Abstract
Aim: Gastrointestinal stromal tumours (GISTs) are uncommon mesenchymal neoplasms. Some metastasise, whereas others remain asymptomatic for years, but it is difficult to distinguish between them histologically. This report analyses the characteristics of seven metastasising GISTs and compares clinicopathological parameters of the metastatic and non-metastatic groups.
Methods/Results: Histology revealed typical GIST features with spindle, epithelioid, or mixed appearance. All seven cases were positive for vimentin, five for neurone specific enolase, six for c-kit, four for S-100, three for PGP-9.5, three for CD-34 and synaptophysin, but all were negative for cytokeratin, neurofilament, chromogranin A, and desmin. Four showed a focal reaction for smooth muscle actin. Three of the tumours were GI, and two each were GII and GIII. The Ki-67 index varied from 4% to 44%, the three GI cases had 4%, 10%, and 16%. Tumours from the metastatic GIST group were significantly larger than those from the non-metastatic group.
Conclusions: Three cases exhibited bland, GI histological features with moderate or low proliferative activity. Among the c-kit positive metastasising stromal tumours, some were low grade, with moderate or low mitotic and Ki-67 indices, emphasising the necessity to develop a reliable grading system for GIST to predict clinical behaviour, the importance of careful analysis of “benign looking” tumours, and the key role of c-kit status in identifying patients who could benefit from treatment with STI-571. Larger tumours had a higher chance of metastasising, and only the size of the primary tumour played a role in predicting metastatic potential.
Keywords: gastrointestinal stromal tumour, metastatic gastrointestinal stromal tumour, grading, c-kit
When compared with epithelial tumours, mesenchymal tumours of the gastrointestinal tract are rare, with gastrointestinal stromal tumours (GISTs) being the most frequent. Possibly derived from the interstitial cells of Cajal of the autonomic nervous system, the tumour cells in GIST characteristically express c-kit (CD-117; a transmembrane tyrosine kinase receptor) and can be detected with an antibody that binds to the C-terminal domain of this molecule, making the diagnosis of this tumour easier than in the past.1–6 In contrast, estimating the possible clinical outcome and predicting the biological behaviour of these tumours remain uncertain. Since 1984, when a subtype of stromal tumours, the so called gastrointestinal autonomic nerve tumour was described, numerous macroscopical, histological, cell kinetic, and molecular parameters, including the size and localisation of the tumours, the Ki-67 and mitotic indices, DNA ploidy, the S phase fraction, and the presence of specific mutations, have been suspected and partly proved to have an impact on the prognosis of the disease.7–18 In spite of extensive research, to date, there is no reliable single predictive factor useful for routine histopathology. Various grading systems have been suggested, which have included histological features such as the phenotype of the tumour cells (epithelioid versus spindle) together with pleomorphism and the mitotic count; or the grade of pleiomorphism and the extent of necrosis together with the mitotic count. This has resulted in several different grading systems, but there is no widely accepted, consistently applied system yet in use.19–23
“In spite of extensive research, to date, there is no reliable single predictive factor useful for routine histopathology”
Recently, a new clinicopathological approach to assess the risk of aggressive behaviour was proposed by Fletcher et al.24 This scheme is based upon the assessment of the two parameters thought to be the most predictive and “simplest to determine”; namely: the mitotic count and the size of the tumour. This resulted in a four grade scale: very low, low, intermediate, and high risk of aggressive behaviour.
