Abstract
Background: Pigment epithelium derived factor (PEDF) was first isolated from medium conditioned by human fetal retinal pigment epithelial cells. PEDF was detected in a broad range of human fetal and adult tissues including almost all brain areas. It can also inhibit the proliferation of cultured rat astrocytes. Recent studies have implicated PEDF in activities that are inhibitory to angiogenesis.
Aims: To investigate the expression of PEDF in gliomas to assess its “gliastatic” effects and its role in anti-angiogenesis.
Methods: PEDF mRNA values were measured by quantitative real time reverse transcription polymerase chain reaction (RT-PCR) analysis of normal brain tissue and tumour specimens from both low and high grade gliomas. In addition, immunohistochemical staining for PEDF and vascular endothelial growth factor (VEGF) was performed on 32 paraffin wax embedded glioma samples, 10 of them grade IV, 10 grade III, seven grade II, and five grade I.
Results: RT-PCR showed that PEDF mRNA values were 5.0 (p < 0.001) and 15.4 (p < 0.001) times higher in normal human brain specimens (n = 5) than in tumour tissue specimens of low grade glioma (grades I and II; n = 15) and high grade glioma (grades III and IV; n = 10), respectively. VEGF was strongly positive in 90% of grade IV, 70% of grade III, 43% of grade II, and 20% of grade I cases. In contrast, PEDF was positive in none of grade IV, 20% of grade III, 43% of grade II, and 60% of grade I tumours. There was an inverse correlation between VEGF and PEDF expression, and a lack of PEDF in advanced grade gliomas.
Conclusions: It is possible that the absence of PEDF expression is a potent factor for the enhancement of angiogenesis in glioma.
Keywords: pigment epithelium derived factor, glioma, angiogenesis, vascular endothelial growth factor, immunohistochemistry
Glioma is characterised by rapid growth, intense angiogenesis, vascular malformations, and poor survival rate. The progressive growth of glioblastoma is thought to be dependent on angiogenesis.1 Angiogenesis is regulated by the local balance between various molecules that induce and suppress neovascularisation. In the microenvironment surrounding glioma, the balance shifts from neutral to angiogenic conditions.2,3 Certain angiogenic factors might be secreted from gliomas and induce a vigorous angiogenic response, overcoming local inhibitors.
“A series of studies have implicated pigment epithelium derived factor in activities that are inhibitory to angiogenesis”
Pigment epithelial derived factor (PEDF) is a 50 kDa glycoprotein initially isolated from retinal pigment epithelial cells, which is recognised for its neurotrophic activity on cells derived from the neural crest.4,5 It shares high sequence homology with other serine proteinase inhibitors (serpins) and behaves like a non-inhibitory serpin.6 PEDF has been detected in many tissues. It is found in a broad range of human fetal and adult tissues, including almost all brain areas,7 and is present in the interphotoreceptor matrix at a high concentration.8,9 Later studies demonstrated that it can promote the survival of cerebellar granule cells (CGCs),10 induce differentiation in retinoblastoma tumour cells in vitro,11 and protect retinal neurones from apoptotic death.12 In addition, a PEDF derived peptide promotes survival and neurite outgrowth in spinal motor neurones, even after axotomy in animal models.13
A series of studies have implicated PEDF in activities that are inhibitory to angiogenesis.14–16 PEDF was shown to inhibit the migration of endothelial cells in vitro in a dose dependent manner and was more effective than other known angiogenesis inhibitors, such as angiostatin, thrombospondin 1 (TSP-1), and endostatin.17 The results of that study placed PEDF among the most potent natural inhibitors of angiogenesis.
In addition, in the presence of PEDF, the growth of astroglia is considerably slowed down. PEDF also completely blocks the granulocyte–macrophage colony stimulating factor stimulated cell division of microglia in rats.18 Because of this “gliastatic” effect of PEDF and the fact that it acts as one of the most potent antiangiogenesis factors, we thought that it would be interesting to evaluate the expression of PEDF and its association with vascular endothelial growth factor (VEGF), a potent angiogenic factor, in glioma. The importance of the local balance of these factors with regard to vascularisation is also discussed.
