Abstract
Background/Aims: Recent studies indicate the presence of reactive oxygen species (ROS) producing homologues of the enzymatic subunit (Nox2) of phagocytic NADPH oxidase in non-phagocytic cells. Interestingly, in these cells, ROS produced by the Nox2 homologue(s) was shown to play a role in various regulatory processes, including cell death, proliferation, and aging. The purpose of this study was to investigate whether human cardiomyocytes express Nox2.
Methods: The expression of Nox2 was studied in human cardiomyocytes using western blot and immunohistochemical analysis. To analyse the putative expression of Nox2 in human heart disease, cardiac samples from patients who had died subsequent to acute myocardial infarction (AMI) were studied.
Results: Both in western blot and immunohistochemical studies, Nox2 expression was found in normal human cardiomyocytes. In patients with AMI, a significant increase in Nox2 expression was found both in viable and in jeopardised cardiomyocytes in the infarcted area. In addition, in the “remote from infarction” area, Nox2 expression was present in cardiomyocytes, but was not increased.
Conclusions: Nox2 or its homologue(s) is expressed in normal and jeopardised human cardiomyocytes. This expression is increased in patients with AMI, suggesting a role for this ROS producing Nox2 homologue(s) in the human heart after AMI.
Keywords: acute myocardial infarction, Nox2, cardiomyocytes, immunohistochemistry, protein expression
Cardiomyocytes produce reactive oxygen species (ROS) under different pathological conditions.1 From studies in non-cardiomyocytes it is known that ROS do not only induce cell damage, but also play a role in processes such as cell proliferation, apoptosis, gene expression, and aging.2–6 Furthermore, recent studies also point to a new, more or less cell specific source of ROS, namely homologues of the NADPH oxidase of phagocytes.7
The catalytic core of the phagocytic NADPH oxidase is a membrane integrated flavocytochrome b558, comprising the p22phox and the enzymatic gp91phox (Nox2) subunits. Recently, several Nox2 homologues have been identified in various human cell types. For instance, in blood vessels Nox2 has been reported in endothelial cells,8 whereas the Nox2 homologues, Nox1 and Nox4, were found in vascular smooth muscle cells.9 In addition, in thyroid, ThOX1 and ThOX2 were identified,10 and in the kidney Nox4.11 In heart homogenates from guinea pigs suffering from experimental hypertension, Nox2 was also found to be expressed, although it was not specified whether the enzyme was located in cardiomyocytes, fibroblasts, or endothelial cells.12
“Reactive oxygen species do not only induce cell damage, but also play a role in processes such as cell proliferation, apoptosis, gene expression, and aging”
To date, there are no data available regarding the expression of Nox2 or its homologues in human cardiomyocytes. Therefore, we have analysed the expression of Nox2 by human cardiomyocytes at the cellular level. Furthermore, we have studied the putative expression of Nox2 in the hearts of 62 patients who died as a result of acute myocardial infarction (AMI), to obtain some insight into the expression of the enzyme in pathological conditions.
MATERIALS AND METHODS
Patients
Our study was carried out using tissue from patients referred to the department of pathology for necropsy. Patients in the AMI model showed signs of a recently developed AMI (table 1) at necropsy; that is, on histochemical examination they had decreased lactate dehydrogenase (LD) staining (decolouration) of the affected myocardium. Clinical data with respect to the time duration of AMI correspond to the time intervals of the different morphological stages of AMI. Necropsies were performed as soon as possible, within 24 hours of death. Our study was approved by the ethics committee of the VU Medical Center, Amsterdam. The investigation conforms with the principles of the Declaration of Helsinki. The use of left over material after the pathological examination is part of the standard patient contract in our hospital.
Table 1.
Patient characteristics
| AMI phase | Infarct age | Number of patients | Male/Female | Age range |
| Early phase | 0–12 h | 20 | 11/9 | 36–88 |
| PMN phase | 12 h to 5 days | 19 | 14/5 | 47–85 |
| Chronic phase | 5–14 days | 6 | 5/1 | 62–75 |
| Reinfarction early phase | 0–12 h | 9 | 6/3 | 45–85 |
| Reinfarction PMN phase | 12 h to 5 days | 8 | 7/1 | 50–83 |
AMI, acute myocardial infarction; h, hours; PMN, polymorphonuclear leucocytes.