MATERIALS AND METHODS
Case selection
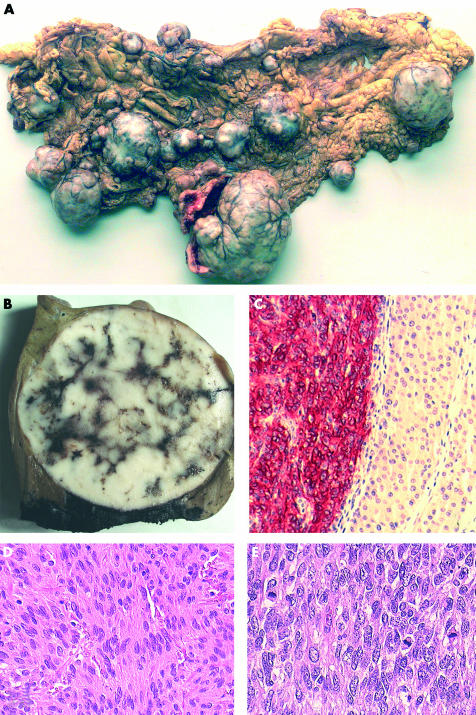
Twenty three gastrointestinal tumours of mesenchymal origin that had been reported in the past 10 years were selected for histopathological review from the archives of the department of pathology, Medical University, Pécs, Hungary. Immunohistochemical examination revealed muscle origin (leiomyosarcoma) in three of the cases showing synchronous c-kit negativity and diffuse, strong smooth muscle actin (SMA) and/or desmin positivity, therefore all have been excluded from the study. Only the cases with evidence of clinically malignant behaviour (metastatic and/or locally recurrent cases) were included for further, detailed examination. Of the seven cases that were analysed, four had multiple liver metastases, two metastasised to the regional lymph nodes, another metastasised to the peritoneal/retroperitoneal regions, and one had multiregional involvement including the omentum, peritoneal layer, mesentery, and retroperitoneum (fig 1A). One of the patients who had liver metastases (case 2) also had recurrent tumour four years after the first presentation and surgery (fig 1B,C). The age of the patients varied from 26 to 77 years (mean, 58). The male to female ratio was 5 : 2.
Figure 1.
(A) Multinodular and multiregional gastrointestinal stromal tumour. The nodules of different sizes are widespread in the omental fat tissue. (B) Macroscopical and (C) microscopical views of a resected liver metastastatic nodule from case 14. Strong c-kit (CD-117) positivity of the tumour cells is seen on the left (red staining), showing that this patient would have been a suitable candidate for treatment with STI-571 (Glivec). Immunohistochemical reaction for c-kit, original magnification, ×200. (D) Microscopical view of a low grade, monomorphic spindle cell tumour and (E) a high grade tumour. Note the differences in the mitotic counts and pleiomorphism of the cells. Haematoxylin and eosin staining; original magnification, ×200 and ×400, respectively.
Histology and immunohistochemistry
The tissue blocks were fixed in buffered formalin, routinely processed, cut, and stained according to the standard haematoxylin and eosin histological protocol. Selected blocks and 13 different antibodies were used to determine the immunophenotype of the tumours. Table 1 summarises the antibodies used. We used the recommended dilutions and microwave pretreatment (700 W, 3 × 5 minutes) in standard citrate buffer for all the antibodies. The slides were developed with the Vectastain universal ABC kit and 3-amino-9-ethylcarbazole was used as substrate.
Table 1.
Summary of the antibodies used
| Antibody/antigen | Type | Clone | Manufacturer |
| c-kit | Polyclonal | – | Santa Cruz Biotechnology |
| CD-34 | Monoclonal | QBEnd/10 | Novocastra |
| Desmin | Monoclonal | D33 | Dako |
| α Smooth muscle actin | Monoclonal | 1A4 | Dako |
| Vimentin | Monoclonal | v9 | Dako |
| Cytokeratin | Monoclonal | AE1-AE3 | Biogenex |
| Chromogranin A | Monoclonal | DAK-A3 | Dako |
| Synaptophysin | Monoclonal | SY38 | Dako |
| S-100 | Polyclonal | – | Dako |
| Neurone specific enolase | Monoclonal | BBS/NC/VI-H14 | Dako |
| PGP-9.5 | Polyclonal | – | Biogenesis |
| Neurofilament | Monoclonal | NRV 170 | Biogenex |
| Ki-67 | Monoclonal | mib-1 | Dako |
The Mib-1 index was determined by counting 1000 tumour cells in the fields showing the highest number of positive nuclei. All the positive cells were counted, regardless of the intensity of staining. The number of mitoses was counted in 30 consecutive high power fields (HPF; ×400 magnification using a Nikon Optiphot 2 microscope).