MATERIALS AND METHODS
Cell culture
Human malignant U251 glioma cells (obtained from the Chinese Academy of Science) were cultured in RPMI 1640 medium and supplemented with 10% fetal bovine serum, 2mM L-glutamine, 100 IU/ml penicillin G, and 100 μg/ml streptomycin at 37°C in a humidified 5% CO2 atmosphere. Normal human SVG glial cells (CRL-8621; ATCC, Rockville, Maryland, USA) were routinely cultured in minimum essential Eagle’s medium with 10% fetal bovine serum, 2mM L-glutamine, 100 IU/ml penicillin G, and 100 μg/ml streptomycin at 37°C in a humidified 5% CO2 atmosphere.
Surgical specimens
Histological specimens were surgically collected from 32 patients with glioma at Hua Shan Hospital, Fudan University from 2000 to 2001. During the histopathology examination the tumours were typed and graded independently by two experienced neuropathologists, using the World Health Organisation classification,19 into glioblastoma, grade 4 (n = 10); anaplastic astrocytoma, grade 3 (n = 10); low grade astrocytoma, grade 2 (n = 7); and pilocytic astrocytoma, grade 1 (n = 5). RNA of sufficient integrity and quality was extracted from 25 of these glioma samples only. Normal brain tissue specimens (n = 5) were surgically obtained from patients with cerebral trauma.
RNA isolation and cDNA synthesis
Total RNA was extracted from specimens and cells by means of TRIzol reagent (Invitrogen, California, USA) according to the standard protocol. The quality of the RNA samples was determined by electrophoresis through agarose gels and staining with ethidium bromide. The 18S and 28S RNA bands were visualised under ultraviolet light. Total RNA (2 μg) was processed directly to cDNA by reverse transcription with superscript II (Invitrogen), according to the manufacturer’s protocol in a total volume of 20 μl.
Probes and primers
Probes and primers for PEDF and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed with the assistance of PRIME 3 software (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi) and synthesised by the Shanghai ShenYou Corporation (Shanghai, China) with the 5′FAM reporter dye and the 3′TAMRA quencher dye. Table 1 shows the sequences. To avoid amplification of contaminating genomic DNA, one of the two primers or the probe was placed at the junction between two exons or in a different exon.
Table 1.
Oligonucleotide primer and probe sequences used
| Gene | Oligonucleotide | Sequence | PCR product size (bp) |
| PEDF | Upper primer | 5′-AGGCCCAGAGTCCTGACGGG-3′ | 151 |
| Lower primer | 5′-CCTTGAAGTGCGCCACACCG-3′ | ||
| Probe | 5′-CGCAGATGAAAGGGAAGCTCGCCAGGT-3′ | ||
| GAPDH | Upper primer | 5′-GAAGGTGAAGGTCGGAGTCA-3′ | 226 |
| Lower primer | 5′-GAAGATGGTGGTGATGGGATTTC-3′ | ||
| Probe | 5′-CAAGCTTCCCGTTCTCAGCC-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PEDF, pigment epithelium derived factor.
Preparation of quantitative standards
Standards were prepared by cloning polymerase chain reaction (PCR) products of the PEDF and GAPDH fragments using the above primers. These clones provided stable standards for long term use and storage. The PCR product was purified using the Wizard PCR Preps DNA Purification System (Promega, Madison, Wisconsin, USA), according to the manufacturer’s instructions. The purified PCR product was cloned using the Promega pGEM-T vector system (Promega), according to the manufacture’s instructions. The recombinant plasmid DNA was isolated and purified using the Qiaprep Plasmid Miniprep Kit (Qiagen, Chatsworth, California, USA). The concentration of plasmid was measured by absorbance at 260 nm and the copy number calculated using the molecular weight of the plasmid. Serial dilutions of each standard were made in the range of 103 to 109 copies/1 μl.
Real time reverse transcription PCR
To measure the PEDF mRNA content, we used a real time PCR approach—the TaqMan PCR method after reverse transcription (RT) of the isolated RNA. In brief, the TaqMan assay uses the 5′-nuclease activity of DNA polymerase to cleave a specific probe that hybridises to the target amplicon during the annealing and extension phase of the PCR. Each probe contains a fluorescent dye reporter at the 5′ end and a quencher dye at the 3′ end that will normally inhibit the reporter emission. Therefore, cleavage of the probe separates the reporter and quencher dyes, resulting in increased fluorescent emission of the reporter, which is monitored by a suitable detector. The probe also provides an added degree of specificity to the assay. To measure cDNA concentrations, the threshold cycle (Ct) at which fluorescence is first detected above baseline is used and a standard curve is drawn between starting cDNA concentrations and the Ct.20 We also quantified transcripts of GAPDH as the endogenous reference control, and each sample was normalised on the basis of its GAPDH content. For each experimental sample, to correct for differences in both RNA quality and quantity between samples, data were normalised by dividing the copy number of the target cDNA by the copy number of GAPDH.
