Abstract
This report describes a case of bacteraemia caused by Anaerobiospirillum succiniciproducens. Anaerobiospirillum succiniciproducens is a rare cause of bacteraemia in humans, and when encountered usually occurs in immunocompromised patients. The organism is an anaerobic, spiral shaped, Gram negative bacillus with bipolar tufts of flagella. In this report, the morphology, with special reference to electron microscopic features, culture characteristics, and antimicrobial susceptibility are described.
Keywords: Anaerobiospirillum, bacteraemia, electron microscopic features, immunocompromised
Anaerobiospirillum was first reported in 1976 by Davis et al as a new genus of spiral shaped bacteria isolated from the throats and faeces of beagle dogs.1 The first case of septicaemia caused by Anaerobiospirillum succiniciproducens was described in 1981 by Rifkin and Opdyke.2 The organism has since been implicated as an occasional cause of bacteraemia in: the USA,3,4 Hong Kong,5 New Zealand,6 Australia,7 the UK,8 Germany,9 Spain,10 South Africa,11 and Israel.12 Most of these patients were immunocompromised.
CASE REPORT
A 25 year old female patient with a malignancy was admitted to hospital for a course of chemotherapy. Twelve hours after chemotherapy, she developed fever, chills, generalised myalgia, non-productive cough, and abdominal cramps, followed by a single episode of loose stools. Her temperature, systemic examination, and chest x ray were within normal limits. Laboratory results were: white blood cell count, 5.2 × 109/litre, with a neutrophilia of 83% and shift to the left; platelet count, 185 × 109/litre; and haemoglobin 113 g/litre. One set of blood cultures, consisting of standard aerobic and anaerobic bottles, was collected. Empirical treatment with amoxicillin–clavulanic acid was initiated. She made an uneventful recovery.
LABORATORY INVESTIGATIONS
Isolation and identification of organism
Blood was collected for culture in a BACTEC PLUS Aerobic/F vial and a BACTEC PLUS Anaerobic/F vial (Becton Dickinson Diagnostic Instrument Systems, USA). The anaerobic bottle gave a positive signal after 48 hours of incubation and Gram stain revealed a Gram negative spiral bacillus. The blood culture broth was subcultured on to 6% sheep blood agar and incubated anaerobically at 37°C using the AnaeroGen™ gas generating kit (Oxoid Ltd, Basingstoke, Hampshire, UK) and also under microaerophilic conditions at 42°C, 37°C, and 25°C using gas generating sachets (Oxoid). Subculture under anaerobic conditions yielded circular, translucent, non-haemolytic colonies, 0.5 mm in diameter. The organism grew poorly at 42°C and failed to grow at 25°C. On darkfield microscopy, the organism showed corkscrew-like motility. The flagella stain as described by Kodaka and colleagues13 showed spiral bacteria with bipolar tufts of flagella. Oxidase and catalase tests were negative. The organism was identified using a rapid ID 32A API (bioMérieux, Marcy L’Etoile, France). The identification was confirmed by the South African Institute for Medical Research in Johannesburg, South Africa, using the API Zym test (bioMérieux) and gas liquid chromatography. The rapid ID 32A and Zym APIs revealed positive enzymatic reactions for leucine arylamidase, phosphohydrolase, α glucosidase and N-acetyl-β-glucosaminidase, and β galactosidase was negative. The major volatile fatty acid was acetic acid and major non-volatile fatty acids were succinic and lactic. The prereduced anaerobic sugar system was used for carbohydrate fermentation determination. Acid was produced from fructose, glucose, maltose, and sucrose, whereas none was produced from lactose and raffinose.
