Abstract
Aims: Hypoxia inducible factors 1α and 2α (HIF1α and HIF2α) are hypoxia regulated transcriptional factors, which control the expression of a variety of genes responsible for angiogenesis, glycolysis, and the inhibition of apoptosis. Because angiogenesis and tissue regeneration are integral components of the inflammatory process, this study was designed to investigate the role of HIFα molecules in inflammatory bowel disease.
Methods: Surgical specimens from patients with active ulcerative colitis (UC) and Crohn’s disease (CD) were assessed immunohistochemically for HIF1α and HIF2α reactivity, and the expression of these molecules was compared with the expression of the angiogenic factors thymidine phosphorylase (TP), vascular endothelial growth factor (VEGF), and VEGF–KDR activated vasculature. The vascular density of the lesions was also assessed using anti-CD31 immunostaining.
Results: HIF1α was expressed focally (epithelial cells, stromal fibroblasts, and myocytes) in both UC and CD, whereas HIF2α was expressed focally in UC and diffusely in CD. TP expression was uniformly positive in both diseases. VEGF expression was absent in CD, and weakly positive in UC. The VEGF–KDR reactivity of the submucosal vasculature was only slightly increased in UC and CD compared with normal tissue. The inflammatory cells stained with HIF2α and TP in all cases, but the reactivity was generalised in CD and focal in UC. In both diseases, vascular density was significantly higher than that seen in normal tissue.
Conclusions: The discordant expression of HIF2α and VEGF in CD suggests an inherent deficiency of the intestine to respond to various stresses by the induction of VEGF. This finding should be investigated further.
Keywords: hypoxia inducible factor; thymidine phosphorylase; vascular endothelial growth factor; IBD, inflammatory bowel disease; KDR; ulcerative colitis; Crohn’s disease
The term inflammatory bowel disease (IBD) comprises two distinct pathological entities, Crohn’s disease (CD) and ulcerative colitis (UC), both of which are characterised by the presence of a granulomatous-type inflammatory process accompanied by systemic manifestations.1 The disorders are of multifactorial aetiology, implying both hereditary and environmental factors. Because ulceration and regeneration of the intestinal epithelium occurs during the course of the disease, angiogenesis and increased cell metabolism are integral to the pathology of IBD. Indeed, some serological studies have suggested that serum concentrations of vascular endothelial growth factor (VEGF), a potent angiogenic factor, are raised in patients with IBD.2,3
In general, angiogenesis is induced by hypoxia, and regulated by two recently described transcriptional factors, namely the hypoxia inducible factors HIF1 and HIF2.4,5. Both proteins are heterodimers of the 1α and the 2α subunit with the 1α (or arylhydrocarbon nuclear receptor translocator) subunit. Increased concentrations of intracellular HIFα molecules occurs after hypoxia, as a result of reduced degradation by the ubiquitin proteasome pathway.6 Binding of HIF to the hypoxia response element of several “HIF regulated genes” results in the increased transcription of several proteins involved in angiogenesis (such as VEGF), glycolysis (glucose transported proteins), and erythropoiesis.7
“Some serological studies have suggested that serum concentrations of vascular endothelial growth factor, a potent angiogenic factor, are raised in patients with inflammatory bowel disease”
The role of hypoxic stimuli or of HIFα proteins in the pathogenesis of IBD has not been studied to date. In our study, we investigated, immunohistochemically, the patterns of HIFα protein expression in patients with active IBD, who were treated with surgery. In addition, the expression of two main angiogenic factors, namely VEGF and thymidine phosphorylase (TP), was assessed in conjunction with the activation status of the VEGF–kinase insert domain containing receptor (KDR) angiogenic pathway.
MATERIALS AND METHODS
Patients and controls
Paraffin wax embedded tissue from 10 patients with active UC and 10 with CD, treated with surgery, were retrieved from the archives of the department of pathology, Democritus University of Thrace, Alexandroupolis, Greece. Additional surgical material from five apparently normal colonic sections (from patients treated for a non-IBD pathology, usually malignant disease) was also obtained. Tissue sections (3 μm thick) were cut and mounted on to poly-L-lysine coated slides. Table 1 shows the patients and their disease characteristics.
Table 1.