All PCR reactions were performed on an ABI Prism5700 sequence detection system. For each PCR run, a master mixture was prepared on ice with 1× PCR buffer; 4mM MgCl2; 200μM dATP, dCTP, dGTP, and dTTP; 300nM each primer; 150nM probe; and 3 U of Takara DNA polymerase (Takara Biotechnology, Dalian, China). A 1 μl aliquot of each reverse transcription sample was added to 49 μl of PCR mixture. The thermal cycling conditions comprised an initial denaturation step at 95°C for 10 minutes, followed by 40 cycles at 94°C for 15 seconds and 60°C for one minute.
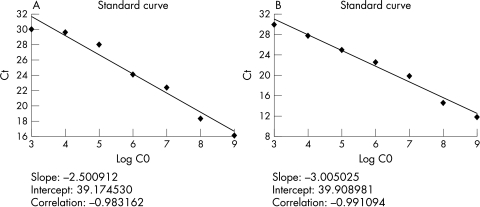
Experiments were performed in triplicate for each data point. Each PCR run included seven points of the calibration curve (fig 1), a no template control, cDNA from the 25 glioma specimens, cDNA from the five normal brain specimens, and cDNA from the U251 and SVG cells.
Figure 1.
The PCR amplification plots of 10 fold serial dilutions of (A) pigment epithelium derived factor (PEDF) cDNA and (B) GAPDH cDNA with known starting number. The dynamic range was seven orders of magnitude. Ct, threshold cycle.
Immunohistochemical analysis
The following primary antibodies were used: a monoclonal antibody specific to human PEDF (1/200 dilution; Chemicon, Temecula, California, USA) and a monoclonal antibody against VEGF (1/100 dilution, VEGF (C-1); sc-7269; Santa Cruz Biotechnology, Santa Cruz, California, USA). Immunoperoxidase staining of formalin fixed, paraffin wax embedded tissue sections was performed using an ordinary biotin–streptavidin method. Briefly, sections were dewaxed, rehydrated in a descending alcohol series, heated in an 800 W microwave oven at maximal power for five minutes in 10mM citric buffer (pH 6.0), and washed with phosphate buffered saline (PBS; pH 7.3). The sections were then immersed in 0.3% hydrogen peroxide in methanol for 20 minutes at room temperature to block endogenous peroxidase activity. After non-specific sites were blocked with 5% normal goat serum in PBS for one hour, the sections were incubated with anti-PEDF and anti-VEGF monoclonal antibodies overnight at 4°C. In subsequent steps, we used the Vectastain ABC kit using DAB as a chromogen (Vector, Burlingame, California, USA). The sections were then counterstained with haematoxylin. In every immunohistochemical staining, we performed additional staining without primary antibody in parallel as a negative control.
Western blot analysis
Subconfluent U251 and SVG cells were washed three times with ice cold PBS, harvested by scraping, and homogenised in an appropriate amount of homogenising buffer (20mM Hepes buffer containing 5mM EGTA, 5mM EDTA, 1mM phenylmethylsulfonyl fluoride, 1mM dithiothreitol, 0.1mM leupeptin, 75μM pepstatin A, 150mM NaCl, and 0.1% Triton X-100). The homogenates were centrifuged at 15 000 ×g for 15 minutes at 4°C, and the supernatants were obtained. After determining the protein concentration by means of the Bio-Rad protein assay, an equal amount of protein (50 μg) from each sample was subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane. After blocking with 5% non-fat dry milk/PBS with 0.1% Tween 20 for one hour at room temperature, the membrane was incubated with anti-PEDF antibody (dilution, 1/400) at 4°C overnight. After incubation with the secondary antibody (rabbit antimouse IgG absorbed horseradish peroxidase labelled (Dako, Glostrup, Denmark; dilution, 1/1000)) for one hour at room temperature, immunoreactive bands were detected using the enhanced chemiluminescence western blotting analysis system (Amersham, Piscataway, New Jersey, USA).
Statistical analysis
Fisher’s exact test was applied for comparisons between group frequencies. The relative expression of PEDF mRNA between two groups was compared using the unpaired Student’s t test. p Values less than 0.05 were considered significant.
