Abstract
Background: Screening for Chlamydia trachomatis specific antibodies is valuable in diagnosing asymptomatic pelvic inflammatory disease (PID) and tubal damage following repeated episodes of PID. The assays in current use are unsuitable for screening large numbers of samples so there is a need to develop more suitable assays.
Aims: To compare the performance of several commercial C trachomatis enzyme immunoassays (EIAs) (SeroCT, C trachopep, Medac p-EIA, Vircell and Labsystems C trachomatis IgG EIAs) using major outer membrane protein (MOMP), an inactivated organism EIA (Genzyme Virotech EIA), and a genus specific EIA (Platelia Chlamydia IgG) with the whole cell inclusion immunofluorescence (WIF) assay. In addition, to adapt, using time resolved fluorescence technology, the assay showing the highest correlation with WIF.
Methods: Ninety sera from patients presenting with ectopic pregnancies, 187 sera from those with a variety of types of infertility, 33 sera from cases of PID where a fourfold rise in WIF titre occurred, and 90 sera from antenatal clinic attenders were tested. A panel of 36 sera from laboratory diagnosed cases of Chlamydia psittaci/Chlamydia pneumoniae infection was also tested.
Results: The Genzyme Virotech EIA showed the highest rank correlation coefficient (0.82) with WIF, particularly at high WIF titres. The MOMP specific assays varied in their correlation with WIF, with rank correlation coefficients ranging from 0.70 (Medac p-EIA) to 0.80 (Vircell EIA). The Genzyme Virotech assay showed poor specificity (5.6%; 95% confidence interval (CI), 0.68% to 18.7%)—it was reactive with 34 of the panel of 36 C psittaci/C pneumoniae positive sera. The MOMP based EIAs showed high specificity, particularly the Medac p-ELISA (97.2%; 95% CI, 85.5% to 99.9%)—only one serum was reactive. In view of the good correlation between WIF and the Genzyme Virotech EIA, a time resolved fluorescence immunoassay (TRFIA) was developed using the Genzyme Virotech antigen. Using an appropriate cut off the TRFIA assay showed excellent correlation with WIF.
Conclusions: The TRFIA assay may be useful as a screening assay, possibly in conjunction with one of the highly specific EIAs studied (for example, Medac p-EIA) to confirm the antibody specificity of sera selected by the screening assay.
Keywords: Chlamydia trachomatis antibody, enzyme immunoassay, time resolved fluorescence immunoassay
Chlamydia trachomatis infection is the most common sexually transmitted bacterial disease in England, Wales, and Northern Ireland, with 64 000 diagnoses made in the year 2000.1 Most C trachomatis lower genital tract infections are asymptomatic and the most common clinical presentation in women is mucopurulent cervicitis, and in men urethritis. For lower genital tract infection, the detection of specific antibodies in a single serum specimen is held to be of little value because such antibodies are frequently found in sera from women who do not have active infection.2 Despite the difficulty of differentiating between previous and current lower genital tract infection, there is a considerable amount of evidence that the presence of C trachomatis specific antibody is significantly associated with upper genital tract infection, particularly when the antibody is at a high titre.3,4 Screening for C trachomatis specific antibodies is valuable in diagnosing asymptomatic pelvic inflammatory disease (PID) and tubal damage following repeated episodes of PID, particularly because it has been shown that C trachomatis is rarely isolated from the upper genital tract and clinical diagnosis requires invasive procedures not routinely available in general practice.5
There are two accepted reference assays for measuring C trachomatis specific antibodies, the microimmunofluorescence assay (MIF) of Wang and colleagues6 and the whole cell inclusion immunofluorescence assay (WIF) of Richmond and Caul.7 The WIF assay is a single antigen immunofluorescence test in which cytochalasin B treated McCoy cells infected with an LGV type 2 strain of C trachomatis are placed in wells on slides coated with polytetrafluoroethylene. In this system, the whole chlamydial inclusion acts as the antigen, in contrast to the MIF test in which elementary bodies act as the antigen. The WIF test detects both genus specific (lipopolysaccharide; LPS) antibody and species specific major outer membrane protein (MOMP) antibody and, like MIF, it is a subjective, labour intensive assay not suited to screening large numbers of sera. Our laboratory uses the WIF assay because we have found it to be more reliable for the diagnosis of upper genital tract infection than MIF, and also because inclusions are easier to visualise than cell free elementary bodies.5
“Time resolved fluorescence immunoassay is suitable for the measurement of low and high amounts of antibody, even with single dilutions of specimen, because of its large linear dynamic range”
The effectiveness of intervention measures to reduce C trachomatis infection in designated female populations can be measured through determining population prevalences of chlamydia mediated upper genital tract infection. For screening activities, in which large numbers of sera need to be tested, we need alternative assays to MIF and WIF and commercial C trachomatis antibody assays may prove to be a useful alternative. Furthermore, for population screening the utilisation of non-invasive sampling techniques, such as collection of oral fluid, is our desired goal, and this approach requires the adoption of specialised technology8 with potential application to oral fluid screening. We favour the use of time resolved fluorescence immunoassay (TRFIA), because in our hands this technology has been found to be highly sensitive.9 TRFIA is suitable for the measurement of low and high amounts of antibody, even with single dilutions of specimen, because of its large linear dynamic range, and has previously been assessed for population screening of anti-C pneumoniae IgG, where its objectivity, reproducibility, and amenability to automation were distinct advantages compared with MIF.10
Our study had two objectives: (1) to compare the performance of several commercial C trachomatis antibody assays in relation to WIF, with a view to assessing their specificity and sensitivity for C trachomatis; and (2) to adapt, using time resolved fluorescence technology, the commercial assay showing highest correlation with WIF.