Processing of tissue specimens
Specimens from the left ventricle were used for isolation of cardiomyocytes from patients who died from a cause not related to heart disease. For the immunohistochemistry of AMI samples, myocardial tissue specimens were obtained from both the infarcted zone and from remote sites of the healthy part of the heart. These remote sites showed normal LD staining patterns and were studied as internal non-infarcted controls. The internal controls were taken from all different phases of infarction and reinfarction. None of these patients had sepsis or malignancy. Before being prepared as cryosections, tissue specimens were stored at −196°C (liquid nitrogen). Frozen sections were mounted on to SuperFrost™ Plus glass slides (Menzel-Gläser, Braunschweig, Germany).
Isolation of human cardiomyocytes
Healthy heart tissue obtained during necropsy, approximately 5 cm3, was cut into small pieces, rinsed twice in phosphate buffered saline (PBS), and pelleted by centrifugation (six minutes at 100 ×g on a low brake). The tissue was then incubated at 37°C in a solution of collagenase type 2 (Worthington Biochemical Corporation, Lakewood, New Jersey, USA) at 0.8 mg/ml in Ca2+ free Krebs Ringer buffer (pH 7.4). After separation of cardiomyocytes, the solution was filtered through a 100 μm filter and centrifuged (six minutes at 100 ×g on a low brake). The pellet contained morphologically purified human cardiomyocytes.
Western blotting
Isolated human cardiomyocytes were dissolved in Laemmli sodium dodecyl sulfate (SDS) sample buffer, stirred and heated at 95°C for 10 minutes. The samples were subjected to SDS polyacrylamide gel electrophoresis (10% gels), transferred to nitrocellulose membranes, and immunoblotted with monoclonal antibody 48 (1/250 dilution) and subsequently with horseradish peroxidase conjugated rabbit antimouse immunoglobulins (RaM-HRP; Dakopatts, Glostrup, Denmark; 1/1000 dilution). The blots were then visualised by enhanced chemiluminescence (ECL; Amersham, Buckinghamshire, UK).
Antibodies
A monoclonal antibody (C3-15) against the complement factor C3d has been used previously for immunohistochemical studies.13 Monoclonal antibodies against CD66b (previously clustered as CD67 (B13.9))14 and against Nox2 (monoclonal antibodies 48 and 7D5)15,16 were obtained from Sanquin Research at CLB, Amsterdam, The Netherlands. Monoclonal antibody KP1 against CD68 was obtained from Dakopatts, Glostrup, Denmark. The monoclonal antibodies were stored at 1 mg/ml in PBS. For all monoclonal antibodies we included controls. Healthy appearing parts of the heart did not stain with C3-15. Irrelevant monoclonal antibodies (two IgG1 and one IgG2a), tested at concentrations similar to those used for the specific monoclonal antibodies, yielded negative results.
Immunohistochemistry
Frozen sections (5 μm thick) were mounted on to glass slides, dried for one hour by exposure to air, and fixed in acetone (“Baker analysed reagent”; Mallinckrodt Baker, Deventer, Netherlands). After rinsing in PBS, the slides were incubated at room temperature for 10 minutes with normal rabbit serum (Dakopatts), diluted 1/50 in PBS containing 1% (wt/vol) bovine serum albumin (PBS-BSA) (BSA from Boehringer, Mannheim, Germany). Incubation of the slides with specific antibody solutions (diluted in PBS-BSA) was performed for 60 minutes (monoclonal antibody 48 was diluted 1/150; CD66b was diluted 1/750; C3–15 was diluted 1/1500; and KP1 was diluted 1/400). The slides were washed for 30 minutes with PBS and incubated with RaM-HRP, diluted 1/25 in PBS-BSA. Thereafter, the slides were washed again in PBS and incubated for three minutes in 0.5 mg/ml 3,3‘-diaminobenzidine tetrahydrochloride (DAB; Sigma, St Louis, Missouri, USA) in PBS, pH 7.4, containing 0.01% (vol/vol) H2O2, washed again, counterstained with haematoxylin for one minute, dehydrated, cleared, and finally mounted.
Microscopic criteria17,18 were used to estimate infarct duration in all myocardial tissue specimens. Because morphological assessment is more reliable in paraffin wax embedded sections, corresponding paraffin wax embedded slides were also made. We characterised jeopardised myocardium by the intensity of eosinophilic staining of involved myofibres, loss of nuclei, and cross striation. We classified the infarction age as follows: no microscopical changes but macroscopically LD-decolouration (early phase (phase 1)), infiltration of polymorphonuclear leucocytes (PMNs) (PMN phase (phase 2)), infiltration of lymphocytes and macrophages and fibrosis (chronic phase (phase 3)). Furthermore, patients with phase 3 morphology and phase 1 morphology were classified as reinfarction early phase (phase 4). Patients with phase 3 morphology and phase 2 morphology were classified as reinfarction PMN phase (phase 5). In all cases, the histologically assessed infarct age corresponded with the clinical course.
