Abstract
Among the family of steroidal molecules, only estrogens have the capability of preventing neuronal cell death caused by increased oxidative burden. Employing neuronal cell lines, brain membrane, and low density lipoprotein oxidation assays, we show that the antioxidant and neuroprotective effects of estrogens are dependent not on their genomic properties as hormones but rather on their basic chemical properties as hydrophobic phenolic molecules. Concentrations of 17β-estradiol of 0.1–500 nM, which confer maximum estrogen receptor-dependent gene transcription in vitro as well as maximum estrogen receptor binding, respectively, do not show antioxidant or neuroprotective effects. In contrast, phenolic compounds such as 2,4,6-trimethylphenol, N-acetylserotonin, and 5-hydroxyindole exhibit neuroprotective effects without any estrogenicity. Comparing various natural and synthetic mono- and polyphenolic compounds, no correlation between their antioxidant cytoprotective effect and their estrogenic potency can be seen. These results call into question the idea of a general correlation between the intended pharmacological effects of estrogens and phenolic compounds and their effect on estrogen receptor-dependent pathways. Furthermore, they may open the door toward the rational design of neuroprotective antioxidants with decreased hormonal side effects.
The number of biological systems that are known to be affected by the steroid estrogen is increasing persistently. Apart from its classical function as a sex steroid (1, 2), estrogen modulates transmembrane receptor function (3, 4), affects intracellular signal transduction cascades (5–8), and shows a variety of other actions (9, 10). Recently, special interest has been attracted to estrogen’s properties as a neurotrophic and neuroprotective effector (11, 12).
There are two lines of argument that assign to estrogen a special function in relation to neurological and neurodegenerative disorders. First, a number of epidemiological and clinical data exist on the beneficial effects of estrogens, for instance, in Alzheimer’s disease (13, 14), in Parkinson’s disease (15), or on mental performance in general (16, 17). Second, estrogen has been shown to have beneficial effects in cellular and molecular systems relevant to neurodegenerative disorders (4, 12, 18–24).
Apart from the steroidal estrogens, it has been long known (25, 26) that a large variety of exogenous compounds, the xenoestrogens and the phytoestrogens, mimic the actions of endogenous estrogen to different extents. Xenoestrogens (27) comprise plastic-material monomers [e.g., bisphenol A (28)], certain polymer plasticizers (29), and detergent-related chemicals (30, 31) as well as special pharmacological molecules such as diethylstilbestrol (32, 33). Major phytoestrogens (34, 35) are the flavonoids, such as quercetin and catechin, and the stilbenes, such as resveratrol.
Besides steroidal estrogen, a number of these environmental estrogens also have been shown to exhibit antioxidative properties (36, 37) or have been suggested to exert beneficial pharmacological effects on neurological disorders on the basis of in vitro observations (38, 39).
The idea of a novel, nonhormonal neuroprotective function of estrogen had arisen because (i) different steroidal estrogens showed comparable neuroprotection (18); (ii) the protective effect could be observed in cells in which 17β-estradiol did not activate estrogen-responsive element (ERE)-directed transcription (40); and (iii) the antiestrogen tamoxifen apparently did not interfere with the neuroprotective effect (41).
To delineate the mechanisms of action of estrogen in experimental systems of oxidative neuronal cell death, we have investigated the neuroprotective effects exhibited by a variety of estrogens from different sources. To this purpose, we have employed mouse hippocampal HT22 cells and human SK-N-MC neuroblastoma cells, two well established systems for the study of oxidative neuronal cell death (18, 22, 42, 43). In addition, we have used two experimental paradigms quantifying estrogenicity in a classical sense [estrogen receptor (ER)-binding activity and induction of ERE-directed gene transcription] and two experimental systems measuring antioxidative activity relevant to the nervous system [brain lipid peroxidation and low density lipoprotein (LDL) oxidation].
MATERIALS AND METHODS
Chemicals.
All chemicals were purchased from Sigma unless otherwise indicated. Phenolic compounds to be tested were ordered in the highest grade available and were assayed for purity by analytical TLC. Compounds presumed to be subject to oxidation by air were checked for contaminating quinones by UV/visible spectroscopy. Where appropriate, compounds were recrystallized from ethanol/water in degassed solvents. Stock solutions of the phenolic chemicals were prepared in ethanol and stored at −20°C. 17β-[2,3,6,7-3H]Estradiol (radiochemical purity, 97.6%; 3.1 TBq/mmol) was from Amersham. Luciferin was from Boehringer Mannheim. ICI 182780 was purchased from Tocris Neuramin (Bristol, U.K.).
Cell Culture.
Media, sera, and supplements were from GIBCO. Glutamate-sensitive murine hippocampal neurons (HT22) were a kind gift from P. Maher (The Scripps Research Institute, La Jolla, CA). Human neuroblastoma cells (SK-N-MC) and human breast carcinoma cells (MCF7) were from the American Type Culture Collection (Manassas, VA). All cell lines were grown in DMEM supplemented with 10% FCS under standard cell culture conditions.