MATERIALS AND METHODS
Sera tested
A set of 310 sera was assembled, comprising 90 sera from ectopic pregnancies, 187 sera from patients with infertility, and 33 sera from cases of PID shown by at least a fourfold increase in titre. In addition, a set of 36 sera from WIF diagnosed cases of C psittaci or C pneumoniae, as shown by rising complement fixation test/WIF titres,7 and a control set of 90 antenatal sera were also assembled to produce a test panel of 436 sera.
Whole cell inclusion immunofluorescence assay (WIF)
This assay was performed as described previously.5,7 WIF testing was performed before the other tests to avoid potential bias in the results. Chlamydia trachomatis L2 was used for C trachomatis specific antibody determination, enzootic abortion ewes C psittaci was used for C psittaci specific antibody determination, and C pneumoniae TW183 was used for C pneumoniae specific antibody determination.
EIA kits tested
The following EIA kits were tested:
Platelia Chlamydia IgG (Sanofi Pasteur), catalogue number 62766, batch number 9K058U. Supplied by Sanofi Diagnostics Pasteur Ltd, Guildford, UK.
SeroCT™-IgG (Savyon Diagnostics Ltd), catalogue number A181–01M, batch number 181–912A. Supplied by Brownes Ltd, Reading, UK.
C trachopep EIA-IgG (PBS Orgenics), catalogue number HX.CTG.096, batch number 990905. Supplied by Quest Biomedical, Knowle, UK.
Chlamydia trachomatis IgG/IgM EIA (Vircell SL), catalogue number G/M1017, batch number 99ECTR104. Supplied by Microgen Bioproducts Ltd, Camberley, UK.
Chlamydia trachomatis-IgG-pEIA (Medac Diagnostica), catalogue number 497/TMB, batch number CTG08. Supplied by Medac Diagnostica, Wedel, Germany.
Chlamydia trachomatis EIA (Genzyme Virotech), catalogue number EC 120.00, batch number 90817–01. Supplied by Diasorin, Wokingham, UK and Genzyme Virotech, Russelsheim, Germany.
Chlamydia trachomatis IgG EIA (Labsystems), catalogue number 6111–101, batch number 112TK2–3. Supplied by Quest Biomedical.
EIA methods
All kits used were of the same batch and testing was performed before their expiry dates. The manufacturers’ instructions were followed, in full, when performing the assays and only IgG values were determined. In each instance, kits were stored at 4°C and allowed to stand at room temperature for one hour before use. Incubations at 37°C were performed in moist chambers unless specifically indicated otherwise. Microtitre plate washing was performed using a Labsystems (Thermo Life Sciences Ltd, Basingstoke, UK) Wellwash 4 Mk 2 and plates were read using a Labsystems Multiskan RC reader. Assays were only regarded as valid if all manufacturers’ validation criteria were satisfied.
