiation-related risk? Ophthalmology 2001;108:1109–14. [DOI] [PubMed] [Google Scholar]
- 3.Abramson DH, Ellsworth RM, Kitchen FD, et al. Second non-ocular tumours in retinoblastoma survivors. Are they radiation-induced? Ophthalmology 1984;91:1351–5. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Hirsch DP, Habashi S, Benbow EW, et al. Post-irradiation leiomyosarcoma of the maxilla. J Laryngol Otol 1991;105:1068–71. [DOI] [PubMed] [Google Scholar]
- 5.Guse TR, Weiss LD. Leiomyosarcoma of the femur in a patient with a history of retinoblastoma. J Bone Joint Surg 1994;6:904–6. [DOI] [PubMed] [Google Scholar]
- 6.Abdelli N, ThiefinG, Diebold MD, et al. Primary leiomyosarcoma of the liver 37 years after successful treatment of hereditary retinoblastoma. Gastroenterol Clin Biol 1996;20:502–5. [PubMed] [Google Scholar]
- 7.Liang SX, Lakshmanan Y, Woda BA, et al. A high grade primary leiomyosarcoma of the bladder in a survivor of retinoblastoma. Arch Pathol Lab Med 2001;125:1231–4. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura J, Sakurai M, Tsukamoto K, et al. Leiomyosarcoma of the bladder eighteen years after cyclophosphamide therapy for retinoblastoma. Urol Int 1993;51:49–53. [DOI] [PubMed] [Google Scholar]
- 9.Marta U, Zsuzsanna S, Joszef B, et al. Rare incidence of three consecutive primary tumours in the maxillofacial region: retinoblastoma, leiomyosarcoma and choriocarcinoma. J Craniofac Surg 2001;12:464–8. [DOI] [PubMed] [Google Scholar]
- 10.Font RL, Jurco S, Brechner RJ. Post-radiation leiomyosarcoma of the orbit complicating bilateral retinoblastoma. Arch Ophthalmol 1983;101:1557–61. [DOI] [PubMed] [Google Scholar]
- 11.Folberg R, Cleasby G, Flanagan JA, et al. Orbital leiomyosarcoma after radiation-therapy for bilateral retinoblastoma. Arch Ophthalmol 1983;101:1562–5. [DOI] [PubMed] [Google Scholar]
- 12.Motta L, Porcaro AB, Ficarra V, et al. Leiomyosarcoma of the bladder fourteen years after cyclophosphamide therapy for retinoblastoma. Scand J Urol Nephrol 2001;35:248–9. [DOI] [PubMed] [Google Scholar]
- 13.Dunkel IJ, Gerald WL, Rosenfield NS, et al. Outcome of patients with a history of bilateral retinoblastoma treated for a second malignancy: the Memorial Sloan-Kettering experience. Med Pediatr Oncol 1998;30:59–62. [DOI] [PubMed] [Google Scholar]
- 14.Klippenstein KA, Wesley RE, Glick AD. Orbital leiomyosarcoma after retinoblastoma. Ophthalmic Surg Lasers 1999;30:579–83. [PubMed] [Google Scholar]
- 15.Schwarz MB, Burgess LPA, Fee WE, et al. Post-irradiation sarcoma in retinoblastoma. Induction or predisposition? Arch Otolaryngol Head Neck Surg 1988;114:640–4. [DOI] [PubMed] [Google Scholar]
- 16.Moll AC, Imhof SM, Bouter LM, et al. Second primary tumours in patients with hereditary retinoblastoma: a register-based follow-up study, 1945–1994. Int J Cancer 1996;67:515–19. [DOI] [PubMed] [Google Scholar]
- 17.Imhof SM, Moll AC, Hofman P, et al. Second primary tumours in hereditary- and nonhereditary retinoblastoma patients treated with megavoltage external beam irradiation. Doc Ophthalmol 1997;93:337–44. [DOI] [PubMed] [Google Scholar]
- 18.Mohney BG, Robertson DM, Schomberg PJ, et al. Second nonocular tumours in survivors of heritable retinoblastoma and prior radiation therapy. Am J Ophthalmol 1998;126:269–77. [DOI] [PubMed] [Google Scholar]
- 19.Abramson DH, Melson MR, Dunkel IJ, et al. Third (fourth and fifth) nonocular tumors in survivors of retinoblastoma. Ophthalmology 2001;108:1868–76. [DOI] [PubMed] [Google Scholar]
- 20.Chauvienc L, Mosseri V, Quintana E, et al. Osteosarcoma following retinoblastoma: age at onset and latency period. Ophthalmic Genet 2001;22:77–88. [DOI] [PubMed] [Google Scholar]
- 21.Weiss SW, Goldblum JR. Leiomyosarcomas. In: Enzinger and Weiss’s soft tissue tumours, 4th ed. St Louis: Mosby, 2001:727–8.
- 22.Tos APD, Maestro R, Doglioni C, et al. Tumor suppressor genes and related molecules in leiomyosarcoma. Am J Pathol 1996;148:1037–45. [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen JA, Geradts J. Loss of RB and MTS1/CDKN2 (p16) expression in human sarcomas. Hum Pathol 1997;28:893–8. [DOI] [PubMed] [Google Scholar]