Cell Viability Assays.
Cellular viability was quantified with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide tests measuring metabolic activity as described (44, 45). The colorimetric tests generally were accompanied by microscopic examination and ionic dye exclusion assays using trypan blue and propidium iodide as indicators of intact cellular membrane structure (18). No significant discrepancies between the different methods to determine cell viability could be observed. The phenolic compounds were nontoxic to the cells at the concentrations used.
ER-Controlled Transcriptional Activation.
ER-controlled gene transcription was measured by transient transfection of MCF7 cells with a luciferase reporter plasmid (MTV-ERE-LUC; courtesy of P. Chambon, College de France, Strasbourg), bearing an ERE, essentially as described (40). The cells were transfected by using 50-kDa polyethylenimine (46). Results were corrected for background luminescence and compared with noninduced control cell expression.
ER Binding.
Rat uterus cytosol was prepared from cycle-matched (day 4) female Sprague–Dawley rats. The uteri were washed with PBS at 4°C and were thoroughly dissected. After centrifugation (4 min at 200 × g) the tissue fragments were suspended in 20 vol of hypotonic TEDG buffer (10 mM Tris, pH 7.5/1 mM EDTA/1 mM DTT/10% glycerol) containing 1 mM PMSF, 20 μg/ml leupeptin, and 20 μg/ml trypsin inhibitor. The tissue fragments were homogenized repeatedly with a Kontes glass homogenizer during 2 h of incubation at 4°C. The homogenate was centrifuged twice (4 min at 1,000 × g, 2 h at 100,000 × g), and the supernatant was stored at −80°C. Western blotting analysis indicated that the uterus preparation was rich in both splice variants of ERα but apparently lacked ERβ, in accordance with observations in mice (47).
The binding assays were performed by coincubation of 200 μg of protein-containing rat uterus extract with 10 nM 17β-[2,3,6,7-3H]estradiol and the respective nonlabeled competitors (final ethanol concentration, 1%) in 100 μl of incubation buffer (10 mM Tris, pH 7.5/2 mM EDTA/1 mM MgCl2/10 mM KCl/1 mM DTT/10 mM Na2MoO4/5% glycerol supplemented with 1 mM PMSF, 20 μg/ml leupeptin, and 20 μg/ml trypsin inhibitor) for 20 h at 4°C. Bound activity was separated from the free radioligand by chromatography on 2.5-ml columns of Sephadex LH 20 (Pharmacia) suspended in incubation buffer. Protein-bound activity was collected to an elution volume of 1.1 ml. Results were calculated after correction for nonspecific binding.
Rat Brain Membrane Oxidation.
Dissected cerebral cortex of adult Sprague—Dawley rats was homogenized in 3 vol of degassed nonreducing lipid buffer (20 mM Tris, pH 7.4/1 mM MgCl2/5 mM KCl) with a Kontes glass homogenizer (all steps were performed at 4°C with degassed liquids). After centrifugation (3,000 × g, 5 min) the pellet was solubilized in lipid buffer supplemented with 0.5 M NaCl by sonication, incubated for 10 min, and centrifuged again (100,000 × g, 20 min). This step was repeated and followed by three analogous washings (without incubation) using water instead of lipid buffer. The pellet was resuspended in water at a concentration of 5 mg/ml protein and frozen at −80°C.
Low-level chemiluminescence occurring during the course of lipid peroxidation was measured as described (48). The rat brain membrane preparation was diluted with PBS to a concentration of 0.6 mg/ml protein and sonicated. Phenolic compounds (final ethanol concentration, 0.4%) were added to the 1-ml aliquots, starting the oxidative chain reaction by the addition of 50 μM ascorbate and transfer to 37°C. Six hours later, single photon counting was done for 1 min (Beckman scintillation counter) after decay of static electricity. Data were corrected for the baseline photocurrent and normalized to control values.
LDL Oxidation.
Fresh human blood plasma LDL was purchased from Sigma. The quality of the lots was tested by measuring their endogenous resistance to oxidation; only lots resistant for at least 20 min at 37°C were used (see below). The copper-catalyzed oxidation of LDL was measured essentially as described (49); the 4°C preparations of LDL were matched to a concentration of 100 μg/ml protein in PBS with 0.5 mM MgCl2. Oxidation was initiated by the addition of 10 μM CuSO4 at 37°C. As indicators of LDL peroxidation, conjugated dienes were measured at 234 nm. Phenolic compounds were added as 100-fold ethanolic solutions, and the increase in absorption after 1 h was plotted vs. concentration. Noninterference of the phenolic compounds with the photometric assay was ensured by quantification (λmax and Amax) of the aromatic longest-wavelength absorption band (π → π*) during the peroxidation process; no change was observed.
Statistics.
Student’s t tests were performed to quantify statistical significance.