Time resolved fluorometric immunoassay (TRFIA)
Sera for testing were diluted 1/64 in DELFIA assay buffer (Wallac Oy, Turku, Finland) and 100 μl was loaded on to Genzyme Virotech C trachomatis coated microtitre plates (Genzyme Virotech). The plates were incubated for two hours at 37°C in a moist chamber and then washed four times with DELFIA wash solution (Wallac Oy) using a DELFIA 1296–026 Platewash (Wallac Oy). Europium labelled antihuman IgG conjugate (Wallac Oy), diluted 1/500 in DELFIA assay buffer, was added (100 μl/well) to the plates, which were then incubated for one hour, at 37°C, in a moist chamber. The plates were washed four times, DELFIA enhancement solution (Wallac Oy) was added (100 μl/well), and the plates were then shaker incubated for 10 minutes at room temperature using a Stuart mini-orbital shaker (Bibby Sterilin, Stone, UK) set at 125 rpm. The plates were then read using a DELFIA 1234 fluorometer (Wallac Oy) and the counts processed by multicalc software, version 2.5 (Wallac Oy).
Statistical methods
Assays were compared on a log scale using Spearman’s coefficient of rank correlation (r) with 95% confidence intervals (CI) calculated by bootstrapping. Best fit regression lines were also plotted. For the 36 sera diagnosed by WIF as C pneumoniae or C psittaci, the specificity of the assays was calculated with 95% confidence intervals.
RESULTS
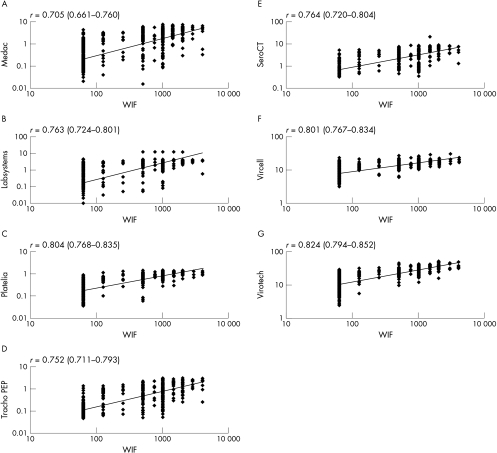
Figure 1 shows the correlation between commercial EIA results (log10 scale) and WIF results. From the graphs, a clustering effect of EIA values could be seen with WIF low and high titre sera to varying degrees, depending on the EIA kit used. A substantial number of sera had low titre WIF and high titre EIA results (false positives) and high titre WIF with low titre EIA results (false negatives). Overall, the Genzyme Virotech assay, which had the highest rank correlation coefficient of 0.82, correlated most closely with WIF, particularly with WIF high titre sera. The Vircell (species specific) and Sanofi Platelia (genus specific) assays had the next highest rank correlation (0.80), and again showed good clustering with WIF high titre sera. The lowest rank correlation coefficient (0.70) was obtained with the Medac p-EIA.
Figure 1.
Correlation between commercial enzyme immunoassay (EIA) results (log10 scale) and whole cell inclusion immunofluorescence (WIF) results. (A) Medac p-EIA v WIF; (B) Labsystems v WIF; (C) Patelia Chlamydia IgG v WIF; (D) C trachopep v WIF; (E) SeroCT v WIF; (F) Vircell v WIF; (G) Genzyme Virotech EIA v WIF.
Using a panel of WIF diagnosed C psittaci and C pneumoniae sera, the C trachomatis antibody specificity of the commercial assays was evaluated (table 1). The Medac p-EIA had the highest specificity when compared with WIF (97.2%; 95% CI, 85.5% to 99.9%), with only one serum testing C trachomatis antibody positive. Both Labsystems and PBS Orgenics assays had specificities of 94.4% (95% CI, 81.3% to 99.3%), followed by the Savyon Sero CT assay with a specificity of 91.7% (95% CI, 77.5% to 98.3%). Despite having the highest rank correlation coefficients with WIF, the Genzyme Virotech and Vircell assays showed the lowest specificities—5.6% and 58.3%, respectively—of the species specific assays tested. The Sanofi Platelia EIA had a specificity of 0%, which reflects the fact that it is a genus specific assay.
Table 1.
Specificity of the commercial enzyme immunoassays (EIAs) for detecting Chlamydia trachomatis antibody when tested against a panel of Chlamydia psittaci/Chlamydia pneumoniae antibody positive sera as diagnosed by whole cell immunofluorescence assay
| Commercial EIA | Number negative out of 36 | Specificity (95% CI) |
| Medac Diagnostica | 35 | 97.2% (85.5% to 99.9%) |
| Labsystems | 34 | 94.4% (81.3% to 99.3%) |
| PBS Orgenics (Tracho PEP) | 34 | 94.4% (81.3% to 99.3%) |
| Savyon Diagnostics (SeroCT) | 33 | 91.7% (77.5% to 98.3%) |
| Vircell SL | 21 | 58.3% (40.8% to 74.5%) |
| Genzyme Virotech | 2 | 5.6% (0.68% to 18.7%) |
| Sanofi Pasteur (Platelia) | 0 | 0% (0% to 9.7%) |
CI, confdence interval.
