Abstract
Background/Aims: Recent reports have suggested that Helicobacter pylori infection induces the mucosal antibiotic peptide human β defensin 2 (HBD-2). Therefore, the present study investigated mRNA and peptide expression of four different defensins in the upper gastrointestinal tract in patients with H pylori positive and negative chronic gastritis.
Materials/Methods: Biopsies from the oesophagus to the duodenum were taken during routine gastroscopy in 71 individuals. Total RNA was extracted and the reverse transcription polymerase chain reaction was performed with primers for human defensins 5 and 6 (HD-5/6) or HBD-1 and HBD-2. Paraffin wax embedded tissue from gastric resections was tested for HD-5, HBD-1, and HBD-2 by immunohistochemistry.
Results: Helicobacter pylori colonisation was associated with an increased percentage of positive biopsies with respect to HBD-2 in the corpus (p < 0.05). Helicobacter pylori had no impact on the gastric expression of HD-5 and HBD-1, whereas HD-6 was increased in the fundus. The abundant expression of α defensins in the duodenum and β defensins in the oesophagus served as a positive control in each individual. Immunohistochemical analysis confirmed the presence of the HD-5, HBD-1, and HBD-2 peptides in gastric resection specimens.
Conclusions: The recently described induction of HBD-2 upon H pylori infection was confirmed in a clinical setting of chronic gastritis. This phenomenon may be mediated by components of the pathogen itself or may occur secondary to immune events in chronic inflammation.
Keywords: antimicrobial peptide, defensins, Helicobacter pylori, gastritis
The Helicobacter pylori organism plays a key role in the pathogenesis of peptic ulcer disease. Although immunological responses such as leucocyte recruitment, interleukin 8 secretion,1 and nitric oxide production2 take place, they are unable to eradicate the pathogen. Defence mechanisms include a non-specific innate antimicrobial system consisting of numerous peptides, which confer epithelial barrier function as an adjunct to specific immunity. One important class of antimicrobial peptides is the family of defensins, small arginine rich peptides with a mass of 3–5 kDa,3–5 conserved throughout phylogeny.
“Bevins and colleagues postulated that defensins participate in the protection of enterocyte stem cells from pathogens in intestinal crypts”
Defensins exert a variable degree of antimicrobial activity against bacteria, fungi, and some enveloped viruses, possibly by perforating the bacterial cell wall through the formation of multimeric pores.6,7 They are classified into α defensins and β defensins, based on the position of three intramolecular disulfide bridges. In recent years, nine different human defensins have been identified, with five of them being of epithelial origin. Human defensins 5 and 6 (HD-5/6) belong to the α defensins and human β defensins 1–3 (HBD-1–3) to the β defensins. The first human intestinal defensins were observed in small intestinal Paneth cells containing HD-5 and HD-6.8,9 Bevins and colleagues postulated that defensins participate in the protection of enterocyte stem cells from pathogens in intestinal crypts.10 Since their initial description, a widespread distribution of these and other defensins has been documented. HBD-1 is thought to be expressed constitutively, whereas HD-5, HD-6, HBD-2, and HBD-3 are inducible under certain conditions.11–14
Recent in vitro investigations have already demonstrated the induction of HBD-2 by H pylori in relation to specific genes.15 The objective of our study was to perform a systematic investigation of defensin expression in response to H pylori colonisation and gastritis in patients.
MATERIALS AND METHODS
Patients
Seventy one patients gave their written informed consent before biopsy sampling during routine gastroscopy. All patients were investigated for peptic ulcer disease, dyspepsia, or gastrointestinal bleeding. The current treatment was recorded, especially with regard to the use of antacids or proton pump inhibitors, antibiotics, and non-steroidal anti-inflammatory drugs (NSAIDs). Two biopsies were drawn from the oesophagus, fundus, corpus, antrum, and duodenum, and immediately snap frozen in liquid nitrogen. To assess the H pylori status, biopsies were taken in parallel for histology and biochemical urease testing from the antrum and corpus.
Paraffin wax embedded tissue sections from gastric resections were provided by the department of pathology (series of four H pylori negative and three H pylori positive).
Histology and urease test
Biopsies were paraffin wax embedded and stained with haematoxylin and eosin. The degree of inflammation was classified according to the Sydney classification16 by an expert pathologist (CW). Helicobacter pylori status was assessed in parallel by methylene blue staining and biochemical analysis of urease activity. The urease kit (CU test) was purchased from Temmler Pharma (Göttingen, Germany) and testing was carried out according to the supplier’s protocol. The H pylori status was considered positive if one of either test was positive.