RESULTS
Various Phenolic Compounds Exhibit Similar Neuroprotective Properties.
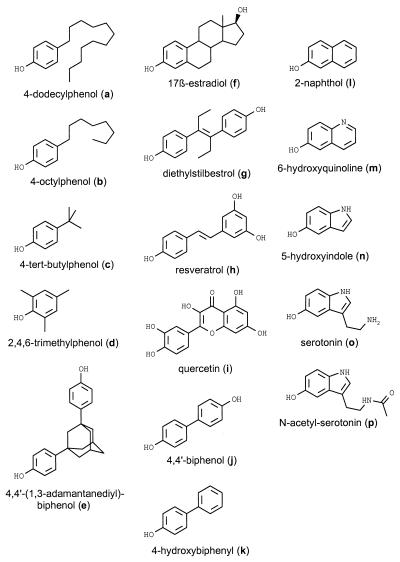
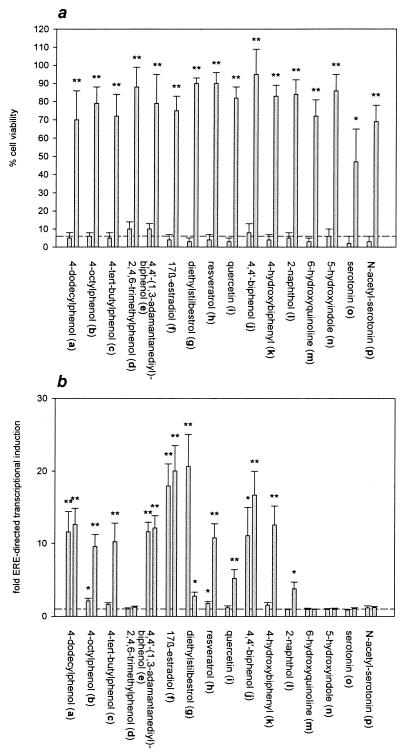
Fig. 1 shows the structures of the phenolic compounds used in this study. All the compounds are effective protectants against oxidative glutamate toxicity in mouse neuronal HT22 cells when employed at a concentration of 20 μM (Fig. 2a). But none of the phenolic compounds, including 17β-estradiol (f), is effective at a concentration of 1 μM. The dose–response curves of the neuroprotective effect appear highly cooperative in the HT22 cellular system as exemplified for selected compounds in Fig. 3c. The compounds’ protective effect on the survival of human neuronal SK-N-MC cells exposed to toxic doses of hydrogen peroxide (H2O2), a mediator of various neurotoxins (50), is completely analogous (not shown).
Figure 1.
Chemical structures of the phenolic compounds.
Figure 2.
(a) Effect of the phenolic compounds on glutamate-treated HT22 cells. For each compound, the left column indicates the relative viability of the cells after treatment with 1 μM of the respective phenolic compound 3 h before the addition of 3 mM glutamate overnight. The right column indicates the incubation with 20 μM of the respective compound given 3 h before glutamate. A quadruplicate determination is shown. The dashed line indicates the viability of nonpretreated cells. (b) Effect of the phenolic compounds on ERE-directed transcriptional activation. Analogously, for each compound, the left column indicates a concentration of 1 μM, and the right column indicates 20 μM. The induction of expression of a luciferase reporter gene in MCF7 cells after transfection and subsequent treatment with the phenolic compounds for 8 h is shown as multiple of the control value (dashed line). Triplicate determinations are shown. Asterisks indicate significant differences of the results vs. the untreated controls (dashed lines): ∗, P < 0.05; ∗∗, P < 0.01.