Two investigators (PAJK and HWMN) each judged and scored independently all slides for infarct age and anatomical localisation of the specific antibodies, as visualised by immunohistochemical staining. Scoring of the slides was performed by first determining complement positive (representing jeopardised cardiomyocytes) and complement negative regions (representing morphologically viable cardiomyocytes) in slides of the macroscopic infarcted area and control area. Thereafter, the number of CD66b and Nox2 positive cardiomyocytes was counted in 25 high power fields (HPF; 400× magnification), both in the complement positive and in the complement negative areas. The slides stained with C3-15, CD66b, monoclonal antibody 48, and KP1 were serial slides. For the final scoring results, consensus was achieved by the two investigators. The average number of positive cardiomyocytes in each HPF was used in the calculations.
Statistics
Statistics were performed with the SPSS statistics program (windows version 9.0). To evaluate whether observed differences were significant, paired or non-paired t tests were used when appropriate. A p value (two sided) of less than 0.05 was considered to be significant.
RESULTS
Nox2 in isolated human cardiomyocytes
Human cardiomyocytes were isolated from the heart of two patients who had died as a result of a condition not related to AMI, and who had suffered from no other forms of heart disease. When analysing the protein expression of Nox2 by human cardiomyocytes with immunoblotting using monoclonal antibody 48 (fig 1), a strong protein band was detected that migrated at a position of approximately 55–65 kDa, presumably representing unglycosylated Nox2. Furthermore, two thinner bands migrating at about 80 kDa were detected, probably representing various degrees of glycosylation of Nox2. These results suggest the presence of Nox2 within human cardiomyocytes.
Figure 1.
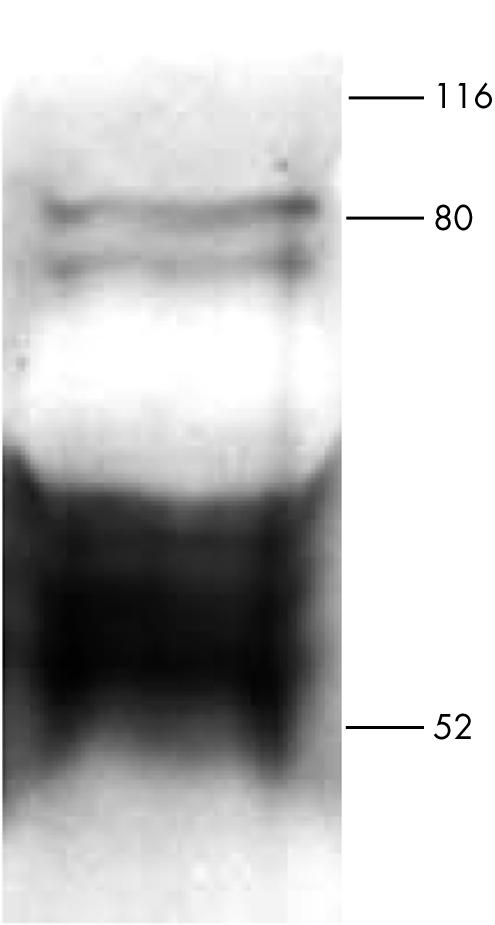
Western blot analysis of Nox2 expression by isolated human cardiomyocytes. Isolated human cardiomyocytes were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins were transferred on to nitrocellulose sheets. Nox2 was visualised with monoclonal antibody 48 (1/250 dilution).