RESULTS
PEDF mRNA expression in normal and glioma specimens and cell lines
Quantitative evaluation after the copy number of PEDF was normalised to that of GAPDH revealed that PEDF mRNA was abundant in the five normal brain tissue samples. The expression coefficients of these normal tissues were in the range of 0.16 to 0.30 (table 2). The amount of PEDF mRNA found in normal tissue was greater than that found in tumour samples. Analysis with grade showed the amount of PEDF mRNA was 5.0 (p < 0.001) and 15.4 (p < 0.001) times higher in normal human brain tissue (n = 5) than in tumour tissue specimens of low grade glioma (grades I and II, n = 15) and high grade glioma (grades III and IV, n = 10), respectively. Moreover, we found a significant difference (p < 0.001) in PEDF mRNA expression between low grade glioma and high grade glioma. In addition, in the cell lines studied, normal SVG glial cells had large amounts of PEDF protein compared with U251 glioma cells. The PEDF transcript was hardly detectable in U251 cells.
Table 2.
PEDF mRNA expression in normals, gliomas, and cell lines
| Sample number | Average PEDF mRNA value normalised to GAPDH | |
| Healthy brain | 5 | 0.23 (0.031) |
| Low grade (grades I, II) | 15 | 0.04* (0.003) |
| High grade (grades I, II) | 10 | 0.015* (0.002) |
| SVG | 1 | 0.12 (0.025) |
| U251 | 1 | 0.009 (0.001) |
Values are shown as mean (SD).
*p<0.001.
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PEDF, pigment epithelium derived factor.
Immunohistochemical assay of VEGF and PEDF
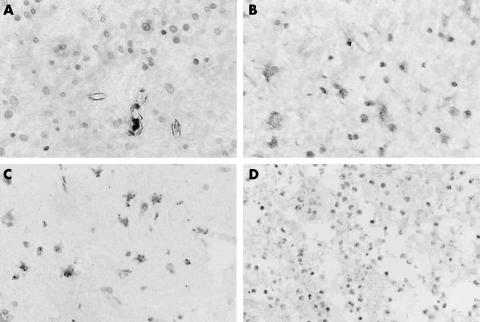
Immunostaining for PEDF and VEGF was also performed on 32 paraffin wax embedded glioma tissue specimens (fig 2). The expression of VEGF varied in the different grades of astrocytoma (table 3). VEGF was strongly positive in nine of the 10 grade IV cases, seven of the 10 grade III tumours, three of the seven grade II tumours, and one of the five grade I tumours. VEGF expression was significantly related to tumour grade (Fisher’s exact test, p = 0.032). The positive signal was most intense in endothelial cells, tumour cells, and vessels around areas of necrosis. In contrast, PEDF was positive in none of grade IV, two of grade III, three of grade II, and three of grade I tumour samples. The frequency and the intensity of PEDF immunostaining decreased greatly from low grade glioma to high grade glioma. A significant inverse relation was demonstrated between PEDF and tumour grade (Fisher’s exact test, p = 0.036). After comparing the expression of VEGF and PEDF in human gliomas of different stages of malignancy, an inverse correlation was found between the two factors (table 4; p = 0.030).
Figure 2.
Representative examples of vascular endothelial growth factor (VEGF) and pigment epithelium derived factor (PEDF) immunohistochemical staining. The positive signal of VEGF was most intense in endothelial cells, tumour cells, and vessels around areas of necrosis. The intensity of PEDF immunostaining decreased greatly from low grade glioma to high grade glioma. (A) VEGF expression in astrocytoma; (B) VEGF expression in glioblastoma; (C) PEDF expression in astrocytoma; (D) PEDF expression in glioblastoma (original magnifications, ×400).
Table 3.
Immunoreactivity for VEGF and PEDF in gliomas
| Tumour type (WHO grade) | Number | VEGF positivity | p Value | PEDF positivity | p Value |
| Pilocytic astrocytoma (I) | 5 | 1 (20%) | 0.032 | 3 (60%) | 0.036 |
| Astrocytoma (II) | 7 | 3 (43%) | 3 (43%) | ||
| Anaplastic astrocytoma (III) | 10 | 7 (70%) | 2 (20%) | ||
| Glioblastoma (IV) | 10 | 9 (90%) | 0 (10%) |
PEDF, pigment epithelium derived factor; VEGF, vascular endothelial growth factor; WHO, World Health Organisation.