Patients and their disease characteristics
| CD | UC | |
| Number of patients | 10 | 10 |
| Mean age in years (range) | 40 (26–56) | 49 (29–69) |
| Sex | ||
| Male | 7 | 6 |
| Female | 3 | 4 |
| Age at diagnosis | ||
| <40 years | 6 | 4 |
| >40 years | 4 | 6 |
| Activity | ||
| Active | 10 | 10 |
| Inactive | 0 | 0 |
| Medical treatment | ||
| Salicylates | 10 | 10 |
| Corticosteroids | 10 | 10 |
| Azathioprine | 4 | 2 |
| Location | ||
| Terminal ileum | 6 | – |
| Colonic | 1 | – |
| Ileocolonic | 3 | – |
| Pancolitis | – | 10 |
CD, Crohn’s disease; UC, ulcerative colitis.
Monoclonal antibodies
The HIF1α protein was detected using the monoclonal antibodies ESEE122 (IgG1 monoclonal antibody; 1/20 dilution; University of Oxford, UK8) and ab463 (Abcam Limited, Cambridge, UK). The HIF2α protein was identified using the antibody EP190b (IgG1 monoclonal antibody; neat; University of Oxford, UK8).
VEGF expression was assessed with the VG1 monoclonal antibody (University of Oxford, UK9), which recognises the 121, 165, and 189 isoforms of VEGF.
The VEGF–KDR complex on the vessels was assessed with the 11B5 monoclonal antibody, an IgM isotype produced using the VEGF Hu NH2-terminus as an immunogen (University of Texas, USA10). In previous studies we showed that the 11B5 monoclonal antibody reacts with the vasculature of subsets of tumours, whereas no more than 5% of normal vessels are stained, confirming that 11B5 recognises activated vasculature.
The PGF-44c monoclonal antibody (University of Oxford, UK11) was used to assess the immunohistochemical expression of TP (also known as platelet derived endothelial cell growth factor PD-ECGF).
Immunohistochemical technique
Sections were dewaxed and endogenous peroxidase activity was quenched with methanol and 3% H2O2 for 15 minutes. Antigen retrieval was achieved by means of microwave treatment (two treatments for four minutes each) for all antibodies except PGF-44c. The primary antibodies were applied overnight, except for PGF-44c (which was applied for one hour only). After washing with Tris buffered saline (TBS), sections were incubated with a secondary rabbit antimouse antibody (Kwik biotinylated secondary; Shandon-Upshaw, Pittsburgh, Pennsylvania, USA) for 15 minutes and washed in TBS. The Kwik streptavidin peroxidase reagent (Shandon-Upshaw) was applied for 15 minutes and sections were again washed in TBS. The colour was developed by a 15 minute incubation with DAB solution and the sections were weakly counterstained with haematoxylin. Normal rabbit immunoglobulin G was substituted for the primary antibody as the negative control (same concentration as the test antibody). As positive controls we used: (1) a case of gastric cancer with intense cancer cell VEGF expression and dense tumoral vascular VEGF–KDR complex expression; (2) a case of breast cancer with intense nuclear HIF accumulation, and (3) a squamous cell lung carcinoma with diffuse nuclear/cytoplasmic TP reactivity. Tissue sections from normal colon were also stained together with the IBD samples.
Assessment of vessel density
The JC70 monoclonal antibody (Dako, Glostrup, Denmark), which recognises the CD31 pan-endothelial antigen (platelet/endothelial cell adhesion molecule12) was used for vessel staining. We used the alkaline phosphatase/antialkaline phosphatase (Dako) procedure, as described previously.12 Sections were dewaxed, rehydrated, and predigested with protease type XXIV (Sigma Chemical Co, St Louis, Missouri, USA) for 20 minutes at 37°C. JC70 (1/50 dilution) was applied at room temperature for 30 minutes and washed in TBS. Rabbit antimouse antibody 1/50 (vol/vol) (Dako) was applied for 30 minutes, followed by application of the mouse APAAP complex 1/1 (vol/vol) for 30 minutes. After washing in TBS, the last two steps were repeated for 10 minutes each. The colour was developed by incubation with new Fuchsin solution for 20 minutes.