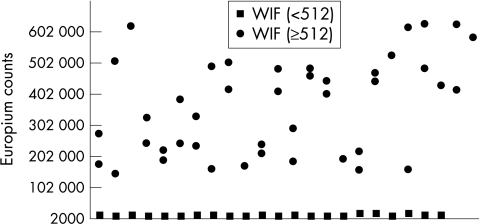
For application to screening activities we considered the Genzyme Virotech assay most suitable for further development because it showed a good correlation with WIF, particularly for sera having WIF titres of 512, or higher, which is the cut off titre used in our laboratory for predicting upper genital tract infection. A limitation of the Genzyme Virotech assay was that the cut offs stipulated by the manufacturer resulted in many sera that had low WIF titres (that is, ≤ 64) being classified as C trachomatis antibody positive. Antibody to C trachomatis could have been present at low levels; however, for our purposes we needed to recalibrate the Genzyme Virotech assay so that the cut off used correlated more closely with the WIF cut off titre of 512. Such a recalibration was achieved by using time resolved fluorescence technology. Forty five sera with WIF titres the same, or higher, than our chosen cut off (that is, WIF titres ≥ 512) and 22 sera with titres below the cut off (that is, WIF titres < 512) were tested (fig 2). A cut off point of 100 000 counts with the modified Genzyme Virotech assay was chosen to differentiate between high WIF titre and low WIF titre sera because it gave excellent discrimination.
Figure 2.
Modified Genzyme Virotech assay showing the excellent discrimination between the 45 sera with whole cell inclusion immunofluorescence (WIF) titres the same, or higher, than the cut off (≥ 512) and 22 sera with titres below the cut off (< 512).
DISCUSSION
A wide range of antibodies has been reported11 to be produced following C trachomatis infection because the organism has a unique biphasic life cycle, alternating between infectious elementary bodies and a replicating, reticulate body. There are three species of chlamydia that cause infection in humans, namely: C trachomatis, C pneumoniae, and C psittaci and several serovars of C trachomatis (D–K) that infect the urogenital tract. Any serological test that is used to detect C trachomatis infection must differentiate between infection by other species of chlamydia, yet retain the capacity to detect antibodies produced at different stages of the life cycle and by different serovars. One advantage of the microimmunofluorescence and whole cell inclusion immunofluorescence assays is that because they use whole organisms/native antigens they detect a wide variety of antibodies and therefore confer enhanced sensitivity. A potential limitation of these assays is that they may detect antibodies unrelated to C trachomatis infection, thereby compromising specificity. For example, crossreaction between the genus specific LPS antigen of Chlamydia spp and the lipopolysaccharides of Porphyromonas gingivalis, Escherichia coli O119, and Salmonella newington has been reported12 for both immunofluorescence and EIAs utilising chlamydial LPS.
“The specificity of the Medac p-EIA may be attributed to its use of a highly immunogenic species specific epitope, which shares no sequence homology with Chlamydia pneumoniae”
MOMP has been used extensively in EIA based tests for C trachomatis because this antigen is considered to be species and serovar specific,13 and the Savyon SeroCT, PBS Orgenics C trachopep, Labsystems C trachomatis, and Medac C trachomatis p-EIA assays use peptides mimicking regions of this protein. From fig 1 it can be seen that the species specific peptide assays performed variably in relation to WIF, with rank correlations ranging from 0.70 to 0.76. The Vircell assay, which uses extracted and purified MOMP, had a higher rank correlation with WIF (0.80) than the synthetic MOMP peptide assays, which may reflect the fact that the antigen used is native, and therefore possesses more epitopes. The assay that had the best correlation with WIF, particularly at high WIF titres, was the Genzyme Virotech C trachomatis EIA, which uses C trachomatis LGV type II strain, cultured in mouse L cells, and inactivated using γ irradiation. It is possible that the reduced correlation of the synthetic peptide assays results from conformational differences in the epitopes presented by the peptides14 compared with native antigens.