Table 4.
Correlation between PEDF expression and VEGF expression in glioma
| PEDF staining | % | ||||
| VEGF staining | Positive (n=9) | Negative (n=23) | Positive | Negative | p Value |
| Positive (n=20) | 2 | 18 | 20 | 80 | 0.030 |
| Negative (n=12) | 6 | 6 | 50 | 50 | |
PEDF, pigment epithelium derived factor; VEGF, vascular endothelial growth factor.
Western blot analysis
The expression of the PEDF protein in normal SVG glial cell and U251 glioma cell lysates was analysed with anti-PEDF monoclonal antibody. The antibody recognised a band at about 50 kDa corresponding to the positive control PEDF from vitreous humour of the human eye (fig 3). Consistent with the observations in vivo, the expression of PEDF in normal glial cells was much higher than that of U251 cells (p < 0.001).
Figure 3.
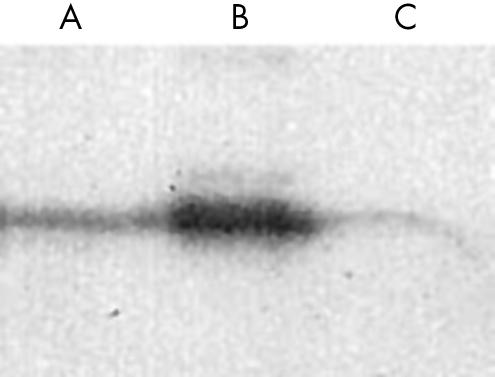
Western blot analysis of the pigment epithelium derived factor protein. Lane A, SVG glial cell lysates; lane B, vitreous humour (1/20 dilution); lane C, U251 glioma cell lysates.
DISCUSSION
Gliomas in the form of pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas, and glioblastomas, which constitute most (65%) primary intracranial neoplasms,21 are typically characterised by rapid cell proliferation and a pronounced propensity to invade and damage surrounding tissues. Any additional prognostic indicator to identify those patients who should be treated aggressively and those who should be treated conservatively would be extremely useful so that the measurement of angiogenesis may have great potential as a prognostic indicator and a therapeutic alternative. Antiangiogenic strategies could offer new ways to treat the malignant brain tumours.22–24 However, the exact role of angiogenesis in glioma has not yet been determined.
PEDF is secreted by many cell types and is a potent inhibitor of neovascularisation. Previous work has shown that PEDF exists in almost all regions of the adult brain,7 indicating that PEDF could be of particular importance in the brain as an autocrine/paracrine factor. However, the expression of PEDF and the association between PEDF and VEGF had not been previously characterised in glioma. We found that PEDF expression was significantly lower in high grade glioma than in low grade glioma at both the mRNA and the protein level.
Although many factors stimulate the proliferation and biological activity of astrocytes and microglial cells, only a few, such as transforming growth factor-α25,26 and TSP-1 can suppress proliferation. Volpert and colleagues27 demonstrated that the two inhibitors of angiogenesis, TSP-1 and PEDF, derived their specificity for remodelling vessels from their dependence on Fas/Fas ligand, which targets endothelial cells for destruction, thereby blocking angiogenesis. This study provided one explanation for the ability of PEDF to select remodelling capillaries for destruction.
PEDF is a multipotent neurotrophic factor that acts upon various types of neurones from different species. For example, it has been reported that PEDF has a potent neuronal differentiating activity on human retinoblastoma28 and neuroblastoma.29 Glioblastoma multiforme is a malignant and highly undifferentiated tumour of the central nervous system (CNS). The observations that the lowest degree of expression was found in those grades of glioma that showed the worst differentiation reinforces the idea that PEDF is an active differentiation factor in vivo. The effects of both native and recombinant PEDF on normal CGCs in primary culture has been examined. Tanawaki and co-workers10 found that PEDF did not induce a more neuronal phenotype in CGCs, but did have a pronounced effect on CGC survival. Araki and colleagues30 found that PEDF was a potent inhibitor of natural and induced apoptosis. The effects of PEDF are not limited to brain neurones. Houenou et al demonstrated that PEDF effectively promotes both the differentiation and survival of developing spinal motor neurons.13 It is clear that PEDF can protect neurones in both longterm and more acute situations and this protection makes PEDF of potential use against diseases of the CNS.