RNA preparation and reverse transcription
Frozen biopsies were disrupted in 1 ml of Trizol (Gibco BRL) with an Ultra-Turrax (Branson, Danbury, Connecticut, USA) until complete fragmentation. Total RNA was extracted according to the supplier’s protocol. RNA quality was determined by electrophoresis and quantified by photometry. Subsequently, 2 μg RNA were reverse transcribed with oligo d T-primers and 200 U reverse transcriptase (RT) (Superscript; Gibco BRL, Eggenstein, Germany), according to routine procedure.
Polymerase chain reaction
A 5 μl aliquot of the cDNA was taken for an established multiplex polymerase chain reaction (PCR). The α defensins (HD-5 and HD-6) were amplified in separate tubes from the β defensins (HBD-1 and HBD-2), each in conjunction with a housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase; GAPDH). Intron spanning primers were as following: HD5 sense, CGCCATCCTTGCTGCCATTCT; HD5 antisense, AACGGCCGGTTCGGCAATAGC; HD6 sense, GTGGGGCAAATGACCAGGACT; HD6 antisense, ATGGCAATGTATGGGACACAC; HBD1 sense, CCTACCTTCTGCTGTTTACTC; HBD1 antisense, ACTTGGCCTTCCCTCTGTAAC; HBD2 sense, CCAGCCATCAGCCATGAGGGT; HBD2 antisense, GGAGCCCTTTCTGAATCCGCA; GAPDH sense, TGCCTCCTGCACCACCAACTG; and GAPDH antisense, CGCCTGCTTCACCACCTTCTT. The PCR products encompassed 203 bp (HD-5), 260 bp (HD-6), 186 bp (HBD-1), 255 bp (HBD-2), and 349 bp (GAPDH). The reaction mix contained 400 nM of each primer, 200 μM of dNTPs, 1.25 U Taq (Gibco BRL), and 10× Tricine buffer (pH 8.4) in a total volume of 50 μl. PCR was performed for 35 cycles in a thermocycler (UNO II; Biometra, Göttingen, Germany). The reaction profile was 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 60 seconds. Aliquots of the PCR products were resolved on agarose gels and stained with ethidium bromide. The correct sequence had been determined by direct sequencing previously.
Immunohistochemistry
Polyclonal rabbit antisera raised against HD-5, HBD-1, and HBD-2 were a generous gift from Dr T Ganz, UCLA, Los Angeles, USA (an antibody directed against HD-6 is not available). A modified alkaline phosphatase anti-alkaline phosphatase (APAAP) protocol was performed in the case of HD-5 and HBD-2.17 Briefly, dewaxed and alcohol fixed tissue sections were boiled for seven minutes twice in citric acid (0.01M; pH 6) in a microwave oven, followed by extensive washing with Tris buffered saline (TBS). In the case of HBD-2, glass slides were incubated in 0.1% trypsin for five minutes at room temperature. Primary antibodies were dissolved in TBS, 2% bovine serum albumin at a dilution of 1/4000 for anti-HD-5 and 1/2000 for anti-HBD-2. Preimmune serum served as a negative control. The first incubation step was carried out in a humid chamber at room temperature for 60 minutes, followed by three washings for five minutes each in TBS. The following steps of the APAAP protocol were performed essentially as outlined by the supplier (Dianova, Hamburg, Germany). The new fuchsin method was used for specific staining and sections were counterstained with haematoxylin.
HBD-1 was processed according to a horseradish peroxidase protocol established by Valore et al.18 Anti-HBD-1 serum was diluted 1/500 to 1/800 in TBS, whereas rabbit serum was diluted 1/1000 in 0.05% Tween 20, 1% gelatin (75 bloom), and 0.01% thimerosal. Specific detection was performed with diaminobenzidine and counterstaining with haematoxylin. As for HBD-2 and HD-5, preimmune serum was used as a negative control. The staining was evaluated by an expert pathologist (CW).
Statistics
Statistical comparisons were based on the two tailed Fisher’s exact test, α = 0.05.
RESULTS
Patient characteristics
Biopsies were taken from 71 individuals. The mean age was 51 years (range, 26–82). All patients had abnormal findings on gastroscopy suggestive of gastritis or duodenitis and 20 patients had active peptic ulcer disease. Proton pump inhibitors were administered in 18 of 51 H pylori negative and five of 20 H pylori positive patients at the time of biopsy sampling. Six and 12 of 71 patients received NSAIDs and antibiotics, respectively.