Sections were scanned at low power (×40 and ×100). The vessel density was assessed in all ×200 optical fields within the submucosa area. The final vessel density was the mean score obtained from three fields of the highest individual score.
Statistical analysis
Statistical analysis was performed using the Graph Pad Prism 2.01 (USA) package software. Differences among groups were assessed using the unpaired two tailed t test. Significance was set at p < 0.05.
RESULTS
Normal tissues
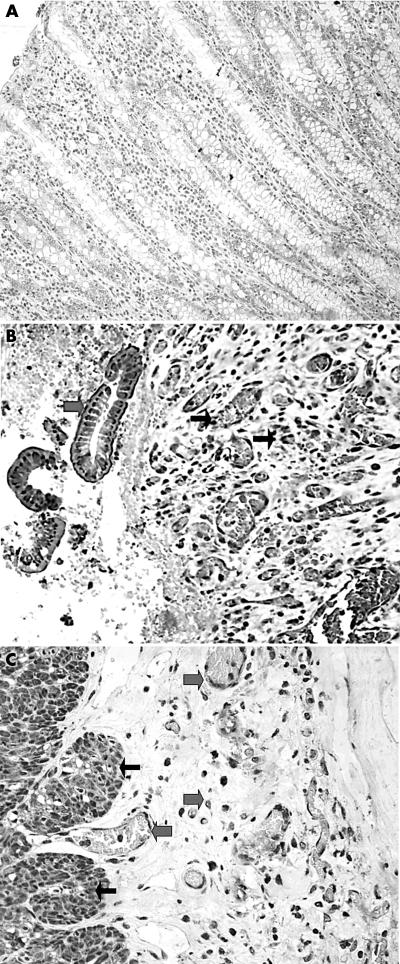
HIF1α, HIF-2α, TP, and VEGF–KDR were consistently unreactive in normal intestinal tissue, and only VEGF showed a weak cytoplasmic positivity in the epithelial cells, both surface and glandular. Figure 1A shows normal intestinal tissue unreactive to HIF2a.
Figure 1.
(A) Normal intestinal mucosa showed no staining for hypoxia inducible factor 2α (HIF2α). (B) Intense and diffuse nuclear/cytoplasmic expression of HIF2α in degenerative epithelium (thick arrows) and the underlining mucosa (vessels and fibroblasts; thin arrows) in Crohn’s disease (CD). (C) Strong and diffuse nuclear/cytoplasmic expression of HIF2α in myocytes (thin arrows), endothelial cells, and serosal stromal cells (thick arrows) in CD. (D) Intense but focal expression of HIF1α (epithelium and fibroblasts; thick arrows) in ulcerative colitis on a background of negative reactivity (epithelium and stroma; thin arrows).
The mean vessel density (SD) was 47 (14) for each ×200 optical field in the normal mucosa and submucosa. VEGF–KDR reactivity was noted in less than 5% of vessels.
Crohn’s disease
HIF-2α and TP was consistently expressed in epithelial cells, stromal fibroblasts, and myocytes throughout the muscle wall (figs 1B,C and 2A). In all cases expression was mixed nuclear/cytoplasmic. HIF1α was expressed focally in the same tissue components (mixed nuclear/cytoplasmic), with the exception of myocytes. HIF1α and HIF2α expression was independent of the presence of necrosis. VEGF was invariably negative in all tissue components. The same pattern of HIF1α expression was obtained for both antibodies used, namely: ESEE122 and Ab463.
The mean vessel density (SD) was 69 (14) for each ×200 optical field in the mucosa and submucosa, which was significantly higher than that seen in normal tissue (p < 0.0001).
VEGF–KDR reactivity was seen focally in no more than 10% of the total submucosal vasculature. Interestingly, vessels involved in the granulomatous process did not express the VEGF–KDR complex. Epithelial and mesenchymal cells were also negative for VEGF–KDR.