Nox2 in the human heart
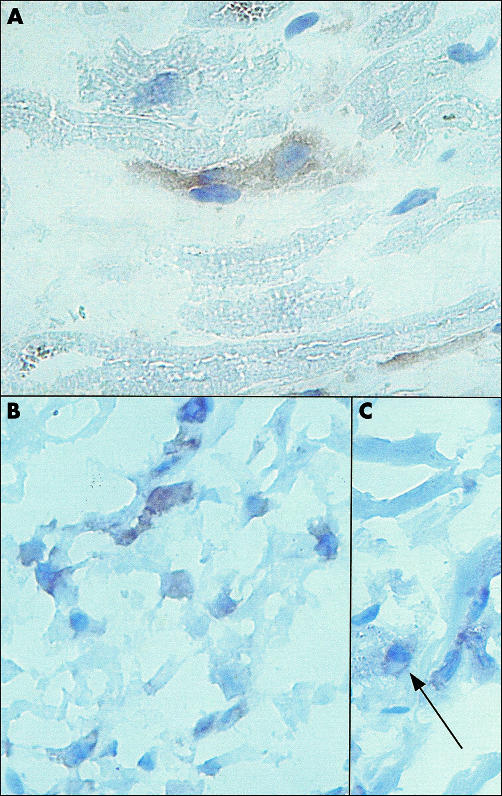
Heart tissue slides, prepared from the hearts of three patients who had died from a cause other than AMI, and did not suffer from any other form of heart disease, were stained with monoclonal antibody 48 to localise Nox2. In agreement with the results obtained by western blotting, Nox2 was detected within human cardiomyocytes (fig 2A), although only limited numbers of cardiomyocytes were positive. Nox2 expression was localised both on the plasma membrane and in the cytosol of cardiomyocytes, including the perinuclear region. Some Nox2 antigen was also found on cross striations.
Figure 2.
Immunohistochemical analysis of human heart tissue. (A) Localisation of Nox2 in the heart of a patient who had died in the absence of heart disease (original magnification, ×630). (B, C) Localisation of Nox2 in a myocardial infarct area in a patient who had died after acute myocardial infarction (original magnification, ×400). Slides were stained with monoclonal antibody 48 (1/150 dilution). (C) The arrow indicates the perinuclear localisation of Nox2.
In addition to monoclonal antibody 48, we also used monoclonal antibody 7D5, which detects the extracellular domain of Nox2.16 A positive signal was also seen in cardiomyocytes with monoclonal antibody 7D5, although this signal was less intense than that obtained with monoclonal antibody 48 (not shown). This was probably because monoclonal antibody 7D5 only stained Nox 2 at the plasma membrane of cardiomyocytes.
Therefore, using western blotting and immunohistochemistry with two different antibodies, we have shown (to our knowledge, for the first time) the presence of Nox2 within human cardiomyocytes.
Expression of Nox2 in infracted myocardium
To characterise the expression pattern of Nox2 in more detail, we subsequently analysed in more detail the expression pattern of Nox2 in heart tissue from patients with AMI by immunohistochemistry, particularly because in these patients there is a dramatic ROS increase within cardiomyocytes after AMI. Myocardial tissue specimens were obtained from 62 patients who died of AMI (table 1), as confirmed by necropsy. Specimens were obtained from the infarcted myocardium as determined macroscopically with LD staining (see “Materials and methods” and fig 3). Infarct age, determined by microscopical criteria,17,18 varied from less than 12 hours up to two weeks. Patients were divided into different groups according to infarct age, notably the early phase (less than 12 hours), PMN phase (12 hours to five days), and the chronic phase (five days to 14 days) (table 1). In addition, patients suffering from reinfarction were divided into separate groups, namely reinfarction in the early phase (12 hours) and reinfarction in the PMN phase (12 hours to five days) (table 1). Tissue specimens from sites distant from the infarct were taken from nine patients who had died after AMI. The infarcts in these patients were at different AMI phases. These specimens were used as an internal negative control.
Figure 3.
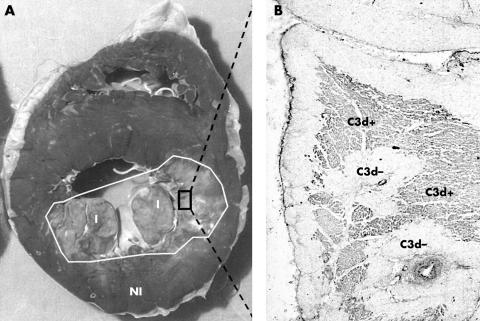
Example of (A) an infarcted human heart and (B) complement deposition in the infarction area. (A) Cross section of the heart of a patient who had died from acute myocardial infarction. Diminished lactate dehydrogenase (LD) staining is indicative of infarction (I), normal LD content is indicative of unaffected myocardium (NI). (B) Microscopical analysis of complement deposition (complement factor C3d) in the macroscopically determined infarcted myocardium.
Because macrophages also express Nox2, we stained serial heart tissue slides from all phases of infarction with the monoclonal antibody KP1 (directed against CD68, a marker for macrophages). Macrophages were indeed positive for Nox2. Nevertheless, there were large areas in which cardiomyocytes were positive for Nox2, whereas only limited numbers of macrophages were found in the interstitium that did not adher to cardiomyocytes. In addition, in the areas in which more macrophages were present, they were almost all localised to the interstitium. Therefore, it is unlikely that macrophages interfered with the Nox2 positivity of cardiomyocytes.