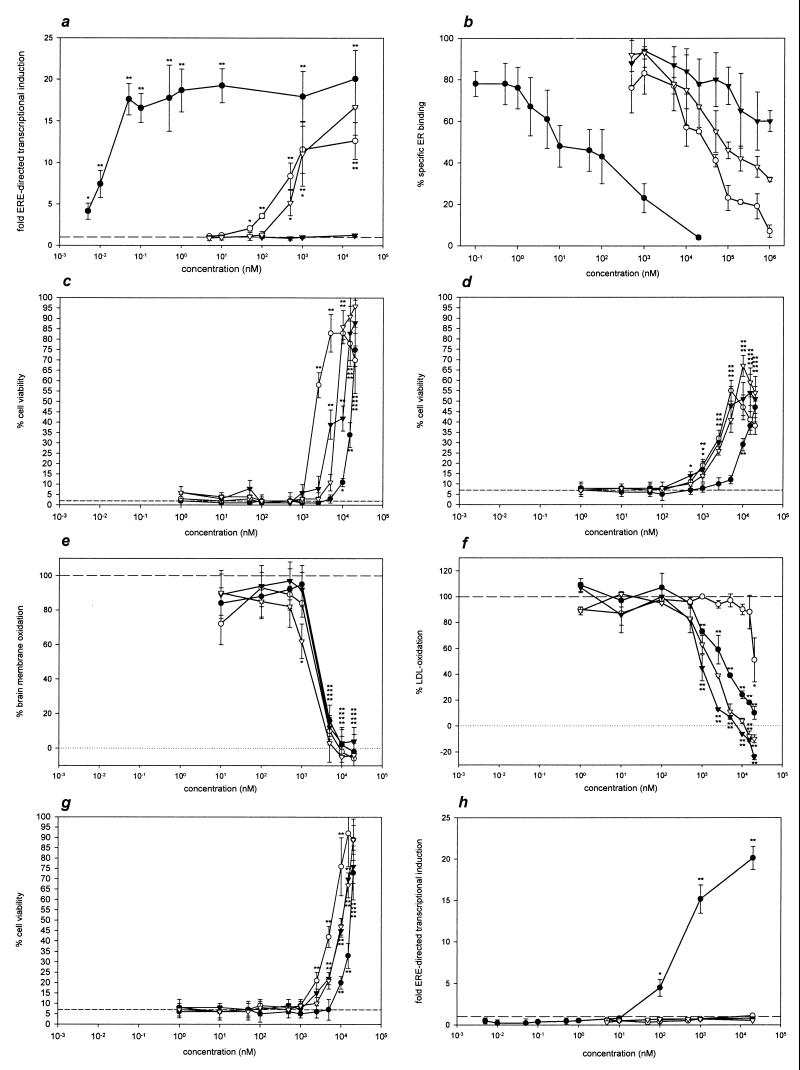
Figure 3.
Dose–response analysis of 17β-estradiol (●), 4-dodecylphenol (○), 4,4′-biphenol (▿), and 2,4,6-trimethylphenol (▾). (a) ERE-directed transcriptional activation in MCF7 cells performed as in Fig. 2b. (b) ER-binding assays using rat uterus cytosol and 10 nM 17β-[2,3,6,7-3H]estradiol as specific radioligand. The relative amount of bound radioactivity is shown, with the indicated concentrations of the nonlabeled phenolic compounds as competitors for ER binding. Triplicate determinations are shown. (c) Cell viabilities of HT22 cells after 3 mM glutamate treatment. The indicated concentrations of the phenolic compounds were added concomitantly with the oxidative stressor. Quadruplicate determinations are depicted. The dashed line indicates the viability of solely glutamate-treated cells. (d) Viabilities of human SK-N-MC neuroblastoma cells after treatment with 160 μM H2O2. Phenolic compounds were added 3 h before the toxin. The experiments were done in quadruplicate. (e) Rat brain membrane oxidation as measured by low-level chemiluminescence. Six hours after addition of the phenolic compounds, light emission was quantified by single photon counting. Duplicate determinations, normalized to control values, are shown. (f) Cu2+-catalyzed oxidation of human LDL after 1 h at 37°C with or without phenolic compounds. Conjugated diene formation was monitored photometrically at 234 nm; triplicate determinations relative to the control values are depicted. (g) Viabilities of glutamate-treated HT22 cells as in c, here with 1 μM of the antiestrogen ICI 182780 added concomitantly with the phenolic compounds. (h) ERE-directed transcriptional activation as in a, with 1 μM of the antiestrogen ICI 182780 added concomitantly with the phenolic compounds. Statistical significance of the results vs. the respective controls (dashed lines) is indicated by asterisks: ∗, P < 0.05; ∗∗, P < 0.01.
Neuroprotective Phenolic Compounds Differ in Their Induction of ER-Dependent Gene Transcription.
Fig. 2b displays the effect of the phenolic compounds on ERE-directed gene transcription as determined by transient transfection of human breast cancer MCF7 cells with a luciferase reporter plasmid bearing an inserted ERE. The compounds’ behavior in this experimental system is highly divergent: 2,4,6-trimethylphenol (d), serotonin (o), and N-acetylserotonin (p), for example, do not induce transcription at a concentration of 1 μM or 20 μM. 4-tert-Butylphenol (c), resveratrol (h), and 4-hydroxybiphenyl (k) induce transcription only at a concentration of 20 μM, and 4-dodecylphenol (a), 4,4′-(1,3-adamantanediyl)-biphenol (e), and 17β-estradiol (f) are inducers at concentrations of 1 and 20 μM. Diethylstilbestrol (g) appears to have a decreased net agonistic effect on transcription at a concentration of 20 μM compared with 1 μM.
ER Binding of Phenolic Compounds Corresponds to Their Induction of ERE-Directed Gene Transcription.
To assign the above data to molecular effects, we have analyzed four compounds in detail [4-dodecylphenol (a), 2,4,6-trimethylphenol (d), 17β-estradiol (f), and 4,4′-biphenol (j)]. For these compounds, Fig. 3a displays the amount of ERE-directed gene transcription as a function of concentration. 17β-Estradiol is, by far, the most potent effector, followed by 4-dodecylphenol and 4,4′-biphenol, which are at least five orders of magnitude less effective. 2,4,6-Trimethylphenol exhibits no inducing effect on ERE-directed transcription.