Ulcerative colitis
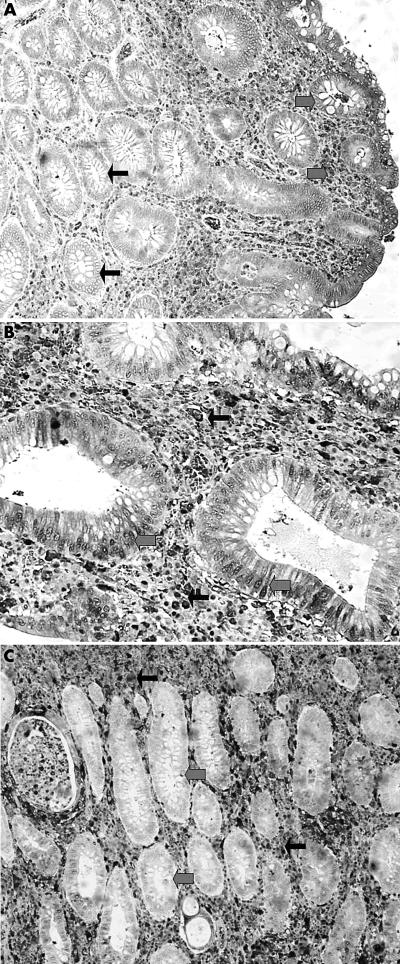
In contrast, UC exhibited focal areas of HIF1α and HIF2α reactivity in epithelial cells, surface and glandular, and in stromal fibroblasts (mixed nuclear/cytoplasmic) (fig 1D). TP was reactive in all mucosal/submucosal fibroblasts, but not in epithelial cells or myocytes (fig 2B). VEGF was weakly reactive in the epithelium (cytoplasmic) in a similar way to that seen in the normal intestine.
Figure 2.
(A) Nuclear/cytoplasmic expression of thymidine phosphorylase (TP) in the intestinal epithelium (thick arrows) and stroma (thin arrows) in Crohn’s disease. (B) Lack of TP expression by epithelial cells (thick arrows) in a case of ulcerative colitis, on a background of strong TP stroma reactivity (thin arrows).
The mean vessel density (SD) was 64 (14) for each ×200 optical field in the mucosa and submucosa, which was similar to that noted in CD (p = 0.68), but significantly higher than that seen in normal tissue (p < 0.0001).
The pattern of VEGF–KDR reactivity in the UC vasculature was similar to that of CD for endothelial, epithelial, and stromal cells.
Inflammatory cells
In both UC and CD, macrophages and lymphocytes were reactive with HIF1α (ESEE122 and ab463 antibodies), HIF2α, TP, and VEGF–KDR (purely cytoplasmic), although staining was generalised in CD and focal in UC.
DISCUSSION
The aetiology and pathogenesis of IBD remains obscure, although consecutive phases of epithelial ulceration and regeneration are known to occur. Neo-angiogenesis is part of the pathology of IBD, as this study confirmed, but it is unclear whether such an angiogenic process is the cause or the consequence of IBD.
Recent studies have indicated that serum VEGF concentrations are increased in patients with IBD,2,3 suggesting that this angiogenic factor is overproduced within the context of an intense angiogenic activity. However, we did not find high amounts of VEGF in our immunohistochemical study, where VEGF expression was absent in patients with CD, and only weakly positive in UC, specifically in the epithelial cells, a finding that was also true in normal intestinal tissues. Furthermore, inflammatory cells (lymphocytes and macrophages), another putative source of VEGF, also failed to react with the VG1 monoclonal antibody (anti-VEGF), and only focally reacted with the 11B5 monoclonal antibody (anti-VEGF–KDR complex). VEGF–KDR activity in IBD may reflect the upregulation of KDR receptor expression rather than VEGF, because the affinity of 11B5 for VEGF is low.10 In agreement with our findings, Kanazawa et al, investigating IBD tissues, found that VEGF reactivity was present in endothelial cells but not in epithelial or inflammatory cells.2
“Angiogenesis is probably not a vascular endothelial growth factor dependent process in inflammatory bowel disease”
The lack of VEGF staining in our material seems to contrast with the results of Griga et al.13 These investigators found that the spontaneous production of VEGF was higher in cultures produced from patients with IBD than in those derived from patients with irritable bowel syndrome. The degree of VEGF production, either stimulated or unstimulated, did not differ between cultures derived from apparently normal mucosa of patients with IBD and cultures derived from controls. However, the high standard error and range of VEGF measurements obtained in their study, together with the limited number of patients recruited, do not allow reliable conclusions to be made. In addition, the mechanism that suppresses VEGF expression in patients with IBD in vivo may be absent in ex vivo situations, which could explain the findings of Griga et al.