In previous studies we have shown that in the macroscopical infarction area viable cardiomyocytes are still present.13 To differentiate within this microscopical infarction area between the expression of Nox2 in non-jeopardised (complement negative) and jeopardised (complement positive) cardiomyocytes, we stained the slides with an antibody against activated complement (C3d) (fig 3).
Specimens obtained from the non-infarcted control areas showed no C3d deposition (data not shown). In the infarction site, extensive complement deposition was found in the PMN phase and PMN reinfarction phase, whereas in the early phase no complement deposition, and in the chronic or early reinfarction phase, only limited areas containing complement, were found (data not shown, also see Lagrand and colleagues13).
Expression of Nox2 in complement negative (viable) areas of the macroscopical infarct
Nox2 was present in cardiomyocytes within the macroscopically determined infarction area of the heart of patients who had died after AMI (fig 2 B,C). Similarly, as in normal cardiomyocytes (see above), Nox2 was localised to the plasma membrane and the cytosol of cardiomyocytes, including the perinuclear region (arrow, fig 2C). Some Nox2 was also found on cross striations.
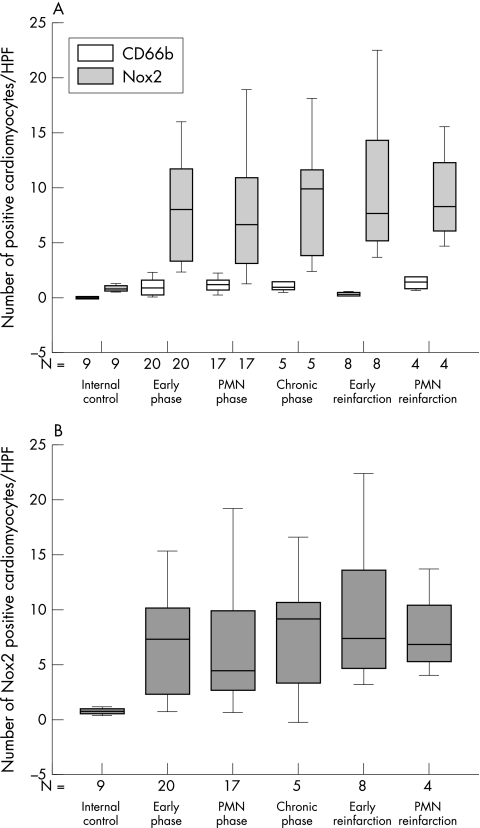
Because PMNs strongly express Nox2 and adhere to cardiomyocytes, possibly resulting in false positive Nox2 staining of cardiomyocytes, especially in the complement positive infarction area,13 we had to correct for their contribution. Therefore, we analysed the presence of CD66b (an immunohistochemical marker for PMNs) on cardiomyocytes. As expected, myofibres did not stain with CD66b in the non-infarcted control areas (fig 4A). However, in these control areas, small numbers of Nox2 positive cardiomyocytes were found, which were significantly higher (p = 0.002) than the numbers of CD66b positive cardiomyocytes (fig 4A). In these internal control areas, the numbers of Nox2 positive cardiomyocytes were comparable to the numbers of Nox2 positive cardiomyocytes in non-infarcted hearts (data not shown).
Figure 4.
Summary of Nox2 expression in relation to the presence of CD66b in complement negative areas within the macroscopical infarction area. Box plots in which the error bars represent minimum and maximum values and in which the boxes represent the lower and upper quartiles. The black lines within the boxes represent the medians. N is the number of patients examined. For each patient the average number of positive cardiomyocytes in each high power field (HPF) was determined from 25 HPFs scored. (A) Box plot of the number of Nox2 (shaded bars) and CD66b (open bars) positive cardiomyocytes in each HPF in the different phases of infarction. (B) Box plot of the number of Nox2 positive cardiomyocytes in each HPF after subtraction of CD66b positive cardiomyocytes. PMN, polymorphonuclear leucocytes.
In the macroscopically infarcted, but microscopically complement negative, areas of all AMI phases, low numbers of CD66b positive cardiomyocytes were also found. In addition, in these areas the number of Nox2 positive myofibres was significantly higher (p < 0.05) than the number of CD66b positive myofibres.