“We found that pigment epithelium derived factor expression was significantly lower in high grade glioma than in low grade glioma at both the mRNA and the protein level”
One of the most important angiogenic factors in solid tumours is VEGF. Our present results showed that VEGF immunoreactivity was most frequent in glioblastomas and least frequent in the lowest grade astrocytomas; there was a significant association between the expression of VEGF and the histological grade of the tumour, suggesting that the expression of VEGF increases during the progression of malignancy in gliomas. We also showed that PEDF values, in contrast to those of VEGF, are negatively correlated with the pathological grade of glioma. Decreased PEDF may be contributing to the development of glioma neovascularisation. It is believed that angiogenesis is regulated by two counter balancing systems: angiogenic stimulators, such as VEGF, and angiogenic inhibitors, such as PEDF. The balance is crucial for the regulation of angiogenesis. Endogenous angiogenic inhibitors are believed to be essential for maintaining the homeostasis of angiogenesis in the glioma. It is hypothesised that in some situations, such as tumour growth, the tissue increases the production of angiogenic stimulators and reduces the production of angiogenic inhibitors, disturbing the balance between the positive and negative regulators of angiogenesis.31,32
In conclusion, we demonstrated a significant correlation between a lack of the PEDF protein and increased VEGF in glioma. The inverse correlation between the expression of PEDF and the degree of malignancy seen in human glioma suggests that PEDF expression might be used as a prognostic marker for this human tumour, indicating that the lack of PEDF expression is a potent factor for enhancement of angiogenesis in gliomas. To our knowledge, this is the first report on PEDF expression in glioma. Its “gliastatic” features, its effects on differentiation, and its antiangiogenesis effects make PEDF an appealing potential agent for the control of tumour growth. We are in the process of studying the regulation of PEDF expression in various human tumours, with the aim of providing more information to help establish new and exciting strategies to inhibit tumour growth.
Take home messages .
There was a significant correlation between a lack of the pigment epithelium derived factor (PEDF) protein and increased vascular endothelial growth factor in glioma
There was also an inverse correlation between the expression of PEDF and the degree of malignancy in human glioma, suggesting that the lack of PEDF expression is a potent factor for the enhancement of angiogenesis in gliomas
PEDF expression might be a useful a prognostic marker for human glioma and might also be a potential agent for the control of tumour growth
Abbreviations
CGC, cerebellar granule cell
CNS, central nervous system
Ct, threshold cycle
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
PBS, phosphate buffered saline
PEDF, pigment epithelium derived factor
RT-PCR, reverse transcription polymerase chain reaction
TSP-1, thrombospondin 1
VEGF, vascular endothelial growth factor
REFERENCES
- 1.Brem S. The role of vascular proliferation in the growth of brain tumors. Clin Neurosurg 1976;23:440–453. [DOI] [PubMed] [Google Scholar]
- 2.Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv Cancer Res 1996;69:135–74. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86:353–64. [DOI] [PubMed] [Google Scholar]
- 4.Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res 1991;53:411–14. [DOI] [PubMed] [Google Scholar]
- 5.Becerra SP, Palmer I, Kumar A, et al. Overexpression of fetal human pigment epithelium-derived factor in Escherichia coli. A functionally active neurotrophic factor. J Biol Chem 1993;268:23148–56. [PubMed] [Google Scholar]
- 6.Steele FR, Chader GJ, Johnson LV, et al. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A 1993;90:1526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tombran-Tink J, Mazuruk K, Rodriguez IR, et al. Organization, evolutionary conservation, expression and unusual Alu density of the human gene for pigment epithelium-derived factor, a unique neurotrophic serpin. Mol Vis 1996;2:11–15. [PubMed] [Google Scholar]