Electron microscopy
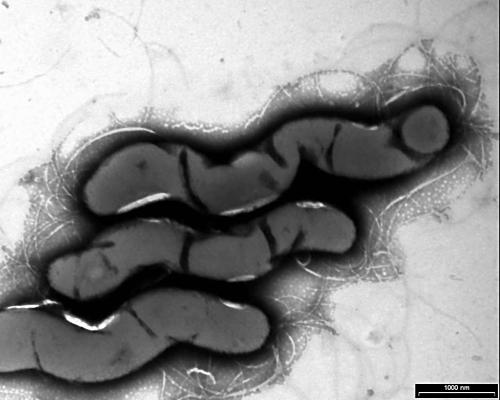
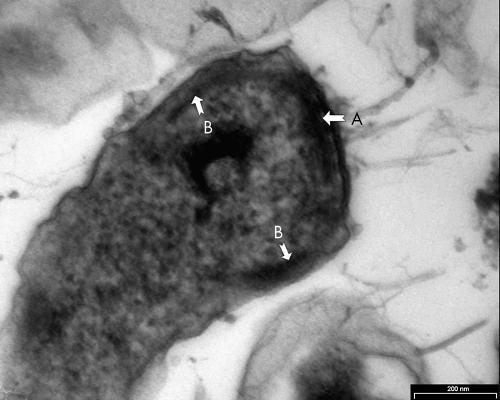
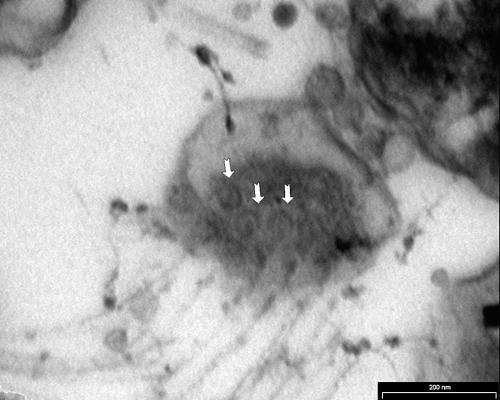
The method described by Wecke and Horbach was used.9 Thin sections were cut using an LKB ultramicrotome and visualised with a Philips Tecnai 10 at 60 kV. The negatively stained micrograph (fig 1) showed spiral bacteria with bipolar tufts of flagella. The bacterial length varied from 3.87 to 7.50 μm, with a mean of 5.43 μm (n = 25). The diameter varied from 0.38 to 0.92 μm, with a mean of 0.69 μm (n = 25). One pole appeared to be flattened (fig 2) and therefore it was not always possible to see the bipolar flagellation. Ultrathin cross sections of the apical end revealed round discs at the flagellar bases (fig 3). The flagella were inserted into an intensely staining platform-like structure in the cytoplasm of the apical end (fig 2). There was also an intensely stained, layered structure at the periphery of the cytoplasm (fig 2).
Figure 1.
Negatively stained Anaerobiospirillum succiniciproducens cells.
Figure 2.
Longitudinal section of apical end of Anaerobiospirillum succiniciproducens. Arrow A indicates a platform-like structure, and arrow B an intensely stained structure at the periphery of the cytoplasm.
Figure 3.
Cross section of the apical end of Anaerobiospirillum succiniciproducens. The arrows indicate disc-like flagella bases.
Antimicrobial susceptibility testing
Minimum inhibitory concentrations (MICs) of amoxicillin–clavulanic acid, cefoxitin, clindamycin, erythromycin, imipenem, metronidazole, and penicillin were determined by an agar dilution method using Wilkins Chalgren agar (Mast Diagnostics, Mast Group Ltd, Bootle, Merseyside, UK) with 5% defibrinated sheep blood according to the recommendations of the National Committee for Clinical Laboratory Standards (NCCLS).14 The organism was suspended in brucella broth (Difco Laboratories, Detroit, Michigan, USA) to a turbidity equivalent to a 0.5 McFarland standard. the MICs were read after 48 hours. Bacteroides fragilis American Type Culture Collection 25285 was used as control.
