Abstract
Background: The biological processes involved in the development of gastric mucosal atrophy and intestinal metaplasia are still incompletely understood. Reports testing the hypothesis that apoptosis leads to atrophy have yielded conflicting results. The availability of new antibodies for the detection of apoptotic cells in tissue sections has facilitated the analysis of the role of apoptosis in the gastritis–atrophy–intestinal metaplasia sequence.
Methods: Archival material from 40 gastric resection specimens with normal mucosa (n = 5), chronic active gastritis (n = 17), or intestinal metaplasia (n = 18) was studied. Immunohistochemistry was performed using antibodies directed against cleaved cytokeratin 18 and active caspase 3. Slides were scored on a 0–3 scale for the presence of apoptotic cells.
Results: Normal gastric mucosa contained low numbers of apoptotic cells at the surface epithelium (mean score, 0.20). This number was significantly increased in cases with chronic gastritis (mean score, 1.06) and in those with intestinal metaplasia (mean score, 2.56). Within the intestinal metaplasia cases, 44 different foci of intestinal metaplasia were identified. In 39 of these 44 areas, concentrations of apoptotic cells were seen immediately adjacent to the foci of intestinal metaplasia, but not in the metaplastic epithelium itself.
Conclusions: Apoptosis is uncommon in normal gastric mucosa. Chronic inflammation and intestinal metaplasia are associated with increased apoptosis, but occur mainly at the mucosal surface and not in the deeper layers. These findings do not support the concept that apoptosis underlies the loss of gastric glands and leads to atrophy, but the observed concentration of apoptotic epithelial cells adjacent to foci of intestinal metaplasia could be related to heterogeneity of epithelial damage, causing apoptosis, to which intestinal metaplasia is a response.
Keywords: apoptosis, atrophic gastritis, cytokeratin 18, intestinal metaplasia
Colonisation of the human stomach with Helicobacter pylori almost invariably induces chronic active gastritis, a condition that predisposes to the development of gastric mucosal atrophy,1 which is thought to be an important precursor of gastric adenocarcinoma. So far, the biological processes involved in the progression from H pylori induced gastritis to glandular atrophy have not been completely elucidated. One of the proposed mechanisms playing a role in this process is an increased apoptosis to proliferation ratio. In normal gastric mucosa, proliferating stem cells are located in the neck region. New cells migrate either upwards, differentiating into foveolar epithelial cells, or downwards, differentiating into glandular cells. After a certain period, both cell types undergo programmed cell death at the surface epithelium or in the gland. It has been hypothesised that colonisation with H pylori leads to a significant increase in the proportion of cells undergoing apoptosis and thus to a change in the balance between proliferation and cell death. Indeed, some authors have reported an increased number of apoptotic cells in H pylori colonised gastric mucosa compared with normal mucosa.2,3 However, these studies were performed by means of the terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL) assay, which has the disadvantage that the results are influenced by the type and duration of fixation.4–7 This might explain some of the previous reports of very high proportions of apoptotic cells in H pylori colonised gastric mucosa.3
“It has been hypothesised that colonisation with Helicobacter pylori leads to a significant increase in the proportion of cells undergoing apoptosis and thus to a change in the balance between proliferation and cell death”
Recently, new antibodies directed against active caspase 3 and cleaved cytokeratin 18 have become available,8,9 enabling the determination of apoptotic cells with a higher sensitivity and specificity than is possible using the TUNEL method. The aim of our present study was to evaluate the role of apoptosis in the development of gastric mucosal atrophy and intestinal metaplasia using these new antibodies.
MATERIALS
Using tissue sections from 40 gastric resection specimens, samples with normal gastric mucosa (n = 5), mild to severe chronic active gastritis (n = 17), and foci of intestinal metaplasia (n = 18) localised in a surrounding of chronic active gastritis were analysed (table 1). Thirty six samples were corpus mucosa, three were antral mucosa, and one was from the cardia. In all cases, (partial) gastrectomy had been performed because of cancer. In addition, 10 samples of gastric adenocarcinoma and three biopsy specimens with normal colorectal mucosa were used as positive controls for M30 CytoDeath (antibody directed against cleaved cytokeratin 18) and active caspase 3 staining.10 Germinal centres of lymphoid follicles served as positive controls for active caspase 3 and negative controls for M30 CytoDeath. All specimens were formaldehyde fixed and paraffin wax embedded, and had been collected from the archives of the department of pathology, VU University Medical Center, Amsterdam, The Netherlands.
Table 1.