Histology and urease test
Twenty of the 71 investigated subjects were identified as H pylori positive by the urease test and/or histology. Table 1 provides the results of the histological graduation of cellular infiltrates and the presence of intestinal metaplasia with respect to H pylori status. Compared with H pylori negative patients, in H pylori carriers chronic gastritis was more often active (18 of 20) and moderate to pronounced, as determined by the presence and extent of a polymorphonuclear infiltrate.
Table 1.
Histological grading of cellular infiltrates and the presence of intestinal metaplasia with respect to Helicobacter pylori status
| Histology | H pylori negative patients (n=51) | H pylori positive patients (n=20) |
| Neutrophils | ||
| Normal | 41 | 2 |
| Mild | 8 | 3 |
| Moderate | 2 | 6 |
| Pronounced | 0 | 9 |
| Mononuclear cells | ||
| Normal | 0 | 0 |
| Mild | 38 | 1 |
| Moderate | 13 | 18 |
| Marked | 0 | 1 |
| Intestinal metaplasia | 11 | 3 |
Type and degree of cellular components of chronic gastritis biopsies graded according to the Sydney classification.16 Intestinal metaplasia was assessed as present or absent only.
Mucosal defensin expression
The multiplex PCR protocol accomplished the separate amplification of α defensin and β defensin mRNA, each in conjunction with a housekeeping gene (GAPDH), which served as an internal control. Equal density of the control band was a prerequisite to compare defensin expression; fig 1 shows a representative electrophoresis gel containing samples from an H pylori negative and an H pylori positive individual. More than 95% of the specimens from the duodenum and oesophagus were positive for α defensins and β defensins, respectively, and were therefore considered as positive external controls.
Figure 1.
Results of a typical multiplex reverse transcription polymerase chain reaction for human defensin 5 (HD-5), HD-6, human β defensin 1 (HBD-1), and HBD-2, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal standard. Lane 1, oesophagus; lane 2, fundus; lane 3, corpus; lane 4, antrum; lane 5, duodenum; MW, molecular weight marker. Biopsies were obtained from two patients with Helicobacter pylori (Hp) negative and H pylori positive chronic gastritis.
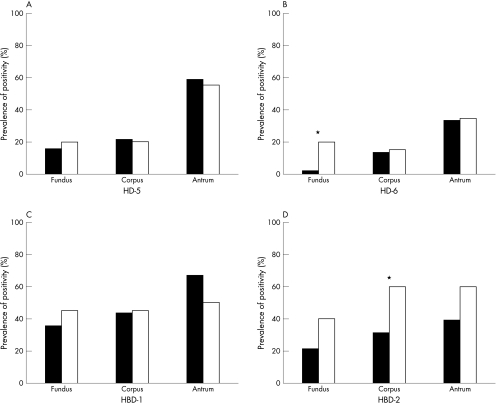
Figure 2 shows the proportions of positive samples at each location of the stomach with respect to H pylori status (51 negative and 20 positive). The expression of HD-5 and HD-6 mRNA was seen more frequently in the distal parts of the stomach (fig 2A,B), whereas this distribution was less pronounced for β defensins HBD-1 and HBD-2 (fig 2C,D).
Figure 2.
Prevalence of defensin transcript detection in biopsies from different sites of the stomach with respect to Helicobacter pylori status (closed bars, H pylori negative; open bars, H pylori positive). Multiplex reverse transcription polymerase chain reaction was performed with strand specific primers for α defensins and β defensins separately, each in conjunction with glyceraldehyde-3-phosphate dehydrogenase as an internal control. Electrophoresis was evaluated qualitatively—a visible band at the predicted position was termed positive. The numbers indicate the prevalence of positive samples for (A) human defensin 5 (HD-5), (B) HD-6, (C) human β defensin 1 (HBD-1), and (D) HBD-2. Helicobacter pylori positive samples expressed higher amounts of HD-6 in the fundus and HBD-2 in the corpus (*p < 0.05).
Similar proportions of positive RT-PCR results were obtained in chronic and chronic active inflammation with regard to HD-5 and HBD-1 (data not shown). Significant differences between H pylori positive and negative cases were found in the stomach with respect to HD-6 in the fundus (p < 0.05) and HBD-2 in the corpus (p < 0.05). HBD-2 was even more strongly related to the presence of neutrophil inflammation in the corpus (HBD-2 positive in 11 of 43 chronic v 17 of 28 chronic active; p < 0.01) and antrum (HBD-2 positive in 15 of 43 chronic v 17 of 28 chronic active; p = 0.05).