To correct for the contribution of PMNs to the presence of Nox2 on cardiomyocytes, the number of CD66b positive cardiomyocytes was subtracted from the number of Nox2 positive cardiomyocytes (fig 4B). In all phases of AMI, the number of Nox2 positive myofibres was significantly higher (p < 0.05) than in the internal control. Notably, no significant differences in the number of Nox2 positive cardiomyocytes were found between the different phases of AMI.
Expression of Nox2 in complement positive (jeopardised) areas of the macroscopical infarct
As mentioned earlier, no complement positivity was found in the early AMI phase. In the chronic phase and in the early reinfarction phase, only two patients showed complement deposition (the area of positivity was maximally 10%, not shown). Therefore, the number of patients was too small for statistical analysis. However, in these patients the number of Nox2 positive myofibres was much lower than that seen in the PMN and PMN reinfarction phase, but was still higher than the number of CD66b positive myofibres (data not shown).
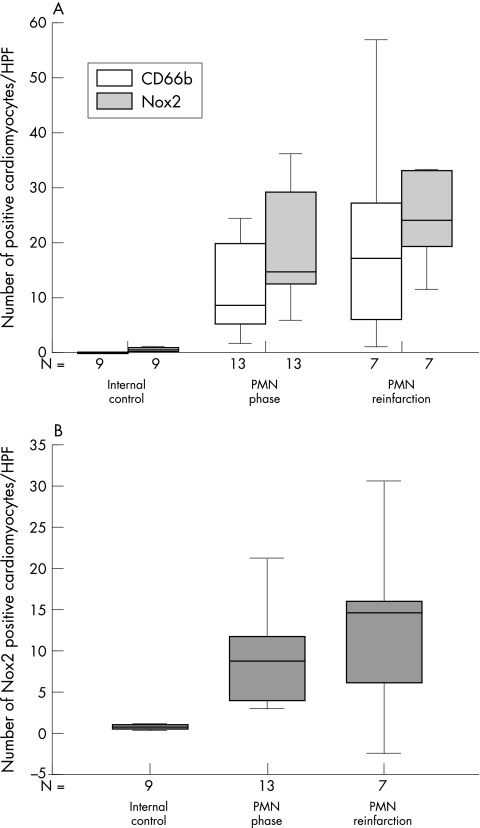
In the two phases of myocardial infarction that are accompanied by the most extensive complement deposition—the PMN and PMN reinfarction phases—the number of Nox2 positive myofibres was higher than the number of CD66b positive myofibres (fig 5A), although this was only significant in the PMN phase.
Figure 5.
Statistical analysis of Nox2 and CD66b in complement positive areas within the macroscopical infarction area. Box plots in which the error bars represent minimum and maximum values and in which the boxes represent the lower and upper quartiles. The black lines within the boxes represent the medians. N is the number of patients examined. For each patient the average number of positive cardiomyocytes in each high power field (HPF) was determined from 25 HPFs scored. (A) Box plot of the number of Nox2 (shaded bars) and CD66b (open bars) positive cardiomyocytes in each HPF in the different phases of infarction. (B) Number of Nox2 positive cardiomyocytes in each HPF after subtraction of CD66b positive cardiomyocytes. PMN, polymorphonuclear leucocytes.
Again, the number of CD66b positive cardiomyocytes was subtracted from the number of Nox2 expressing cardiomyocytes to correct for interference by PMNs (fig 5B). In both the PMN and the PMN reinfarction phases, the number of Nox2 positive cardiomyocytes was significantly higher (p < 0.05) than in the internal control. However, the number of Nox2 positive myofibres did not significantly differ between the PMN and PMN reinfarction phases. The number of cardiomyocytes positive for Nox2 in the complement positive areas of the infarction site was higher than was seen in the complement negative areas (1.1 fold for the PMN phase and 1.6 fold for the PMN reinfarction phase), although this difference was not significant. However, it should be noted that in both the PMN and the PMN reinfarction phase, the number of CD66b positive cardiomyocytes was significantly (p < 0.05) higher in complement positive areas than in complement negative areas (approximately seven and 17 fold, respectively). Despite this high number of CD66b positive cardiomyocytes, the number of cardiomyocytes positive for Nox2 but negative for CD66b in complement positive areas during both the PMN and PMN reinfarction phases was higher than that seen in the complement negative areas, although not significantly. Because we excluded the cardiomyocytes that were positive for CD66b and thus also for Nox2, our data probably represent an underestimation of Nox2 expressing cardiomyocytes in complement positive areas of the infarct as compared with that in complement negative areas.