Overview of cases included in our study and mean immunoscores for cleaved cytokeratin 18 in normal mucosa, chronic gastritis, and intestinal metaplasia
| Type of lesion | N (slides/areas) | Mean M30 score (range) |
| Normal gastric mucosa | 5/5 | 0.20 (0–1) |
| Chronic gastritis | 17/27 | 1.06 (0–3) |
| Intestinal metaplasia | 18/44 | 2.56 (1–3) |
M30 score = cleaved cytokeratin 18 score.
METHODS
Serial sections (4 μm thick) were cut and stained using a standard three step streptavidin–biotin complex method. First, slides were dewaxed for 3 × 10 minutes in xylol and rehydrated in 100%, 96%, and 70% ethanol and water. Endogenous peroxidase was blocked for 30 minutes in methanol with 0.3% H2O2. After rinsing in water, antigen retrieval was achieved by microwaving for 10 minutes at 360 W in citrate buffer (10 mmol/litre, pH 6.0). Slides were rinsed in phosphate buffered saline (PBS).
For the detection of cleaved cytokeratin 18, slides were preincubated with normal rabbit serum (Dako, Glostrup, Denmark; 1/50 dilution) for 10 minutes and then incubated for 60 minutes with the mouse monoclonal antibody M30 CytoDeath (Roche, Mannheim, Germany; 1/100 dilution). After rinsing in PBS, slides were incubated with biotin labelled rabbit antimouse antibody (Dako; 1/500 dilution) for 30 minutes, again rinsed in PBS, and incubated with streptavidin–biotin complex (Dako; 1/200 dilution) for 60 minutes.
For the detection of active caspase 3, slides were preincubated with normal swine serum (Dako; 1/10 dilution) for 10 minutes and then incubated with the rabbit monoclonal anti-active caspase 3 antibody (BD PharMingen, San Diego, USA; 1/400 dilution) for 60 minutes. After rinsing in PBS, slides were incubated with biotin labelled swine antirabbit antibody (Dako; 1/300 dilution) for 30 minutes. Next, the slides were incubated in streptavidin–biotin complex (Dako; 1/1000 dilution) for 30 minutes, rinsed in PBS, followed by incubation with biotinylated tyramine for 10 minutes, rinsed in PBS again, and incubated with streptavidin–biotin complex (Dako; 1/200 dilution).
After rinsing in PBS, peroxidase was developed with the DAB (diaminobenzidine) chromogen for three minutes. After rinsing in water, slides were counterstained with haematoxylin for 30 seconds.
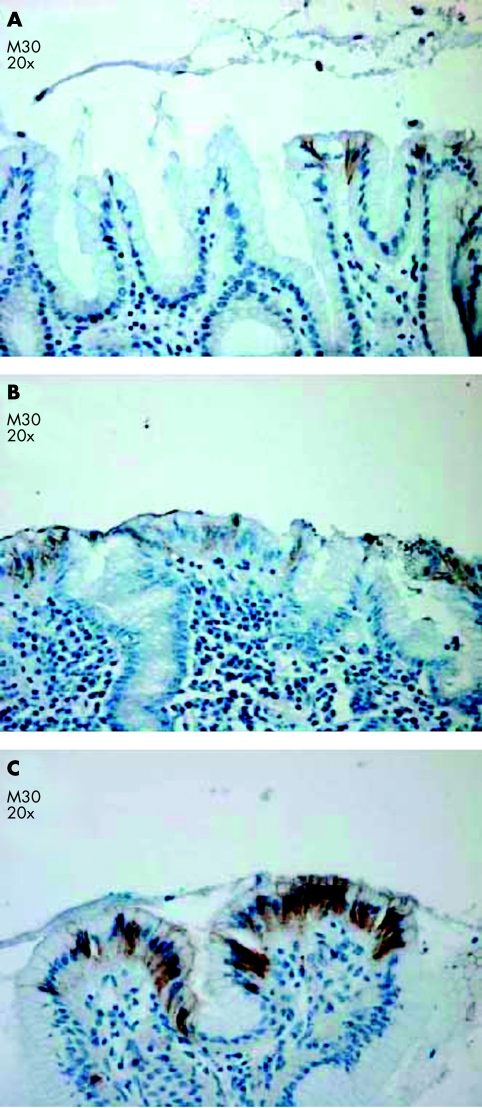
Staining was evaluated by light microscopy and the number of positive cells was assessed by two observers (NvG, GA), who discussed the case until they reached consensus. The samples were scored on a 0–3 scale, as follows: 0, no positive cells; 1, single positive cells; 2, small groups of positive cells; and 3, large areas of adjacent positive cells. Figure 1 shows examples of these scores.