The Medac p-EIA showed the highest specificity for C trachomatis specific antibody (97.2%) with WIF when tested against sera from WIF diagnosed C psittaci and C pneumoniae infection. The specificity of the Medac p-EIA may be attributed to its use of a highly immunogenic species specific epitope, which shares no sequence homology with C pneumoniae.15 The Labsystems C trachomatis EIA was the next most specific assay, and this test is based on four synthetic peptides derived from the variable domain IV of the MOMP of C trachomatis serotypes C, G, E, and L2.16 The Genzyme Virotech EIA, although showing the best correlation with WIF, particularly at high titres, had a specificity of only 5.6% with the C psittaci/C pneumoniae antibody positive sera. This would be expected because the antigen used has not had chlamydial LPS, which is a group specific antigen, extracted. The antigen used for WIF7 is also not LPS extracted and LPS antibodies will be detected in both these assays, but not in the MOMP synthetic peptide assays. There is evidence to suggest17 that, in many instances, anti-LPS antibody rapidly declines following C trachomatis infection, and in cases of chronic infection associated with tubal factor infertility and ectopic pregnancy little LPS antibody is found. In our laboratory,18 comparison of complement fixing antibodies (that is, anti-LPS antibody) with WIF titres is used to differentiate recent and chronic active infections in patients with high WIF titres (≥ 512). The absence of complement fixing antibody or its presence at low titres in sera with WIF titres ≥ 512 is suggestive of tubal damage with no evidence of active infection; however, if complement fixing antibody is found at titres ≥ 12, PID is suspected.
The Genzyme Virotech EIA was selected as our screening assay; however, we needed to refine the assay so that the cut off used correlated with that used for WIF (≥ 512) in our laboratory. The assay was modified using time resolved fluorescence technology because this technique, as a result of its large linear dynamic range, has particular application to screening single serum dilutions.9 This approach appeared to be highly successful (fig 2) in differentiating between WIF positive sera (titres ≥ 512) and WIF negative sera (titres < 512). Using the modified, time resolved Genzyme Virotech assay, high chlamydial antibody titre sera can be selected from our screening programmes for further testing for C trachomatis specific MOMP antibody. Our studies of specificity have shown that the Medac p-EIA is the most appropriate assay to use; however, there are occasions when sera have high screening antibody titres but are not p-EIA positive. Such incidents may be the result of genuine C psittaci/C pneumoniae infection, crossreactivitity with LPS from Gram negative bacteria,12 or a failure of C trachomatis antibodies in the serum to recognise the MOMP epitopes in the Medac p-EIA.
Our ultimate goal is to develop a non-invasive screening assay for chlamydial infection which, with appropriate refinements, will be specific for C trachomatis mediated upper genital tract disease. The amount of IgG in whole saliva is about one thousandth of that in plasma,8 and using the TRFIA cut off count of 100 000 (when testing sera at a 1/64 dilution), a theoretical cut off with saliva would be 100 counts. We normally test saliva at a 1/4 dilution; therefore, the theoretical cut off would be 1600 counts, which is measured with ease by TRFIA.
Take home messages
Screening for Chlamydia trachomatis specific antibodies is valuable in diagnosing asymptomatic pelvic inflammatory disease (PID) and tubal damage following repeated episodes of PID
Because the currently used assays are unsuitable for screening activities in which large numbers of sera need to be tested, we evaluated several commercial C trachomatis specific enzyme immunoassays (EIAs) against the most commonly used assay (WIF)
The best correlation (0.82) was seen with an EIA using a cultured, inactivated organism and this assay has been modified, using time resolved fluorescence technology, for use as a screening assay
Some of the EIAs studied (for example, Medac p-EIA) were highly specific for C trachomatis antibody and can be used to confirm the antibody specificity of sera selected by the screening assay
Further studies have been initiated to assess the usefulness of this screening approach
“Our ultimate goal is to develop a non-invasive screening assay for chlamydial infection which, with appropriate refinements, will be specific for Chlamydia trachomatis mediated upper genital tract disease”
To conclude, we have evaluated several commercial C trachomatis specific EIAs against WIF and the correlation has ranged between 0.70 and 0.80. The best correlation (0.82) was seen with an EIA using a cultured, inactivated organism and this assay has been modified, using time resolved fluorescence technology, for use as a screening assay. Some of the EIAs studied (for example, Medac p-EIA) were highly specific for C trachomatis antibody and can be used to confirm the antibody specificity of sera selected by the screening assay. Further studies have been initiated to assess the usefulness of this screening approach.