Our results with regard to the VEGF–KDR activation status of the neovasculature are very interesting. Indeed, although the vessel density was significantly higher in UC and CD than in normal mucosa/submucosa, the activated VEGF–KDR reactivity was only slightly increased (10% in UC and CD v 5% in normal vasculature) and, certainly, not within areas of intense inflammation. This low VEGF–KDR activation status indicates that angiogenesis is probably not a VEGF dependent process in IBD. In contrast, the consistent expression of TP in the stromal fibroblasts of both CD and UC and, to a lesser extent, its detection in epithelial cells, the vascular endothelium, and the inflammatory component, strongly suggests that TP is an important member of the molecular cascade stimulating angiogenesis in these diseases. The increased amounts of 2-deoxyribose-1-phosphate, released from epithelial and stromal cells with high TP activity, provide a potent chemotactic stimulus for endothelial cells14 and, at the same time, exert a strong oxidative stress on surrounding cells15; this may account for the increased angiogenesis and the upregulation of HIF found in all cellular components of the intestine in patients with IBD.
This discrepancy between immunohistochemical and serological findings raises the question of whether serum VEGF values are informative. Indeed, platelets are the major source of VEGF in the human body,16,17 and during ex vivo platelet aggregation VEGF is released into the supernatant.18 Therefore, serum VEGF concentrations do not reflect VEGF production by the intestinal epithelium or the related inflammation. Plasma concentrations of VEGF are several times lower than those obtained from the sera of patients.19 Because platelet counts are high, especially in active IBD,20 it is possible that serum VEGF concentrations reflect VEGF of platelet origin rather than that produced by the intestine. In a recently submitted study of ours, not only were plasma concentrations of VEGF not increased in patients with IBD, but they were also reduced in patients with active CD (Koukourakis et al, 2002, unpublished data).
The observed upregulation of HIFα molecules in IBD raises two main questions regarding (1) the cause, and (2) the pathological relevance of this event. Certainly, HIFα molecules are hypoxia regulated proteins. However, in the case of quiescent IBD, the intestinal mucosa seems histologically similar to normal tissue and the high degree of vascularisation of the mucosa in active disease does not support an obvious causative link between HIF upregulation and protracted hypoxia of vascular origin. Indeed, HIF expression was not related to the presence of necrosis. Although vasoconstriction or microthrombosis of the intestinal vessels (a theory supported by the high platelet counts, the prevalence of activated platelets, and the increased thrombopoietin values seen in patients with IBD2,20,21) may cause focal hypoxia and HIF upregulation, other mechanisms may also contribute towards this observation. HIFα proteins are also induced by cytokines, released by the inflammatory cell component.22 The upregulation of TP expression by stromal fibroblasts may also occur as a consequence of cytokine stimulation,23 and the oxidative stress produced by 2-deoxyribose may directly upregulate the expression of HIFα molecules or even contribute to the release of cytokines from fibroblasts, which further induce the expression of HIFα molecules. A strong association between TP expression and the accumulation of HIF2α has been also confirmed in previous studies of ours in various human carcinomas.24,25
The pathological relevance of HIFα overexpression in IBD should be examined in relation to the lack of VEGF reactivity observed. Both HIF1α and HIF2α are potent inducers of VEGF gene expression.26,27 The rather focal expression of HIF1α in the intestinal mucosal and submucosal cells is compatible with the lack of VEGF upregulation. The diffuse expression of HIF2α by all cellular components in CD, including the muscular layer and serosa, conforms with an intensively activated HIF2α pathway, which nonetheless fails to induce VEGF. Therefore, the eventual disruption of the HIF2α–VEGF pathway is probably part of the pathogenesis of CD. Whether upregulation of the recently discovered HIF inhibitory protein (IPAS), or the decreased individual ability to express VEGF as a result of a specific VEGF gene polymorphism, accounts for this event remains to be investigated.28,29 Unlike CD, overexpression of HIF2α in UC affects mainly the inflammatory component and not the intestinal cell population. This HIF2α prevalence in CD may reflect the increased severity of the inflammatory process, which extends throughout the intestinal wall and is not necessarily confined to the surface.