Figure 1.
Examples of gastric tissue sections stained for M30 CytoDeath immunoscored as (A) 1, (B) 2, and (C) 3.
RESULTS
In all control specimens, a specific staining pattern was seen for both the M30 CytoDeath and active caspase 3 antibodies. M30 CytoDeath and anti-active caspase 3 typically produced a cytoplasmic staining pattern with highest intensity along the cell membrane. On serial sections, M30 CytoDeath and anti-active caspase 3 colocalised. When present, lymph follicles showed high numbers of cells positive for active caspase 3, but not for M30 CytoDeath, which is compatible with the fact that cytokeratin 18 is only present in epithelial cells and not in lymphocytes. In gastric adenocarcinoma samples, morphologically well recognisable apoptotic cells showed positive staining with both antibodies. In biopsy specimens of the colon, positive staining was seen in the surface epithelium of normal colorectal mucosa, as was expected.
First, M30 CytoDeath and anti-active caspase 3 positivity was scored in five samples with normal gastric mucosa, 17 with chronic active gastritis, and 18 with intestinal metaplasia. In normal gastric mucosa, low numbers of immunopositive cells for both cleaved cytokeratin 18 and active caspase 3 were seen (mean score for the number of positive cells, 0.20; range, 0–1) (fig 2). These cells were almost exclusively seen at the surface epithelium (fig 3A) with a patchy distribution. No positive cells were seen in the foveola and glandular layer.
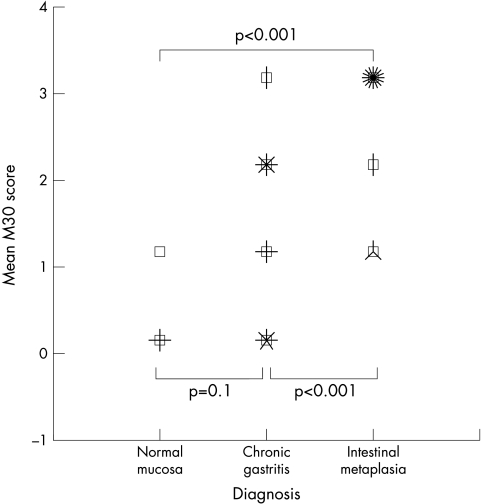
Figure 2.
Scatter (sunflower) plot showing M30 CytoDeath immunopositivity scores (0–3) for normal mucosa, chronic gastritis, and intestinal metaplasia, with each petal of a sunflower representing one case.
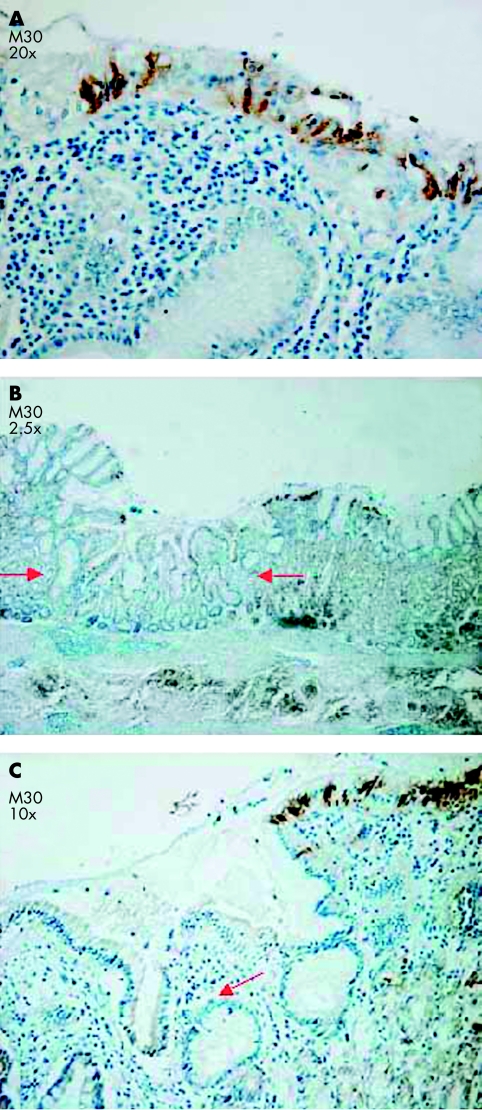
Figure 3.