Abbreviations
CI, confidence interval
EIA, enzyme immunoassay
LPS, lipopolysaccharide
MIF, microimmunofluorescence assay
MOMP, major outer membrane protein
PID, pelvic inflammatory disease
TRFI, time resolved fluorescence immunoassay
WIF, whole cell inclusion immunofluorescence
REFERENCES
- 1.PHLS, DHSS and PS and the Scottish ISD(D)5 Collaborative Group. Sexually transmitted infections in the UK: new episodes seen at Genitourinary Medicine Clinics, 1995 to 2,000. London: Public Health Laboratory Service, 2001.
- 2.Caul EO. Laboratory diagnosis of Chlamydia trachomatis infection. PHLS Microbiology Digest 1990;7:65–72. [Google Scholar]
- 3.Gump DW, Gibson M, Ashikaga T. Evidence of prior pelvic inflammatory disease and its relationship to Chlamydia trachomatis antibody and intrauterine contraceptive device use in infertile women. Am J Obstet Gynecol 1983;146:153–9. [DOI] [PubMed] [Google Scholar]
- 4.Moore DE, Spadoni LR, Foy HM, et al. Increased frequency of serum antibodies to Chlamydia trachomatis in infertility due to distal tubal disease. Lancet 1982;ii:574–7. [DOI] [PubMed] [Google Scholar]
- 5.Chernesky M, Luinstra K, Sellors J, et al. Can serology diagnose upper genital tract Chlamydia trachomatis infection? Studies on women with pelvic pain, with or without chlamydial plasmid DNA in endometrial biopsy tissue. Sex Transm Dis 1998;25:14–19. [DOI] [PubMed] [Google Scholar]
- 6.Wang SP, Grayston JT, Alexander ER, et al. Simplified microimmunofluorescence test with trachoma-lymphogranuloma venereum (Chlamydia trachomatis) antigens for use as a screening test for antibody. J Clin Microbiol 1975;1:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richmond SJ, Caul EO. Fluorescent antibody studies in chlamydial infections. J Clin Microbiol 1975;1:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKie A, Vyse A, Maple C. Novel methods for the detection of microbial antibodies in oral fluid. Lancet Infectious Diseases 2002;2:18–24. [DOI] [PubMed] [Google Scholar]
- 9.Maple PAC, Jones CS. Time-resolved fluorometric immunoassay for rubella antibody—a useful method for serosurveillance studies. Vaccine 2002;20:1378–82. [DOI] [PubMed] [Google Scholar]
- 10.Wong YK, Sueur JM, Fall CHD, et al. The species specificity of the microimmunofluorescence antibody test and comparisons with a time resolved fluoroscopic immunoassay for measuring IgG antibodies against Chlamydia pneumoniae. J Clin Pathol 1999;52:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black CM. Current methods of laboratory diagnosis of Chlamydia trachomatis infections. Clin Microbiol Rev 1997;10:160–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haralambieva I, Iankov I, Petrov D, et al. Cross-reaction between the genus-specific lipopolysaccharide antigen of Chlamydia spp. and the lipopolysaccharides of Porphyromonas gingivalis, Escherichia coli O119 and Salmonella newington: implications for diagnosis. Diagn Microbiol Infect Dis 2001;41:99–106. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz L, Angevine M, Kim S-Y, et al. T-cell epitopes in variable segments of Chlamydia trachomatis major outer membrane protein elicit serovar-specific immune responses in infected humans. Infect Immun 2000;68:1719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mygind P, Christiansen G, Persson K, et al. Detection of Chlamydia trachomatis-specific antibodies in human sera by recombinant major outer-membrane protein polyantigens. J Med Microbiol 2000;49:457–65. [DOI] [PubMed] [Google Scholar]
- 15.Paukku M, Narvanen A, Puolakkainen M, et al. Detection of Chlamydia trachomatis antibodies by 2 novel tests: rELISA and peptide EIA. Int J STD AIDS 1998;9:604–7. [DOI] [PubMed] [Google Scholar]
- 16.Clad A, Kunze FM, Schnoeckel U, et al. Detection of seroconversion and persistence of Chlamydia trachomatis antibodies in five different serological tests. Eur J Clin Microbiol Infect Dis 2000;19:932–7. [DOI] [PubMed] [Google Scholar]
- 17.Puolakkainen M, Vesterinen E, Purola E, et al. Persistence of chlamydial antibodies after pelvic inflammatory disease. J Clin Microbiol 1986;23:924–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway D, Glazener CMA, Caul EO, et al. Chlamydial serology in fertile and infertile women. Lancet 1984;i:191–3. [DOI] [PubMed] [Google Scholar]