The MICs of the isolate were: amoxicillin–clavulanic acid, 2.0 mg/litre; cefoxitin, 1.0 mg/litre; clindamycin, 16 mg/litre; erythromycin, 32 mg/litre; imipenem, 0.06 mg/litre; metronidazole, 16 mg/litre; and penicillin, 0.5 mg/litre. According to the NCCLS breakpoints for anaerobes, the isolate was susceptible to amoxicillin–clavulanic acid, cefoxitin, imipenem, and penicillin, intermediately resistant to metronidazole, and resistant to clindamycin. Nitrocefin (Oxoid) was used to test for β lactamase production. The organism did not produce β lactamase.
Take home messages .
We describe a case of bacteraemia caused by Anaerobiospirillum succiniciproducens in an immunocompromised patient with malignancy
We characterised the organism and found it to be an anaerobic, spiral shaped, Gram negative bacillus with bipolar tufts of flagella
Interestingly, similar to the other African isolate, our isolate was β galactosidase and lactose negative, unlike previous reports, and it failed to ferment raffinose, whereas most isolates previously reported were raffinose positive
DISCUSSION
Anaerobiospirillum spp and Campylobacter spp are morphologically similar and can be confused. Anaerobiospirillum spp are oxidase and catalase negative, whereas Campylobacter spp are oxidase and catalase positive. Anaerobiospirillum demonstrates corkscrew-like motility, whereas campylobacter displays darting motility. Anaerobiospirillum has bipolar tufts of flagella, whereas campylobacter has a single flagellum on one or both poles. Our strain, and the first African isolate, were β galactosidase and lactose negative. According to previous reports, A succiniciproducens is β galactosidase and lactose positive.1,2,5,7,10,15,16 An additional unusual biochemical finding of our isolate was that it failed to ferment raffinose, whereas most isolates previously reported were raffinose positive.1,11,15,16
The bipolar lophotrigous flagellation is characteristic of A succiniciproducens.9 The length and diameter of this isolate fall within the same range of A succiniciproducens as measured by other authors.1,7,9,15,16 The flagellar bases of A succiniciproducens in the cytoplasm are disc-like structures in contrast to comparable structures in Helicobacter pylori, which are club-like.9,17 Wecke and Horbach described an electron dense ring situated under the flagellated pole in close connection with the outer sheath.9 This structure is unique to A succiniciproducens. Ultrastructurally, this organism fits the description of A succiniciproducens.
“To our knowledge, this is only the second case of bacteraemia caused by Anaerobiospirillum succiniciproducens reported from Africa, both being from South Africa”
There are two known anaerobiospirillum species that infect humans: A succiniciproducens causes both bacteraemia2–12 and diarrhoea,15 and A thomasii has only been implicated as a cause of diarrhoea.16 Anaerobiospirillum succiniciproducens is more likely to cause bacteraemia than diarrhoea. McNeil et al showed that 17 of 22 patients infected with A succiniciproducens had gastrointestinal signs and symptoms and suggested that the gastrointestinal tract was the likely portal of entry.4 Although the organisms can be isolated from the rectal swabs of cats and dogs, they have not been isolated from faeces of asymptomatic individuals.15 Therefore, they are unlikely to form part of the normal gastrointestinal flora of humans. Most patients with A succiniciproducens bacteraemia had underlying disorders, such as chronic alcoholism,3,4,7,8,10 atherosclerosis,2,4 malignancies, 3,4,6,7 recent surgery,4 diabetes mellitus,4,6 dental caries,4 and AIDS.7,9 The patient we report had an underlying malignancy and presented with gastrointestinal signs and symptoms in addition to bacteraemia. Owing to the rare reports of bacteraemia caused by this organism, the optimal antimicrobial treatment for A succiniciproducens still remains to be determined.
To our knowledge, this is only the second case of bacteraemia caused by A succiniciproducens reported from Africa, both being from South Africa.
Acknowledgments
We wish to thank Professor LD Liebowitz and Dr GN Rolinson for reviewing the manuscript and A Sooka of SAIMR in Johannesburg, South Africa for confirming the identity of our isolate.