Thus, our data indicate that Nox2 expression is upregulated, both in jeopardised and in non-jeopardised cardiomyocytes within the macroscopic infarction area of patients with AMI. The number of Nox2 expressing cardiomyocytes was significantly higher in this macroscopic infarction area than in the macroscopic control areas.
DISCUSSION
Recent studies have described cell specific homologues of gp91phox (Nox2) as a new source of ROS.8–11 To the best of our knowledge, we have shown for the first time: (1) the presence of Nox2 in non-pathological human cardiomyocytes, using western blot and immunohistochemical analysis and two different antibodies for detection; and (2) an increase in expression of Nox2 in human cardiomyocytes in a pathological condition, namely AMI.
On western blotting, Nox2 was found in human cardiomyocytes as multiple bands; that is, an intense band between 55 kDa and 65 kDa and two bands around 80 kDa. The signal between 55 and 65 kDa is probably the unglycosylated protein, because the predicted molecular weight of unglycosylated Nox2 protein is approximately 58 kDa16 to 65 kDa in phagocytes.19,20 In human B cells21 and rat endothelial cells,19 Nox2 was also detected at approximately 60 to 65 kDa. The bands around 80 kDa probably represent glycosylated Nox2, as has also been shown in human umbilical vein endothelial cells.8
“Nox2 might play a role in signal transduction processes in normal cardiomyocytes”
Although two phagocyte Nox2 specific antibodies yielded comparable results in human cardiomyocytes, we cannot exclude the possibility that the epitope detected by the antibodies in the cardiomyocytes actually belongs to one or more homologue(s) of Nox2. Also unknown is the function of Nox2 or its homologue(s) in cardiomyocytes. Although Nox2 was present in some of the non-pathological cardiomyocytes, a significant increase in Nox2 positivity was detected in patients with AMI.
Take home messages.
Nox2 or its homologue(s) is expressed in both normal and jeopardised human cardiomyocytes
This expression is increased in patients with acute myocardial infarction (AMI), suggesting a role for this reactive oxygen species producing Nox2 homologue(s) in the human heart after AMI
AMI induced upregulation of Nox2 is probably related to ROS production. Indeed, in rat cardiomyoblast cells (H9c2) it has already been shown that diphenylene iodonium, a non-specific inhibitor of NADPH oxidase, results in a decrease in ROS production.22 Studies of Mox1 (= Nox1) suggested that these Nox2 homologues produce much lower quantities of ROS when compared with the amount of ROS produced by Nox2 in phagocytes.23 Because in our study Nox2 was found also in the jeopardised (complement positive) cardiomyocytes, the possibility that ROS produced via Nox2 in cardiomyocytes plays a role in the process of cell damage cannot be excluded. However, cardiomyocytes in the non-jeopardised (complement negative) part of the macroscopical AMI infarction area also strongly expressed Nox2. Because some of these cardiomyocytes become secondarily hypertrophic in time, a role for ROS produced via Nox2 in the process of hypertrophy can be postulated. This is in line with other studies in which Nox2 or Nox2 homologues were shown to be involved in the process of hypertrophy.24–27 The role of Nox2 in non-infarcted, morphological normal cardiomyocytes at this moment is difficult to predict. However, as suggested in studies of Nox2 homologues in other cell types, Nox2 might play a role in signal transduction processes in normal cardiomyocytes.
In conclusion, we have shown the expression of Nox2 in human cardiomyocytes, which increases during myocardial infarction. The pathophysiological role of this myocardial Nox2 is the subject of further study.
Acknowledgments
Dr Niessen is a recipient of the Dr E Dekker program of the Netherlands Heart Foundation (D99025). This study was financially supported by the Netherlands Heart Foundation, grant 2002B010.