“The reduced ability of a tissue to produce vascular endothelial growth factor (VEGF) or to respond to VEGF upregulation may result in reduced endothelial and epithelial cell viability”
Alternatively, upregulation of HIF2α in CD may represent the trend of the intestinal tissues to induce the production of VEGF, which, apart from being angiogenic, is a major survival factor inhibiting the apoptosis of both endothelial and epithelial cells.30,31 Constant, although low, amounts of VEGF production may be essential to maintain tissue integrity by regulating the delicate balance between proliferation and apoptosis. This has been shown in studies on lung embryogenesis and physiology.32,33 After an insult (inflammatory, chemical, or physical), VEGF upregulation may be required to facilitate tissue regeneration and repair. Certainly, this occurs during oesophageal mucosa healing, following radiation damage.34 Therefore, the reduced ability of a tissue to produce VEGF or to respond to VEGF upregulation may result in reduced endothelial and epithelial cell viability. The discordance between the expression of HIF2α and VEGF found in our study, predominantly concerning CD, suggests an inherent deficiency of the intestine to respond to various stresses by inducing VEGF. The precise mechanism of the disrupted HIF–VEGF intercommunication in IBD pathology requires further investigation.
Take home messages
Hypoxia inducible factor 1α (HIF1α) was expressed focally in both ulcerative colitis (UC) and Crohn’s disease (CD), whereas HIF2α was expressed focally in UC and diffusely in CD
Thymidine phosphorylase (TP) was expressed uniformly in both UC and CD
Vascular endothelial growth factor (VEGF) expression was absent in CD, and weakly positive in UC
The discordant expression of HIF2α and VEGF in CD suggests an inherent deficiency of the intestine to respond to various stresses by the induction of VEGF
The precise mechanism of the disrupted HIF–VEGF intercommunication in CD and UC requires further investigation
Acknowledgments
The study was financially supported by the Tumour and Angiogenesis Research Group and Cancer Research UK.
Abbreviations
APAAP, alkaline phosphatase/antialkaline phosphatase
CD, Crohn’s disease
HIF, hypoxia inducible factor
KDR, kinase insert domain containing receptor
TBS, Tris buffered saline
TP, thymidine phosphorylase
UC, ulcerative colitis
VEGF, vascular endothelial growth factor
REFERENCES
- 1.Maini RN. Autoimmunity in rheumatoid arthritis. An approach via a study of B lymphocytes. Rheum Dis Clin North Am 1987;13:319. [PubMed] [Google Scholar]
- 2.Kanazawa S, Tsunoda T, Onuma E, et al. VEGF, basic-FGF, and TGF-beta in Crohn’s disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am J Gastroenterol 2001;96:822–8. [DOI] [PubMed] [Google Scholar]
- 3.Bousvaros A, Leichtner A, Zurakowski D, et al. Elevated serum vascular endothelial growth factor in children and young adults with Crohn’s disease. Dig Dis Sci 1999;44:424–30. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 1992;12:5447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiesener MS, Turley H, Allen WE, et al. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1α. Blood 1998;92:2260–8. [PubMed] [Google Scholar]
- 6.Huang LE, Gu J, Scheau M, et al. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitine–proteasome pathway. Proc Natl Acad Sci U S A 1998;95:7987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 1998;8:588–94. [DOI] [PubMed] [Google Scholar]
- 8.Talks KL, Turley H, Gatter KC, et al. The expression and distribution of the hypoxia inducible factors HIF-1α and HIF-2α in normal human tissues, cancers and tumor associated macrophages. Am J Pathol 2000;157:2411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Scott P, Turley H, et al. Validation of anti-vascular endothelial growth factor (anti-VEGF) antibodies for immunohistochemical localization of VEGF in tissue sections: expression of VEGF in the human endometrium. J Pathol 1998;185:402–8. [DOI] [PubMed] [Google Scholar]
- 10.Brekken RA, Huang X, King SW, et al. Vascular endothelial growth factor as a marker of tumor endothelium. Cancer Res 1998;58:1952–9. [PubMed] [Google Scholar]