Fig. 3b shows the results of ER-binding assays performed with a rat uterus cytosol preparation employing 10 nM 17β-[2,3,6,7-3H]estradiol as specific radioligand. Nonlabeled 17β-estradiol competes for the radioligand in an approximate concentration ratio of 1:1 as expected. 4-Dodecylphenol and 4,4′-biphenol are more than three orders of magnitude less effective in competing with the radioligand for ER binding. 2,4,6-Trimethylphenol in 105-fold excess is able to substitute for only about 30% of the labeled 17β-estradiol and, therefore, is the least potent competitor.
The Neuroprotective Activity of Phenolic Compounds Against Oxidative Stress Corresponds to Their Direct Antioxidant Activity.
The concentration-dependent neuroprotective activities of the selected phenolic compounds against glutamate-induced oxidative HT22 cell death are shown in Fig. 3c, and the protective effects against H2O2 toxicity in SK-N-MC cells are presented in Fig. 3d. All compounds exhibit similar protective properties against oxidative glutamate toxicity as well as H2O2 toxicity, with half-maximal effective concentrations ranging from 3 to 18 μM.
Apart from the different maximum viabilities, there is no significant difference between the results of the two experimental cellular systems. None of the compounds shows any significant protective effect at a concentration of 500 nM or less in either system. A kinetic analysis of the protective effect reveals that the phenolic compounds act immediately and have a protective effect even when added concomitantly with the oxidative stressors (not shown).
Fig. 3e shows the peroxidation of cell-free rat brain membranes induced by low concentrations of ascorbate. In this system, measuring low-level chemiluminescence as an indicator of actual peroxidation processes (48), all of the four compounds exhibit a very similar antioxidant activity with nearly identical half-maximal effective concentrations of about 3 μM.
Another biologically relevant system to measure antioxidant activity is LDL oxidation (49). Again, concentrations of the four compounds of 500 nM or less are ineffective in preventing the oxidative modification of LDL (Fig. 3f). The half-maximal effective concentration for the best antioxidant in this experimental paradigm, 2,4,6-trimethylphenol, is 1.5 μM. 4,4′-Biphenol and 17β-estradiol are slightly less effective, and 4-dodecylphenol is the least effective LDL antioxidant.
The Antiestrogen ICI 182780 Does Not Decrease the Antioxidant Neuroprotective Effect of Phenolic Compounds.
Fig. 3g shows the neuroprotective effect of the compounds on HT22 cells when added concomitantly with 1 μM ICI 182780. There is no difference from the results without antiestrogen (Fig. 3c). ICI 182780, an exclusive antiestrogen (51) that is not protective for HT22 cells by itself (not shown), nevertheless drastically decreases ER-driven gene transcription. In Fig. 3h, the MCF7 transcriptional induction assay is performed with 1 μM ICI 182780 being added to the cells concomitantly with the phenolic compounds. Apart from 17β-estradiol at concentrations of 100 nM or higher, there is no longer any induction of ER-controlled transcription by the phenolic compounds.
DISCUSSION
A pathogenetic role of oxidative stress has been described for various nonneuronal and neuronal disorders (52–54). Many approaches toward neuroprotection against neurodegenerative events currently are focusing on antioxidative defense systems and antioxidants. These efforts have been fueled further by a recent first success of a multicenter trial in moderately severe Alzheimer’s disease patients, using vitamin E as an antioxidant (55). Therefore, the aim of our study was to elucidate the antioxidant neuroprotective mechanism of estrogen and to identify the role of ER-mediated effects in neuroprotection.
Comparing the chemical structures of the molecules shown in Fig. 1 with their protective characteristics against oxidative glutamate toxicity in HT22 cells shown in Fig. 2a, it is remarkable that (i) all these molecules are protective against glutamate and (ii) they all behave very similarly with respect to the concentration needed to afford the protective effect. Neither the number of phenolic hydroxyl groups nor the nature of the aromatic moiety seems to play an important role. A neuroprotective antioxidant effect in HT22 cells thereby is shared by the sex hormone estrogen (f), the neurotransmitter serotonin (o), the pineal gland hormone precursor N-acetylserotonin (p), the red wine phenol resveratrol (h), the Gingko biloba flavonoid quercetin (i), and also simple alkylphenols such as 4-dodecylphenol (a).
In stark contrast to the compounds’ neuroprotective activity is their potential to activate ER-driven gene transcription as determined by employing human breast carcinoma MCF7 cells. The two profiles of the panel of phenolic compounds (Fig. 2 a and b) are clearly divergent, and there are different neuroprotective compounds that show no ER-controlled transcriptional activation at all. Whereas 17β-estradiol shows a half-maximal rate of ER-dependent induction of transcription at a concentration of approximately 20 pM, any significant cytoprotective (Fig. 3 c and d) or biochemically antioxidant (Fig. 3 e and f) effects of 17β-estradiol require concentrations that are more than five orders of magnitude higher. In contrast, the neuroprotective properties of estrogen and its effects in cell-free assays of antioxidant activity show almost identical dose-response curves.