(A) Apoptotic epithelial cells were detected at the gastric surface mucosa with M30 CytoDeath. (B) Apoptotic cells were found at the transitional margins between intestinal metaplasia (arrows) and gastritis with M30 CytoDeath. (C) The same area shown in (B) but at a higher magnification (×10).
In comparison with normal gastric mucosa, significantly higher numbers of M30 CytoDeath and active caspase 3 scores were seen in cases with chronic active gastritis (mean score for the number of positive cells, 1.06; range, 0–3; p < 0.001) and intestinal metaplasia (mean score for the number of positive cells, 2.56; range, 1–3; p < 0.001). Positive cells were found almost exclusively in the foveolar layer and the surface epithelium. M30 CytoDeath and active caspase 3 positivity in the glandular layer was rare. In only two samples, a few apoptotic epithelial cells could be detected in the glandular layer of the gastric mucosa.
Next, the relation between apoptosis and intestinal metaplasia was studied in more detail. Within the intestinal metaplasia cases, 44 different foci of intestinal metaplasia were identified, and in 39 of these cases increased concentrations of apoptotic cells were seen in the foveolar and surface epithelium, immediately adjacent to these foci of intestinal metaplasia (fig 3B,C). Only two of these 39 foci contained apoptotic cells within the intestinal metaplastic epithelium itself.
DISCUSSION
Helicobacter pylori induced gastritis is strongly associated with the development of gastric mucosal atrophy and intestinal metaplasia,1 conditions that are thought to increase the risk for the development of gastric adenocarcinoma. It has been hypothesised that H pylori infection disturbs the balance between cell proliferation and apoptosis, in particular by inducing apoptosis. Indeed, increased numbers of apoptotic cells of up to 16.8% have been reported in H pylori infected gastric mucosa, compared with 1–3% in normal mucosa.2,3 However, others reported that the number of apoptotic cells in H pylori gastritis was within normal limits.11–13 These studies were based on the use of a TUNEL assay, and the discrepancies between these studies may be explained, at least in part, by the limited specificity and sensitivity of this method. The TUNEL assay has been shown to be very sensitive to fixation. Furthermore, pretreatment of the tissue induces general labelling of normal cells.4,5,14 With the new immunohistochemical antibodies—M30 CytoDeath, which is directed against cleaved cytokeratin 18, and anti-active caspase 3,9 directed against a key enzyme in the final common pathway of apoptosis—it is now possible to study apoptosis reliably in relation to tissue morphology in H pylori gastritis.14 Cytokeratin 18 is present in several types of epithelial cells and is cleaved during apoptosis, and as such M30 CytoDeath positivity can be regarded as a functional read out of apoptosis in tissue sections. In our present study, in non-neoplastic gastric mucosa, both active caspase 3 and M30 CytoDeath microscopically showed very specific positive staining in the cytoplasm of epithelial cells in which the nucleus, on haematoxylin and eosin staining, did not yet show a typical apoptotic phenotype. In addition, immunopositive staining was found at the surface of both colorectal and gastric mucosa, where it would be expected considering the normal physiology of these tissues. Furthermore, M30 CytoDeath and active caspase 3 colocalised in almost all cases. In lymph follicles in the gastric mucosa, a subset of lymphocytes showed positivity for active caspase 3, but not for M30 CytoDeath, as was expected, because cytokeratin 18 is not present in lymphocytes. In addition, morphologically recognisable apoptotic cells in tissue sections with gastric adenocarcinoma also showed clear staining with M30 CytoDeath and anti-active caspase 3. These observations provide further evidence of the reliability of these antibodies as histological markers of apoptosis. In theory, H pylori may induce apoptosis either directly or indirectly via inflammatory factors, such as cytokines, released during H pylori induced chronic gastritis. This would result in caspase 9 mediated, stress induced apoptosis. Alternatively, the caspase 8 mediated pathway may be activated via induction of a cytotoxic T cell response.2,15–20 The fact that in our present study apoptosis was seen mainly at the surface of the gastric mucosa favours stress induced apoptosis, rather than T cell mediated apoptosis. In both cases, a cascade of caspases is cleaved and thus activated, with caspase 3 as a common final pathway. Caspase 3 finally cleaves target proteins, such as structural proteins in the cytoplasm, including cytokeratins, or proteins involved in homeostasis and repair mechanisms in the nucleus.8
“If an increased apoptotic rate does play a role in the development of glandular atrophy, an increased number of M30 CytoDeath and active caspase 3 positive cells would be expected in the glandular layer and/or the neck region of the gastric mucosa”
In a subset of H pylori infected subjects, longterm chronic gastritis will finally lead to mucosal atrophy; that is, the loss of specific glandular epithelium. It has been hypothesised that apoptosis of epithelial cells is the cause of this atrophy. However, this concept could not unambiguously be confirmed by our present data. First, the high numbers of apoptotic cells in the gastric mucosa previously reported were not confirmed by our findings.2,3 Second, in our present study, apoptotic cells were almost always located in the surface epithelium of the gastric mucosa. If an increased apoptotic rate does play a role in the development of glandular atrophy, an increased number of M30 CytoDeath and active caspase 3 positive cells would be expected in the glandular layer and/or the neck region of the gastric mucosa. Similar to uninfected surface epithelium, apoptotic cells were found only rarely in the glandular layer of H pylori colonised gastric mucosa, suggesting that H pylori colonisation and the resulting gastritis do not result in increased apoptosis in the glandular layer and the neck region.