Immunohistochemistry of gastric defensins
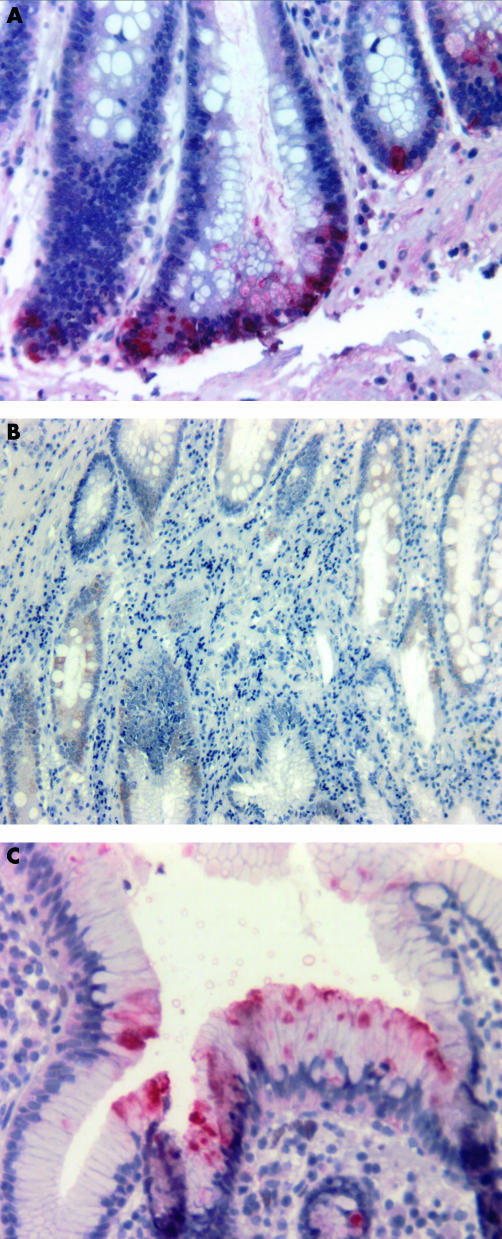
Seven surgical resections of the stomach carried out because of peptic ulcer disease and carcinoma were obtained from the department of pathology (four H pylori negative, three H pylori positive). The antrum disclosed a variable degree of chronic and active gastritis and intestinal metaplasia was present in two of seven. Immunohistochemical staining in the antrum was restricted to the epithelial lining. HD-5 positive cells occurred at the bottom of gastric glands (fig 3A). In contrast, HBD-1 showed a broad epithelial distribution (fig 3B), whereas the HBD-2 peptide was sparse and confined to cells of the apical foveolae (fig 3C). Thus, synthesis and possibly storage may be attributed to different cell populations.
Figure 3.
Immunohistochemical detection of defensin peptides in selected gastric tissue sections. (A) Epithelial cells of the basal crypts stained positive for human defensin 5 (HD-5) with the alkaline phosphatase anti-alkaline phosphatase (APAAP) method (dilution of the polyclonal antiserum, 1/4000). (B) Human β defensin 1 (HBD-1) was detected by the peroxidase method (dilution of the polyconal antiserum, 1/1000) disclosing a broad epithelial distribution. (C) The APAAP method was performed for HBD-2 detection (dilution of the polyclonal antiserum, 1/2000). Positive cells were confined to the epithelial cells of the apical foveolae.
DISCUSSION
This is the first systematic study of differential defensin expression in the stomach with respect to gastric pathology and H pylori status. A multiplex PCR protocol allowed the simultaneous detection of α defensin and β defensin mRNA in the upper gastrointestinal tract. Because of the uncertainties of PCR quantitation in a multiplex approach we expressed the findings as the proportion of individuals with a distinctly positive PCR signal. The abundant expression of α defensins in the duodenum and β defensins in the oesophagus served as an external positive control in each individual. Whereas HD-5, HD-6, and HBD-1 were almost absent or of low abundance irrespective of the presence of H pylori, the induction of HBD-2 during H pylori associated chronic active gastritis was noted. We reconfirmed an association between H pylori positive gastritis and more severe histological inflammation, judging that medication was irrelevant at the time of biopsy sampling.