The neuroprotective activities of 17β-estradiol and the other phenolic compounds are not diminished by the concomitant administration of high doses of antiestrogens. With 1 μM ICI 182780, 17β-estradiol’s half-maximal transcriptional effect is shifted more than four orders of magnitude toward higher concentrations, and all the other phenolic compounds do not retain any transcriptional effect at all (Fig. 3 h vs. a). Nevertheless, their neuroprotective activities remain completely unaffected (Fig. 3 g vs. c).
The exact nature of the experimental system used for determining the neuroprotective or antioxidant potential of the phenolic compounds including estrogen did not influence the EC50 values found, e.g., disturbance of intracellular antioxidant metabolism in HT22 cells, exogenous reactive oxygen species overload in SK-N-MC neuroblastoma cells, on-line quantification of oxidation reactions in brain membranes, and monitoring of peroxidation end products in lipoproteins (Fig. 3 c–f, respectively). Therefore, this stepwise transition from a biologically relevant system (HT22) (56) toward a biochemically clearly defined system (LDL) allows us to hypothesize that the similar EC50 values of 17β-estradiol indeed may result from a similar mechanism of action in all of these systems.
The structural requirements for antioxidative neuroprotection appear to be low compared with the prerequisites of an estrogenic effect. Especially, there is no necessity for neuroprotective molecules to have more than one cyclic structure of the original 17β-estradiol molecule retained, which is in contrast to the proposition of Green et al. (41). This can be exemplified by the pronounced neuroprotective effect of 4-dodecylphenol (a), with an EC50 value of about 2 μM in both assays of cellular neuroprotection, thereby exceeding 17β-estradiol’s protective potency.
Compounds that are to be considered candidates for neuroprotective antioxidants in vivo should be able to cross the blood–brain barrier (BBB) readily. Estrogen is actively sequestered by the brain (57), and it can be estimated from their physicochemical properties that the lipophilic and small compounds 2,4,6-trimethylphenol (d), 6-hydroxyquinoline (m), and 5-hydroxyindole (n) are capable of passing through the BBB, at least to some extent (58). For parenterally administered serotonin (o) and N-acetylserotonin (p), effects on the central nervous system are known (59, 60), but they may require specific BBB transport.
Another critical feature concerning neuroprotective antioxidants is the compounds’ potential toxicity, because their required concentrations may be high. The above compounds were tested up to 200 μM and found to be nontoxic to cultivated cells, but there also are in vivo examples of phenolic antioxidants that were nontoxic to mice when given as 0.5% of the total diet (61). Nevertheless, phenolic compounds in high doses may be hepatotoxic, but toxicity seems to arise from hydroxylation and conjugation reactions performed by cytochrome P450 enzymes, and these reactions can be minimized by an appropriate molecular design, e.g., substitution of the phenolic core (62).
Flavonoids probably are not optimal structures for use as antioxidants because of their ability to participate in redox cycling and because of their pronounced estrogenic properties. Higher concentrations of flavonoids usually are required to observe antioxidant effects (36, 63) compared with estrogenic effects (27). Furthermore, a wealth of other biochemical activities of flavonoids have been reported whose in vivo consequences cannot be adequately assessed (64–66).
In any case, ER activation during the course of antioxidant neuroprotective treatment should be avoided because of a possible involvement of ER activation in neoplastic processes (67). We conclude that although the female sex hormone 17β-estradiol has a potent intrinsic antioxidant capacity, it has a uniquely high affinity to its cognate cellular hormone receptors that in vivo may prevail over its antioxidant effect. Here, we present examples of molecules that lack estrogenic hormonal effects but nevertheless are equally effective neuroprotective antioxidants compared with 17β-estradiol. These compounds may serve as a structural basis for the design of improved neuroprotective antioxidants.
Ultimately, our results may have important implications for the prevention and therapy of oxidative stress-related disorders such as Alzheimer’s disease.
Acknowledgments
This work was supported partly by a grant from the Peter und Beate Heller Stiftung to C.B.