A remarkable finding was the observation that concentrations of immunopositive cells occurred in the foveolar epithelium immediately adjacent to foci of intestinal metaplasia, whereas in the metaplastic epithelium itself, apoptotic cells were seen in only two foci. Most of the 44 foci of intestinal metaplasia that we studied did not show apoptotic cells, whereas the adjacent epithelium did contain these cells (fig 3B,C). This distribution pattern has been described previously with the TUNEL assay,21 and a similar staining pattern was described for Fas (CD95) expression in Barrett’s metaplasia.22
Intestinal trefoil factors (TFF1, TFF2, and TFF3), which may be involved in the development of intestinal metaplasia,23 are also thought to prevent apoptosis at sites of mucosal injury (for example, peptic ulcer disease, inflammatory bowel disease).24,25 Therefore, a possible mechanism could be that H pylori induces an increased apoptotic rate in the gastric surface epithelium, which is a major threat for the integrity of the gastric mucosa. In response to this, the upregulation of trefoil factors would cause both a reduction of apoptosis of epithelial cells and a change in epithelial differentiation towards intestinal metaplasia. As a result, the gastric mucosal epithelium would be better able to resist the environmental pressure leading to apoptosis and its integrity would be better preserved. However, in the long run, this metaplasia would result in an increased risk of cancer.26,27 Because intestinal metaplasia is patchy and apoptosis appears to concentrate around these patches, it seems that this apoptotic pressure is heterogeneous throughout the gastric epithelium. However, our present data are also consistent with a mechanism in which intestinal metaplasia is not preceded by mucosal atrophy, as is proposed by Correa et al, but occurs directly in response to the mucosal damage that induces programmed cell death.28,29
In conclusion, apoptosis is uncommon in normal gastric mucosa. Chronic inflammation and intestinal metaplasia are associated with increased apoptosis at the mucosal surface, but not in the deeper layers. These findings do not support a role for apoptosis in the development of atrophy. The observed concentration of apoptotic epithelial cells adjacent to foci of intestinal metaplasia could be related to heterogeneously distributed epithelial damage, to which intestinal metaplasia is a response.
Take home messages.
Apoptosis occurs only rarely in normal gastric mucosa
Apoptosis is increased in chronic inflammation and intestinal metaplasia, although it occurs mainly at the mucosal surface and not in the deeper, glandular layers
Thus, apoptosis does not appear to underlie the loss of gastric glands that leads to atrophy
However, high numbers of apoptotic epithelial cells were found adjacent to foci of intestinal metaplasia and could be the result of epithelial damage causing apoptosis, to which intestinal metaplasia is a response
Abbreviations
PBS, phosphate buffered saline
TUNEL, terminal deoxynucleotidyl transferase mediated dUTP nick end labelling
REFERENCES
- 1.Kuipers EJ, Uyterlinde AM, Peña AS, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet 1995;345:1525–8. [DOI] [PubMed] [Google Scholar]
- 2.Rudi J, Kuck D, Strand S, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Invest 1998;102:1506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moss SF, Calam J, Agarwal B, et al. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut 1996;38:498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willingham MC. Cytochemical methods for the detection of apoptosis. J Histochem Cytochem 1999;47:1101–10. [DOI] [PubMed] [Google Scholar]
- 5.Labat-Moleur F, Guillermet C, Lorimier P, et al. TUNEL apoptotic cell detection in tissue sections: critical evaluation and improvement. J Histochem Cytochem 1998;46:327–34. [DOI] [PubMed] [Google Scholar]
- 6.Cervos-Navarro J, Schubert TE. Pitfalls in the evaluation of apoptosis using TUNEL. Brain Pathol 1996;6:347–48. [PubMed] [Google Scholar]
- 7.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, et al. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 1995;21:1465–8. [DOI] [PubMed] [Google Scholar]