Statistical analysis
Statistical analysis (t test and χ2 test) was carried out using Microsoft Excel software. Significance was set at p < 0.05.
RESULTS
The primary tumours were between 35 and 140 mm in the largest diameter (mean, 83). Four of the seven tumours were solitary, and three were multinodular. Two of the tumours localised to the stomach, four to the small bowel, and as mentioned earlier one (case 6) showed multiregional involvement. The macroscopic details are summarised in the table 2.
Table 2.
Clinicopathological data of the analysed gastrointestinal stromal tumours
| Case | Age/sex | Localisation | Metastasis | Size (mm) | Type |
| 2 | 62/M | Small bowel | Liver | 55 | S |
| 3 | 61/M | Small bowel | Lymph node | 50 | S |
| 5 | 26/F | Stomach | Liver | 50* | MN |
| 6 | 61/M | Multiregional | Multiorgan | 115* | MN† |
| 14 | 50/M | Small bowel | Liver | 35 | S |
| 20 | 77/F | Stomach | Liver, lymph node | 140 | S |
| 22 | 67/M | Small bowel | Peri-retroperitoneal | 135 | MN‡ |
Size refers to the largest diameter of the tumour.
F, female; M, male; MN, multinodular tumour; S, solitary tumour.
*The largest nodule; †multiple involvement in the omentum, peritoneum, retroperitoneum, and mesentery; ‡multinodular small bowel tumour.
Histologically, two of the tumours had an epithelioid phenotype, four had a spindle cell phenotype, and one showed a mixed phenotype. The FNCLCC (French Federation of Cancer Centers) system (originally created for soft tissue sarcomas) and others, such as the schemes established by Newman et al and de Saint Aubain Somerhausen and Fletcher were used to grade the cases.19, 23 The FNCLCC system uses phenotypical and proliferative parameters, such as the mitotic count, the presence and grade of atypia, and necrosis, creating a scoring system with four categories: G0 (benign, score 3), GI (low grade, score 4–5), GII (intermediate grade, score 6–7), and GIII (high grade, score 8–10). The other two schemes, which are very similar to one another, discriminate three categories: benign, borderline, and malignant, combining the epithelioid/spindle cell features with atypia and the number of the mitoses.19, 20, 22, 23 According to the FNCLCC system, the cases were classified as three GI, two GII, and two GIII, but as seven malignant (M) tumours according to the other two systems (table 3; fig 1D,E). When a recently published new system, which is based upon the combination of mitotic count and tumour size, was used all of the cases were graded as high risk tumours.24 The mitotic index varied from eight in each 30 HPF to 171 in each 30 HPF, being eight, nine and 10 in each 30 HPF in the GI tumours, 30 and 15 in each 30 HPF in the GII tumours, and 45 and 171 in each 30 HPF in the GIII tumours (table 3). Immunohistochemically, all but one case showed moderate to strong reactivity for c-kit (CD-117). The negative case coexpressed synaptophysin, PGP-9.5, and S-100, thereby demonstrating its neurogenic origin. All the cases were positive for vimentin, five of five for neurone specific enolase, four of seven for S-100, three of five for PGP-9.5, and three of six for CD-34 and synaptophysin; they were all negative for chromogranin A, neurofilament, cytokeratin, and desmin. There was weak and focal reactivity for SMA in four of seven cases. The recurrent tumour seen in case 2 had the same phenotypical features as the original tumour, although the histological grade changed to GII, and immunophenotypically focal, weak SMA and diffuse, moderate S-100 and chromogranin A positivity appeared. The Ki-67 index varied from 4% to 44% (4%, 10%, and 16% in the GI tumours, 15% in both of the GII tumours, and 30% and 44% in the GIII tumours).
Table 3.