ABBREVIATIONS
- ER
estrogen receptor
- ERE
estrogen-responsive element
- LDL
low density lipoprotein
References
- 1.Evans R M. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beato M. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 3.Wetzel C H R, Hermann B, Behl C, Pestel E, Rammes G, Zieglgänsberger W, Holsboer F, Rupprecht R. Mol Endocrinol. 1998;12:1441–1451. doi: 10.1210/mend.12.9.0163. [DOI] [PubMed] [Google Scholar]
- 4.Gu Q, Korach K S, Moss R L. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- 5.Curtis S W, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse J F, Korach K S. Proc Natl Acad Sci USA. 1996;93:12626–12630. doi: 10.1073/pnas.93.22.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auriccio F. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 7.Watters J J, Dorsa D M. J Neurosci. 1998;18:6672–6680. doi: 10.1523/JNEUROSCI.18-17-06672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marino M, Pallottini V, Trentalance A. Biochem Biophys Res Commun. 1998;245:254–258. doi: 10.1006/bbrc.1998.8413. [DOI] [PubMed] [Google Scholar]
- 9.Revelli A, Massobrio M, Tesarik J. Endocr Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- 10.Moss R L, Gu Q, Wong M. Recent Prog Horm Res. 1997;52:33–68. [PubMed] [Google Scholar]
- 11.Wickelgren I. Science. 1997;276:675–678. doi: 10.1126/science.276.5313.675. [DOI] [PubMed] [Google Scholar]
- 12.Woolley C S. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- 13.Paganini-Hill A, Henderson V W. Am J Epidemiol. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 14.Tang M X, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 15.Marder K, Tang M X, Alfaro B, Mejia H, Cote L, Jacobs D, Stern Y, Sano M, Mayeux R. Neurology. 1998;50:1141–1143. doi: 10.1212/wnl.50.4.1141. [DOI] [PubMed] [Google Scholar]
- 16.Sherwin B B. Neurology. 1997;48:S21–S26. doi: 10.1212/wnl.48.5_suppl_7.21s. [DOI] [PubMed] [Google Scholar]
- 17.Miles C, Green R, Sanders G, Hines M. Horm Behav. 1998;34:199–208. doi: 10.1006/hbeh.1998.1478. [DOI] [PubMed] [Google Scholar]
- 18.Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton C, Holsboer F. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- 19.Xu H, Gouras G K, Greenfield J P, Vincent B, Naslund J, Mazzarelli L, Friend G, Jovanovic J N, Seeger M, Relkin N R, et al. Nat Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 20.Woolley C S, Weiland N G, McEwen B S, Schwartzkroin P A. J. Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dulzen D. Brain Res. 1997;767:340–344. doi: 10.1016/s0006-8993(97)00630-6. [DOI] [PubMed] [Google Scholar]
- 22.Green P S, Gordon K, Simpkins J W. J Steroid Biochem Mol Biol. 1997;63:229–235. doi: 10.1016/s0960-0760(97)00124-6. [DOI] [PubMed] [Google Scholar]
- 23.McEwen B S, Alves S E, Bulloch K, Weiland N G. Neurology. 1997;48:S8–S15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 24.Dubal D B, Kashon M L, Pettigrew L C, Ren J M, Finklestein S P, Rau S W, Wise P M. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Loewe S, Lange F, Spohr E. Biochem Z. 1927;180:1–26. [Google Scholar]
- 26.Cook J W, Dodds E C, Hewett C L. Nature (London) 1933;131:56–57. , 205–206. [Google Scholar]
- 27.Kuiper G G J M, Lemmen J G, Carlsson B, Corton J C, Safe S H, van der Saag P T, van der Burg B, Gustafsson J A. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 28.Steinmetz R, Mitchner N A, Grant A, Allen D L, Bigsby R M, Ben-Jonathan N. Endocrinology. 1998;139:2741–2747. doi: 10.1210/endo.139.6.6027. [DOI] [PubMed] [Google Scholar]
- 29.Nakai M, Tabira Y, Asai D, Yakabe Y, Shimyozu T, Noguchi M, Takatsuki M, Shimohigashi Y. Biochem Biophys Res Commun. 1999;254:311–314. doi: 10.1006/bbrc.1998.9928. [DOI] [PubMed] [Google Scholar]
- 30.Mueller G C, Kim U H. Endocrinology. 1978;102:1429–1435. doi: 10.1210/endo-102-5-1429. [DOI] [PubMed] [Google Scholar]
- 31.Routledge E J, Sumpter J P. J Biol Chem. 1997;272:3280–3288. doi: 10.1074/jbc.272.6.3280. [DOI] [PubMed] [Google Scholar]
- 32.Marselos M, Tomatis L. Eur J Cancer. 1992;28A:1182–1189. doi: 10.1016/0959-8049(92)90482-h. [DOI] [PubMed] [Google Scholar]
- 33.Marselos M, Tomatis L. Eur J Cancer. 1992;29A:149–155. doi: 10.1016/0959-8049(93)90597-9. [DOI] [PubMed] [Google Scholar]