- 8.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998;281:1312–16. [DOI] [PubMed] [Google Scholar]
- 9.Leers MP, Kolgen W, Bjorklund V, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol 1999;187:567–72. [DOI] [PubMed] [Google Scholar]
- 10.Backus HH, Van Groeningen CJ, Vos W, et al. Differential expression of cell cycle and apoptosis related proteins in colorectal mucosa, primary colon tumours, and liver metastases. J Clin Pathol 2002;55:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahm KB, Lee KJ, Choi SY, et al. Possibility of chemoprevention by the eradication of Helicobacter pylori: oxidative DNA damage and apoptosis in H. pylori infection. Am J Gastroenterol 1997;92:1853–7. [PubMed] [Google Scholar]
- 12.Mannick EE, Bravo LE, Zarama G, et al. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res 1996;56:3238–43. [PubMed] [Google Scholar]
- 13.Yoshimura T, Shimoyama T, Tanaka M, et al. Gastric mucosal inflammation and epithelial cell turnover are associated with gastric cancer in patients with Helicobacter pylori infection. J Clin Pathol 2000;53:532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr NJ. M30 expression demonstrates apoptotic cells, correlates with in situ end-labeling, and is associated with Ki-67 expression in large intestinal neoplasms. Arch Pathol Lab Med 2000;124:1768–72. [DOI] [PubMed] [Google Scholar]
- 15.Houghton J, Korah RM, Condon MR, et al. Apoptosis in Helicobacter pylori-associated gastric and duodenal ulcer disease is mediated via the Fas antigen pathway. Dig Dis Sci 1999;44:465–78. [DOI] [PubMed] [Google Scholar]
- 16.Smoot DT. How does Helicobacter pylori cause mucosal damage? Direct mechanisms. Gastroenterology 1997;113:S31–4. [DOI] [PubMed] [Google Scholar]
- 17.Ernst PB, Crowe SE, Reyes VE. How does Helicobacter pylori cause mucosal damage? The inflammatory response. Gastroenterology 1997;113:S35–42. [DOI] [PubMed] [Google Scholar]
- 18.Ishihara S, Fukuda R, Kawashima K, et al. T cell-mediated cytotoxicity via Fas/Fas ligand signaling in Helicobacter pylori-infected gastric corpus. Helicobacter 2001;6:283–93. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree JE, Shallcross TM, Heatley RV, et al. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut 1991;32:1473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata J, Goto H, Arisawa T, et al. Regulation of tumour necrosis factor (TNF) induced apoptosis by soluble TNF receptors in Helicobacter pylori infection. Gut 1999;45:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scotiniotis IA, Rokkas T, Furth EE, et al. Altered gastric epithelial cell kinetics in Helicobacter pylori-associated intestinal metaplasia: implications for gastric carcinogenesis. Int J Cancer 2000;85:192–200. [PubMed] [Google Scholar]
- 22.Younes M, Lechago J, Ertan A, et al. Decreased expression of Fas (CD95/APO1) associated with goblet cell metaplasia in Barrett’s esophagus. Hum Pathol 2000;31:434–8. [DOI] [PubMed] [Google Scholar]
- 23.Taupin D, Pedersen J, Familari M, et al. Augmented intestinal trefoil factor (TFF3) and loss of pS2 (TFF1) expression precedes metaplastic differentiation of gastric epithelium. Lab Invest 2001;81:397–408. [DOI] [PubMed] [Google Scholar]
- 24.Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci U S A 2000;97:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong WM, Poulsom R, Wright NA. Trefoil peptides. Gut 1999;44:890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiting JL, Sigurdsson A, Rowlands DC, et al. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut 2002;50:378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu MS, Shun CT, Lee WC, et al. Gastric cancer risk in relation to Helicobacter pylori infection and subtypes of intestinal metaplasia. Br J Cancer 1998;78:125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res 1992;52:6735–40. [PubMed] [Google Scholar]
- 29.Meining A, Morgner A, Miehlke S, et al. Atrophy–metaplasia–dysplasia–carcinoma sequence in the stomach: a reality or merely an hypothesis? Best Pract Res Clin Gastroenterol 2001;15:983–98. [DOI] [PubMed] [Google Scholar]