Histological parameters and grading according to the different systems
| Case | Phenotype | Mitosis | Atypia | Necrosis | Grade20 | Grade19 | Grade23 | Risk24 | Ki-67 |
| 2 | Spindle | 9/30 | + | – | GI (2+1+2) | M | M | H | 4% |
| 3 | Spindle | 171/30 | + | + | GIII (2+2+4) | M | M | H | 44% |
| 5 | Epithelioid | 8/30 | + | – | GI (2+1+2) | M | M | H | 10% |
| 6 | Epithelioid | 30/30 | + | – | GII (2+1+3) | M | M | H | 15% |
| 14 | Spindle | 10/30 | + | – | GI (2+1+2) | M | M | H | 16% |
| 20 | Spindle | 45/30 | + | + | GIII (2+2+4) | M | M | H | 30% |
| 22 | Mixed | 15/30 | + | + | GII (2+3+2) | M | M | H | 15% |
Mitosis refers to the number of mitoses in 30 high power fields (×400 magnification). Atypia: –, no atypia; +, atypia in less than 50% of the tumour cells. Necrosis: –, no necrosis; +, less than 50% of the tumour is necrotic. Grade20,19,23 : grading according to the FNCLCC system; GI, GII, and GIII corresponds to grade I, II, and III (scores for atypia, necrosis, and mitosis, respectively)20,22. Grading according to Newman and colleagues19 and de Saint Aubain Somerhausen and colleagues23 : M, malignant. Risk assessment according to Fletcher and colleagues24 : H, high risk of malignant behaviour. Ki-67 represents the number of positive nuclei/1000 cells expressed as a percentage.
Twelve of the 23 gastrointestinal tumours of mesenchymal origin were non-metastasising GISTs. Seven localised to the stomach, four to the small intestine, and one to the large intestine. The size of the tumours varied from 10 to 135 mm (mean, 45). Ten of the 12 cases were solitary and the remaining two were multinodular. Nine had a spindle cell phenotype and two were mixed tumours. The mitotic index varied from 0 in each 30 HPF to 48 in each 30 HPF, and the Ki-67 index varied from 0% to 45%. All the tumours showed histological signs of malignancy: eight were GI, three were GII, and one was GIII. The different clinicopathological variables, such as the size of the tumour, the multinodular/solitary appearance, the localisation, the mitotic and Ki-67 indices, the phenotype, and the age of the patient were compared and analysed in the two (metastatic and non-metastatic) groups. Using the t test, there were no significant differences in the mitotic and Ki-67 indices (p = 0.08 and p = 0.09, respectively) or the age of the patients (p = 0.16) between the two groups, but tumour size was significantly greater in the metastatic group compared with the non-metastatic group (p = 0.05). According to the χ2 test, there were no significant differences in the occurrence of multinodular tumours (p = 0.21), the localisation (p = 0.46), the phenotype (p = 0.14), or the histological grade (p = 0.45) between the metastatic and non-metastatic groups.
DISCUSSION
GISTs account for only a small proportion of all gastrointestinal tumours, but make up most of the mesenchymal group.4, 23 In addition to their rare occurrence, predicting their possible biological behaviour poses a major problem. Large size, intestinal localisation, high mitotic activity and Ki-67 index, aneuploidy of the tumour cells, point mutations or deletions in the c-kit gene, and high histological grade are the major indicators of poor prognosis.9–17, 19, 20, 22, 25 Three of our cases were low grade (GI histomorphology), with low or moderate mitotic activity (eight, nine, and 10 mitoses in each 30 HPF) and Ki-67 index (4%, 10%, and 16%). The remaining cases were intermediate or high grade tumours and had higher proliferative parameters (15–171 mitoses in each 30 HPF, 15–44% Ki-67 index) with an obvious malignant phenotype. The mitotic index is thought to be of more value than the Ki-67 index in discriminating between low and high grade cases. However, we found that it was not only those tumours with a high mitotic count and obvious high grade features that behave in a malignant manner, but that low grade neoplasms are also capable of metastasising.