Abbreviations
MIC, minimum inhibitory concentration
NCCLS, National Committee for Clinical Laboratory Standards
REFERENCES
- 1.Davis CP, Cleven D, Brown J, et al. Anaerobiospirillum, a new genus of spiral-shaped bacteria. Int J Syst Bacteriol 1976;26:498–504. [Google Scholar]
- 2.Rifkin GD, Opdyke JE. Anaerobiospirillum succiniciproducens septicemia. J Clin Microbiol 1981;13:811–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lally RT, Woolfrey BF. Anaerobiospirillum succiniciproducens bacteremia. Clinical Microbiology Newsletter 1988;10:87–8. [Google Scholar]
- 4.McNeil MM, Martone WJ, Dowell VR, Jr. Bacteremia with Anaerobiospirillum succiniciproducens. Rev Infect Dis 1987;9:737–42. [PubMed] [Google Scholar]
- 5.Yuen KY, Yung WH, Seto WH. A case report of Anaerobiospirillum causing septicemia. J Infect Dis 1989;159:153–4. [DOI] [PubMed] [Google Scholar]
- 6.Henry J. Anaerobiospirillum succiniciproducens septicaemia—“a case of red herrings”. New Zealand Journal of Medical Laboratory Technology 1989;43:113. [Google Scholar]
- 7.Tee W, Korman TM, Waters MJ, et al. Three cases of Anaerobiospirillum succiniciproducens bacteremia confirmed by 16S rRNA gene sequencing. J Clin Microbiol 1998;36:1209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goddard WW, Bennett SA, Parkinson C. Anaerobiospirillum succiniciproducens septicaemia: important aspects of diagnosis and management. J Infect 1998;37:68–70. [DOI] [PubMed] [Google Scholar]
- 9.Wecke J, Horbach I. Ultrastructural characterization of Anaerobiospirillum succiniciproducens and its differentiation from campylobacter species. FEMS Microbiol Lett 1999;170:83–8. [DOI] [PubMed] [Google Scholar]
- 10.Saavedra JM, Naranjo C, Vega D, et al. Bacteriemia por Anaerobiospirillum succiniciproducens asociada a íleo paralítico. Enferm Infecc Microbiol Clin 1996;14:513. [PubMed] [Google Scholar]
- 11.Marcus L, Gove EW, van der Walt ML, et al. First reported African case of Anaerobiospirillum succiniciproducens septicemia. Eur J Clin Microbiol Infect Dis 1996;15:741–4. [DOI] [PubMed] [Google Scholar]
- 12.Rudensky B, Wachtel D, Yinnon AM, et al. Anaerobiospirillum succiniciproducens bacteremia in a young child. Pediatr Infect Dis J 2002;21:575–6. [DOI] [PubMed] [Google Scholar]
- 13.Kodaka H, Armfield AY, Lombard GL, et al. Practical procedure for demonstrating bacterial flagella. J Clin Microbiol 1982;16:948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing of anaerobic bacteria: approved standard, 3rd ed. NCCLS document M11-A3. Villanova, PA: National Committee for Clinical Laboratory Standards, 1993.
- 15.Malnick H, Williams K, Phil-Ebosie J, et al. Description of a medium for isolating Anaerobiospirillum spp., a possible cause of zoonotic disease, from diarrheal feces and blood of humans and use of the medium in a survey of human, canine, and feline feces. J Clin Microbiol 1990;28:1380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malnick H. Anaerobiospirillum thomasii sp. nov., an anaerobic spiral bacterium isolated from the feces of cats and dogs and from diarrheal feces of humans, and emendation of the genus anaerobiospirillum. Int J Syst Bacteriol 1997;47:381–4. [DOI] [PubMed] [Google Scholar]
- 17.Geis G, Leying H, Suerbaum S, et al. Ultrastructure and chemical analysis of Campylobacter pylori flagella. J Clin Microbiol 1989;27:436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]