- 11.Fox SB, Moghaddam A, Westwood M, et al. Platelet derived endothelial cell growth factor/thymidine phosphorylase expression in normal tissues an immunohistochemical study. J Pathol 1995;176:183–90. [DOI] [PubMed] [Google Scholar]
- 12.Parums DV, Cordell JL, Micklem K, et al. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol 1990;43:752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griga T, Voigt E, Gretzer B, et al. Increased production of vascular endothelial growth factor by intestinal mucosa of patients with inflammatory bowel disease. Hepatogastroenterology 1999;46:920–3. [PubMed] [Google Scholar]
- 14.Brown NS, Bicknell R. Thymidine phosphorylase, 2-deoxy-D-ribose and angiogenesis. Biochem J 1998;334:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown NS, Jones A, Fujiyama C, et al. Thymidine phosphorylase induces carcinoma cell oxidative stress and promotes secretion of angiogenic factors. Cancer Res 2002;60:6298–302. [PubMed] [Google Scholar]
- 16.Gunsilius E, Petzer A, Stockhammer G, et al. Thrombocytes are the major source for soluble vascular endothelial growth factor in peripheral blood. Oncology 2000;58:169–74. [DOI] [PubMed] [Google Scholar]
- 17.Verheul HM, Hoekman K, Luykx-de Bakker S, et al. Platelet transporter of vascular endothelial growth factor. Clin Cancer Res 1997;3:2187–90. [PubMed] [Google Scholar]
- 18.Maloney JP, Silliman CC, Ambruso DR, et al. In vitro release of vascular endothelial growth factor during platelet aggregation. Am J Physiol 1998;275:1054–61. [DOI] [PubMed] [Google Scholar]
- 19.Webb NJ, Bottomley MJ, Watson CJ, et al. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci (Colch) 1998;94:395–404. [DOI] [PubMed] [Google Scholar]
- 20.Kapsoritakis AN, Koukourakis MI, Sfiridaki A, et al. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol 2001;96:776–81. [DOI] [PubMed] [Google Scholar]
- 21.Kapsoritakis AN, Potamianos SP, Sfiridaki AI, et al. Elevated thrombopoietin serum levels in patients with inflammatory bowel disease. Am J Gastroenterol 2000;95:3478–81. [DOI] [PubMed] [Google Scholar]
- 22.Thornton RD, Lane P, Borghaei RC, et al. Interleukin 1 induces hypoxia-inducible factor 1 in human gingival and synovial fibroblasts. Biochem J 2000;1:307–12. [PMC free article] [PubMed] [Google Scholar]
- 23.Braybrooke JP, Propper DJ, O’Byrne KJ, et al. Induction of thymidine phosphorylase as a pharmacodynamic end point in patients with advanced carcinoma treated with 5-fluorouracil, folinic acid and interferon-alpha. Br J Cancer 2000;83:219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 2001;85:881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivridis E, Giatromanolaki A, Gatter KC, et al. Hypoxia inducible factor (1-alpha and 2-alpha) expression in endometrial cancer relates with angiogenesis and prognosis. Cancer 2002;95:1055–63.12209691 [Google Scholar]
- 26.Ema M, Taya S, Yokotani N, et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A 1997;94:4273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996;16:4604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makino Y, Cao R, Svensson K, et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 2001;414:550–4. [DOI] [PubMed] [Google Scholar]
- 29.Watson CJ, Webb NJ, Bottomley MJ, et al. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein production. Cytokine 2000;12:1232–5. [DOI] [PubMed] [Google Scholar]
- 30.Abu-Ghazaleh R, Kabir J, Jia H, et al. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J 2001;360:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachelder RE, Crago A, Chung J, et al. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res 2001;61:5736–40. [PubMed] [Google Scholar]
- 32.Brown KR, England KM, Goss KL, et al. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol 2001;281:1001–10. [DOI] [PubMed] [Google Scholar]
- 33.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koukourakis MI, Flordellis CS, Giatromanolaki A, et al. Oral administration of recombinant human granulocyte–macrophage colony stimulating factor (rhGM-CSF) in the management of radiotherapy induced esophagitis. Clin Cancer Res 1999;5:3970–6. [PubMed] [Google Scholar]