- 8.Tombran-Tink J, Shivaram SM, Chader GJ, et al. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci 1995;15:4992–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu YQ, Notario V, Chader GJ, et al. Identification of pigment epithelium-derived factor in the interphotoreceptor matrix of bovine eyes. Protein Expr Purif 1995;6:447–56. [DOI] [PubMed] [Google Scholar]
- 10.Taniwaki T, Becerra SP, Chader GJ, et al. Pigment epithelium-derived factor is a survival factor for cerebellar granule cells in culture. J Neurochem 1995;64:2509–17. [DOI] [PubMed] [Google Scholar]
- 11.Seigel GM, Tombran-Tink J, Becerra SP, et al. Differentiation of Y79 retinoblastoma cells with pigment epithelial-derived factor and interphotoreceptor matrix wash: effects on tumorigenicity. Growth Factors 1994;10:289–97. [DOI] [PubMed] [Google Scholar]
- 12.Cao W, Tombran-Tink J, Chen W, et al. Pigment epithelium-derived factor protects cultured retinal neurons against hydrogen peroxide-induced cell death. J Neurosci Res 1999;57:789–800. [PubMed] [Google Scholar]
- 13.Houenou LJ, D’Costa AP, Li L, et al. Pigment epithelium-derived factor promotes the survival and differentiation of developing spinal motor neurons. J Comp Neurol 1999;412:506–14. [PubMed] [Google Scholar]
- 14.Renno RZ, Youssri AI, Michaud N, et al. Expression of pigment epithelium-derived factor in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2002;43:1574–80. [PubMed] [Google Scholar]
- 15.Gao G, Li Y, Zhang D, Gee S, et al. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett 2001;489:270–6. [DOI] [PubMed] [Google Scholar]
- 16.Ogata N, Wada M, Otsuji T, et al. Expression of pigment epithelium-derived factor in normal adult rat eye and experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2002;43:1168–75. [PubMed] [Google Scholar]
- 17.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 1999;285:245–8. [DOI] [PubMed] [Google Scholar]
- 18.Sugita Y, Becerra SP, Chader GJ, et al. Pigment epithelium-derived factor (PEDF) has direct effects on the metabolism and proliferation of microglia and indirect effects on astrocytes. J Neurosci Res 1997;49:710–18. [DOI] [PubMed] [Google Scholar]
- 19.Kleiheus P, Burger PC, Scheithauer BW. Histological typing of tumours of the central nervous system, 2nd ed. London: Springer Verlag, 1993.
- 20.Heid CA, Stevens J, Livak KJ, et al. Real time quantitative PCR. Genome Res 1996;6:986–94. [DOI] [PubMed] [Google Scholar]
- 21.Mahaley MS, Jr, Mettlin C, Natarajan N, et al. National survey of patterns of care for brain-tumor patients. J Neurosurg 1989;71:826–36. [DOI] [PubMed] [Google Scholar]
- 22.Kirsch M, Santarius T, Black PM, et al. Therapeutic anti-angiogenesis for malignant brain tumors. Onkologie 2001;24:423–30. [DOI] [PubMed] [Google Scholar]
- 23.Eikesdal HP, Bjorkhaug ST, Dahl O. Hyperthermia exhibits anti-vascular activity in the s.c. BT4An rat glioma: lack of interaction with the angiogenesis inhibitor batimastat. Int J Hyperthermia 2002;18:141–52. [DOI] [PubMed] [Google Scholar]
- 24.Griscelli F, Li H, Cheong C, et al. Combined effects of radiotherapy and angiostatin gene therapy in glioma tumor model. Proc Natl Acad Sci U S A 2000;97:6698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzumura A, Sawada M, Yamamoto H, et al. Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. J Immunol 1993;151:2150–8. [PubMed] [Google Scholar]
- 26.Zenni GC, Ellinger J, Lam TM, et al. Biomaterial-induced macrophage activation and monokine release. J Invest Surg 1994;7:135–41. [DOI] [PubMed] [Google Scholar]
- 27.Volpert OV, Zaichuk T, Zhou W, et al. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med 2002;8:349–57. [DOI] [PubMed] [Google Scholar]
- 28.Tombran-Tink J, Johnson LV. Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest Ophthalmol Vis Sci 1989;30:1700–7. [PubMed] [Google Scholar]
- 29.Crawford SE, Stellmach V, Ranalli M, et al. Pigment epithelium-derived factor (PEDF) in neuroblastoma: a multifunctional mediator of Schwann cell antitumor activity. J Cell Sci 2001;114:4421–8. [DOI] [PubMed] [Google Scholar]
- 30.Araki T, Taniwaki T, Becerra SP, et al. Pigment epithelium-derived factor (PEDF) differentially protects immature but not mature cerebellar granule cells against apoptotic cell death. J Neurosci Res 1998;53:7–15. [DOI] [PubMed] [Google Scholar]
- 31.Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev 1997;13:37–50. [DOI] [PubMed] [Google Scholar]
- 32.Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci 1997;22:251–6. [DOI] [PubMed] [Google Scholar]