- 34.Murkies A L, Wilcox G, Davies S R. J Clin Endocrinol Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 35.Bravo L. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 36.Fauconneau B, Waffo-Teguo P, Huguet F, Barrier L, Decendit A, Merillon J M. Life Sci. 1997;61:2103–2110. doi: 10.1016/s0024-3205(97)00883-7. [DOI] [PubMed] [Google Scholar]
- 37.Lien E J, Ren S, Bui H H, Wang R. Free Radical Biol Med. 1999;26:285–294. doi: 10.1016/s0891-5849(98)00190-7. [DOI] [PubMed] [Google Scholar]
- 38.Oyama Y, Fuchs P A, Katayama N, Noda K. Brain Res. 1994;635:125–129. doi: 10.1016/0006-8993(94)91431-1. [DOI] [PubMed] [Google Scholar]
- 39.Skaper S D, Fabris M, Ferrari V, Dalle Carbonare M, Leon A. Free Radical Biol Med. 1997;22:669–678. doi: 10.1016/s0891-5849(96)00383-8. [DOI] [PubMed] [Google Scholar]
- 40.Behl C, Widmann M, Trapp T, Holsboer F. Biochem Biophys Res. Commun. 1995;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- 41.Green P S, Bishop J, Simpkins J W. J Neurosci. 1997;17:511–515. doi: 10.1523/JNEUROSCI.17-02-00511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies J B, Maher P. Brain Res. 1994;652:169–173. doi: 10.1016/0006-8993(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Maher P, Schubert D. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 44.Mosmann T. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 45.Moosmann B, Uhr M, Behl C. FEBS Lett. 1997;413:467–472. doi: 10.1016/s0014-5793(97)00961-7. [DOI] [PubMed] [Google Scholar]
- 46.Boussif O, Lezoualc’h F, Zanta M A, Mergny M D, Scherman D, Demeneix B, Behr J-P. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couse J F, Lindzey J, Grandien K, Gustafsson J A, Korach K S. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 48.Reiber H, Martens U, Prall F, Uhr M. J Neurochem. 1994;62:608–614. doi: 10.1046/j.1471-4159.1994.62020608.x. [DOI] [PubMed] [Google Scholar]
- 49.Parthasarathy S, Morales A J, Murphy A A. J Clin Invest. 1994;94:1990–1995. doi: 10.1172/JCI117551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coyle J T, Puttfarcken P. Science. 1993;262:689–694. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 51.Osborne C K, Coronado-Heinsohn E B, Hilsenbeck S G, McCue B L, Wakeling A E, McClelland R A, Manning D L, Nicholson R I. J Natl Cancer Inst. 1995;87:746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- 52.Reiter R J. FASEB J. 1995;9:526–533. [PubMed] [Google Scholar]
- 53.Beal M F. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 54.Behl C. Prog Neurobiol. 1999;57:301–323. doi: 10.1016/s0301-0082(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 55.Sano M, Ernesto C, Thomas R G, Klauber M R, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman C W, Pfeiffer E, et al. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 56.Tan S, Wood M, Maher P. J Neurochem. 1998;71:95–105. doi: 10.1046/j.1471-4159.1998.71010095.x. [DOI] [PubMed] [Google Scholar]
- 57.Pardridge W M, Moeller T L, Mietus L J, Oldendorf W H. Am J Physiol. 1980;239:E96–E102. doi: 10.1152/ajpendo.1980.239.1.E96. [DOI] [PubMed] [Google Scholar]
- 58.Seelig A, Gottschlich R, Devant R M. Proc Natl Acad Sci USA. 1994;91:68–72. doi: 10.1073/pnas.91.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen Z, Bonvento G, Lacombe P, Hamel E. Prog Neurobiol. 1996;50:335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- 60.Winters W D, Petit J P, Lakin M L, Miller C H. J Pharmacol Exp Ther. 1984;230:69–74. [PubMed] [Google Scholar]
- 61.Cynshi O, Kawabe Y, Suzuki T, Takashima Y, Kaise H, Nakamura M, Ohba Y, Kato Y, Tamura K, Hayasaka A, et al. Proc Natl Acad Sci USA. 1998;95:10123–10128. doi: 10.1073/pnas.95.17.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson D C, Perera K, London R. Chem Res Toxicol. 1995;8:55–60. doi: 10.1021/tx00043a007. [DOI] [PubMed] [Google Scholar]
- 63.Terao J, Piskula M, Yao Q. Arch Biochem Biophys. 1994;308:278–284. doi: 10.1006/abbi.1994.1039. [DOI] [PubMed] [Google Scholar]
- 64.Ohshima H, Yoshie Y, Auriol S, Gilibert I. Free Radical Biol Med. 1998;25:1057–1065. doi: 10.1016/s0891-5849(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 65.Ji X D, Melman N, Jacobson K A. J Med Chem. 1996;39:781–788. doi: 10.1021/jm950661k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agullo G, Gamet-Payrastre L, Manenti S, Viala S, Remsey C, Chap H, Payastre B. Biochem Pharmacol. 1997;53:1649–1657. doi: 10.1016/s0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- 67.Colditz G A. J Natl Cancer Inst. 1998;90:814–823. doi: 10.1093/jnci/90.11.814. [DOI] [PubMed] [Google Scholar]