The aim in most malignancies is to find one or more prognostic clinicopathological factor that can aid in predicting the biological behaviour or metastatic capability of the tumour, and this is the case with GISTs. In our study, several clinicopathological variables were analysed and compared between the metastatic and non-metastatic groups of stromal tumours, but only the size of the primary tumour showed a significant difference (p = 0.05) between the two groups. This means that bigger tumours have a greater chance of disseminating or forming metastases, which fits in with the every day experience of malignant tumours in general. Although the difference between the groups was significant, the results should be interpreted with caution because the number of cases investigated was small.
“We found that it was not only those tumours with a high mitotic count and obvious high grade features that behave in a malignant manner, but that low grade neoplasms are also capable of metastasising”
Recently, Joensuu et al used the tyrosine kinase inhibitor, STI-571 (Glivec), in a patient with a metastatic GIST and saw a significant reduction in the mass of this otherwise chemoresistant metastatic tumour.26 In another recent phase I study, STI-571 was used successfully (25 of 36 patients had objective tumour regression) and safely with only minor and manageable side effects.27 These successes have intensified the research in this area and increased the number of clinical trials on c-kit positive GISTs. With regard to this therapeutic possibility, it is important that among the metastatic cases in our study the frequency of low grade, c-kit positive tumours with bland histological features was relatively high. Thus, we stress the importance of a careful histological analysis, the necessity of a widely accepted standard grading or risk assessment system (the Fletcher risk assessment system seems to be good at predicting metastatic potiential because all of our cases were of “high” risk tumours), and the importance of clinical follow up (including the regular checking of the liver status) even in “benign looking” GISTs, because these cases are potential candidates for this promising new treatment either in adjuvant, neoadjuvant, or palliative settings. Apart from this, a reliable grading system that would help in predicting the biological behaviour of these tumours would help to avoid overtreatment in the future.
Take home messages .
Some of the c-kit positive metastasising gastrointestinal stromal tumours (GISTs) in this study were of low grade, with moderate or low mitotic and Ki-67 indices
Thus, a reliable grading system is needed for GIST to predict clinical behaviour and even “benign looking” tumours should undergo a careful histological analysis
The c-kit status plays a key role in identifying those patients who could benefit from treatment with STI-571
Larger tumours had a higher chance of metastasising, and the size of the primary tumour was the only factor that was found to predict metastatic potential
Acknowledgments
The authors wish to thank Novartis Hungária Kft. Pharma for technical assistance.
Abbreviations
FNCLCC, French Federation of Cancer Centers
GIST, gastrointestinal stromal tumour
HPF, high power fields
SMA, smooth muscle actin
REFERENCES
- 1.Hurlimann J, Gardiol D. Gastrointestinal stromal tumours: an immunohistochemical study of 165 cases. Histopathology 1991;19:311–20. [DOI] [PubMed] [Google Scholar]
- 2.Seidal T, Edvardsson H. Expression of c-kit (CD-117) and Ki-67 provides information about the possible cell of origin and clinical course of gastrointestinal stromal tumours. Histopathology 1999;34:416–24. [DOI] [PubMed] [Google Scholar]
- 3.Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT). Gastrointestinal stromal tumors show phenotypic characteristics of interstitial cells of Cajal. Am J Pathol 1998;152:1259–69. [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JKC. Mesenchymal tumors of the gastrointestinal tract: a paradise for acronyms (STUMP, GIST, GANT and now GIPACT), implication of c-kit in genesis and yet another of many emerging roles for the interstitial cell of Cajal in the pathogenesis of gastrointestinal diseases. Adv Anat Pathol 1999;6:19–40. [DOI] [PubMed] [Google Scholar]