Abbreviations
AMI, acute myocardial infarction
BSA, bovine serum albumin
HPF, high power field
LD, lactate dehydrogenase
PBS, phosphate buffered saline
RaM-HRP, horseradish peroxidase conjugated rabbit antimouse immunoglobulin
ROS, reactive oxygen species
SDS, sodium dodecyl sulfate
REFERENCES
- 1.Duranteau J, Chandel NS, Kulisz A, et al. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 1998;273:11619–24. [DOI] [PubMed] [Google Scholar]
- 2.Rao GN, Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res 1992;70:593–9. [DOI] [PubMed] [Google Scholar]
- 3.Abe J, Berk BC. Reactive oxygen species as mediators of signal transduction in cardiovascular disease. Trends Cardiovasc Med 2002;8:59–64. [DOI] [PubMed] [Google Scholar]
- 4.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J 1997;11:118–24. [PubMed] [Google Scholar]
- 5.Katusic ZS. Superoxide anion and endothelial regulation of arterial tone. Free Radic Biol Med 1996;20:443–8. [DOI] [PubMed] [Google Scholar]
- 6.von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 1999;99:2934–41. [DOI] [PubMed] [Google Scholar]
- 7.Lambeth JD, Cheng G, Arnold RS, et al. Novel homologs of gp91phox. Trends Biochem Sci 2000;25:459–61. [DOI] [PubMed] [Google Scholar]
- 8.Gorlach A, Brandes RP, Nguyen K, et al. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res 2000;87:26–32. [DOI] [PubMed] [Google Scholar]
- 9.Lassegue B, Sorescu D, Szocs K, et al. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 2001;88:888–94. [DOI] [PubMed] [Google Scholar]
- 10.De Deken X, Wang D, Many MC, et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 2000;275:23227–33. [DOI] [PubMed] [Google Scholar]
- 11.Shiose A, Kuroda J, Tsuruya K, et al. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem 2001;276:1417–23. [DOI] [PubMed] [Google Scholar]
- 12.MacCarthy PA, Grieve DJ, Li JM, et al. Impaired endothelial regulation of ventricular relaxation in cardiac hypertrophy: role of reactive oxygen species and NADPH oxidase. Circulation 2001;104:2967–74. [DOI] [PubMed] [Google Scholar]
- 13.Lagrand WK, Niessen HW, Wolbink GJ, et al. C-reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation 1997;95:97–103. [DOI] [PubMed] [Google Scholar]
- 14.Niessen HW, Kuijpers TW, Roos D, et al. Release of azurophilic granule contents in fMLP-stimulated neutrophils requires two activation signals, one of which is a rise in cytosolic free Ca2+. Cell Signal 1991;3:625–33. [DOI] [PubMed] [Google Scholar]
- 15.Verhoeven AJ, Bolscher BG, Meerhof LJ, et al. Characterization of two monoclonal antibodies against cytochrome b558 of human neutrophils. Blood 1989;73:1686–94. [PubMed] [Google Scholar]
- 16.Yamauchi A, Yu L, Potgens AJ, et al. Location of the epitope for 7D5, a monoclonal antibody raised against human flavocytochrome b558, to the extracellular peptide portion of primate gp91phox. Microbiol Immunol 2001;45:249–57. [DOI] [PubMed] [Google Scholar]
- 17.Mallory GK, White PD, Salcedo-Salgar J. The speed of healing of myocardial infarction. Am Heart J 1939;18:647–51. [Google Scholar]
- 18.Cotran SC, Kumar V, Robbins LR. The heart. In: Robbins pathologic basis of disease, 4th ed. Philadelphia PA: WB Saunders, 1989:605–14.
- 19.Bayraktutan U, Blayney L, Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol 2000;20:1903–11. [DOI] [PubMed] [Google Scholar]
- 20.Cheng G, Cao Z, Xu X, et al. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001;269:131–40. [DOI] [PubMed] [Google Scholar]
- 21.Porter CD, Kuribayashi F, Parkar MH, et al. Detection of gp91-phox precursor protein in B-cell lines from patients with X-linked chronic granulomatous disease as an indicator for mutations impairing cytochrome b558 biosynthesis. Biochem J 1996;315:571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souren JE, Van Der Mast C, Van Wijk R. NADPH-oxidase-dependent superoxide production by myocyte-derived H9c2 cells: influence of ischemia, heat shock, cycloheximide and cytochalasin D. J Mol Cell Cardiol 1997;29:2803–12. [DOI] [PubMed] [Google Scholar]
- 23.Suh YA, Arnold RS, Lassegue B, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999;401:79–82. [DOI] [PubMed] [Google Scholar]
- 24.Bendall JK, Cave AC, Heymes C, et al. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 2002;105:293–6. [DOI] [PubMed] [Google Scholar]
- 25.Zafari AM, Ushio-Fukai M, Minieri CA, et al. Arachidonic acid metabolites mediate angiotensin II-induced NADH/NADPH oxidase activity and hypertrophy in vascular smooth muscle cells. Antioxid Redox Signal 1999;1:167–79. [DOI] [PubMed] [Google Scholar]
- 26.Zafari AM, Ushio-Fukai M, Akers M, et al. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension 1998;32:488–95. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K, Honda M, Takabatake T. Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J Am Coll Cardiol 2001;37:676–85. [DOI] [PubMed] [Google Scholar]