- 5.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577–80. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M, Lasota J. Gastrointestinal stromal tumors—definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001;438:1–12. [DOI] [PubMed] [Google Scholar]
- 7.Herrera GA, Moraes HP, Grizzle WE, et al. Malignant small bowel neoplasm of enteric plexus derivation (plexosarcoma). Dig Dis Sci 1984;29:275–84. [DOI] [PubMed] [Google Scholar]
- 8.Herrera GA, Cerezo L, Jones JE, et al. Gastointestinal autonomic nerve tumours. “Plexosarcomas”. Arch Pathol Lab Med 1989;113:846–53. [PubMed] [Google Scholar]
- 9.Franquemont DW. Differentiation and risk assessment of gastrointestinal stromal tumours. Am J Clin Pathol 1995;103:41–7. [DOI] [PubMed] [Google Scholar]
- 10.Carillo R, Candia A, Rodriguez-Peralto JL, et al. Prognostic significance of DNA ploidy and proliferative index (MIB-1 index) in gastrointestinal stromal tumours. Hum Pathol 1997;28:160–5. [DOI] [PubMed] [Google Scholar]
- 11.Cooper PN, Quirke BM, Hardy GJ, et al. A flow cytometric, clinical, and histological study of stromal neoplasms of the gastrointestinal tract. Am J Surg Pathol 1992;16:163–70. [DOI] [PubMed] [Google Scholar]
- 12.Franquemont DW, Frierson HF, Jr. Proliferating cell nuclear antigen immunoreactivity and prognosis of gastrointestinal stromal tumours. Mod Pathol 1995;8:473–7. [PubMed] [Google Scholar]
- 13.Franquemont DW, Geary WA. Gastrointestinal stromal tumours and proliferating cell nuclear antigen. Prognostic challenges. Am J Clin Pathol 1993;100:369–70. [DOI] [PubMed] [Google Scholar]
- 14.Yu CCW, Fletcher CDM, Newman PL, et al. A comparison of proliferating nuclear antigen (PCNA) immunostaining, nucleolar organizer region (AgNOR) staining, and histological grading in gastrointestinal stromal tumours. J Pathol 1992;166:147–52. [DOI] [PubMed] [Google Scholar]
- 15.Lasota J, Jasinski M, Sarlomo-Rikala M, et al. Mutations in exon 11 of c-kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol 1999;154:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst SI, Hubbs AE, Przygodzki RM, et al. Kit mutation portends poor prognosis in gastrointestinal stromal/smooth muscle tumors. Lab Invest 1998;78:1633–6. [PubMed] [Google Scholar]
- 17.Tornóczky T, Kálmán E, Hegedus G, et al. High mitotic index associated with poor prognosis in gastrointestinal autonomic nerve tumour. Histopathology 1999;35:121–8. [DOI] [PubMed] [Google Scholar]
- 18.Amin MB, Ma CK, Linden MD, et al. Prognostic value of proliferating cell nuclear antigen index in gastric stromal tumors. Correlation with mitotic count and clinical outcome. Am J Clin Pathol 1993;100:428–32. [DOI] [PubMed] [Google Scholar]
- 19.Newman PL, Wadden C, Fletcher CDM. Gastointestinal stomal tumours: correlation of immunophenotype with clinicopathological features. J Pathol 1991;164:107–17. [DOI] [PubMed] [Google Scholar]
- 20.Rudolph P, Gloeckner K, Parwaresch R, et al. Immunophenotype, proliferation, DNA ploidy, and biological behaviour of gastrointestinal stromal tumors: a multivariate clinicopathologic study. Hum Pathol 1998;29:791–800. [DOI] [PubMed] [Google Scholar]
- 21.Berman JJ, O’Leary TJ. Gastrointestinal stromal tumor workshop. Hum Pathol 2001;32:578–82. [DOI] [PubMed] [Google Scholar]
- 22.Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcoma of adults: study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984;33:37–42. [DOI] [PubMed] [Google Scholar]
- 23.de Saint Aubain Somerhausen N, Fletcher CDM. Gastrointestinal stromal tumours: an update. Sarcoma 1998;2:131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fletcher CDM, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002;33:459–65. [DOI] [PubMed] [Google Scholar]
- 25.van Roggen GJF, van Velthuysen MLF, Hogendoorn PCW. The pathological differential diagnosis of gastrointestinal stromal tumours. J Clin Pathol 2001;54:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joensuu H, Robers PJ, Sarlomo-Rikala M, et al. Brief report: effect of the tyrosine kinase inhibitor STI-571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001;344:1052–6. [DOI] [PubMed] [Google Scholar]
- 27.van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 2001;358:1421–3. [DOI] [PubMed] [Google Scholar]