In our present study, a variable degree of gastric defensin mRNA expression was encountered even in the absence of H pylori colonisation confirmed by immunohistochemistry. This is in contrast to a recent report by Frye and colleagues,19 who used uninflamed mucosa only and found no HD-5 or HD-6 mRNA in the stomach and no HBD-2 mRNA in gastric or duodenal biopsies. It could be that the different populations studied—inflamed versus non-inflamed tissue—and the different methodological approaches taken account for these observed differences. The origin of HD-5 in the gastric epithelium was confined to cells of the basal gastric glands, which parallels its intestinal localisation.20 Recently, metaplastic Paneth cells have been identified as the source of colonic HD-5.21 Therefore, intestinal metaplasia would be an acceptable explanation for the occurence of HD-5 and possibly HD-6 in chronic gastritis. The divergent expression of HD-5 and HD-6 in the fundus of H pylori positive patients is less clear and small numbers suggest a statistical error. However, it might reflect a spread of inflammation involving the fundus enabling the preferential induction of HD-6, but this remains speculative.
Take home messages.
We have confirmed that the expression of human β defensin (HBD-2) is induced upon Helicobacter pylori infection in a clinical setting of chronic gastritis
This induction may be mediated by components of the pathogen itself or may occur secondary to immune events in chronic inflammation
HBD-2 might play a role in controlling this organism and preventing invasion
“The induction of human β defensin 2 is not unique to cag pathogenicity island positive Helicobacter pylori because other bacteria, such as salmonella, are also active in human gastric cell lines”
HBD-1 was expressed in a uniformly low manner, providing further evidence for its constitutive nature.11 This is in accordance with the lack of transcription factor regulatory elements for proinflammatory signals in the HBD-1 gene. We found a considerable difference between the expression of HBD-2 in the gastric corpus of H pylori carriers compared with H pylori negative patients. Although the appearance of HBD-2 was related to H pylori status it was even more strongly associated with the degree and activity of microscopic inflammation. Because these parameters are interdependent, it is difficult to conceive that HBD-2 expression is solely based on the presence of H pylori. The cellular source differed from that of HD-5 because HBD-2 was identified in the apical region, similar to that reported in the colon.22 In a recent paper, Hamanaka and colleagues23 detected epithelial HBD-2 expression in a small set of patients with H pylori positive gastritis, which subsided upon eradication treatment, and this expression was not seen in H pylori negative patients. Interestingly, in vitro experiments revealed that HBD-2 has direct antimicrobial activity against H pylori, strengthening its role in mucosal defence.
In human gastric epithelial cell lines, only those strains of H pylori carrying the cytotoxin associated gene (cag) pathogenicity island (PAI) induced the pronounced expression of HBD-2.15,24 However, the induction of HBD-2 is not unique to cag PAI positive H pylori because other bacteria, such as salmonella, are also active in human gastric cell lines.24 A comparable HBD-2 response is seen in airway epithelial cells following mucoid Pseudomonoas aeruginosa infection25 and in intestinal epithelial cells upon infection with Salmonella spp and Escherichia coli.26 Although cag PAI was not determined in our cohort the results clearly indicate an augmented HBD-2 response as a result of H pylori in chronic active gastritis. However, as mentioned above, it is difficult to determine whether this induction is mediated by the pathogen itself or is secondary to the inflammatory response. For instance, interleukin 1α has been found to promote HBD-2 production in gastric epithelial cells.24 Substantial in vitro information suggests that a direct H pylori mediated effect is responsible. Nuclear factor κB (NFκB) and activator protein 1 consensus sites in the HBD-2 promoter are believed to participate in the activation of gene transcription,25,27 and it has been recognised that cag A positive H pylori induce NFκB.28 Interestingly, reporter gene assays with an HBD-2 promoter fragment demonstrated that cag PAI positive H pylori enhance gene transcription, whereas cag PAI negative strains do not. This ability is lost upon truncation or mutation of the NFκB consensus site, strenthening the importance of cag PAI in the induction of HBD-2.29 Whether the activation pathway is receptor mediated or directly linked to the incorporation of microbial components, especially cag A, remains to be determined.
In conclusion, H pylori positive chronic gastritis leads to a significant rise in HBD-2 expression in the gastric mucosa, which might play a role in controlling the pathogen and preventing invasion.
Abbreviations
APAAP, alkaline phosphatase anti-alkaline phosphatase
cag A, cytotoxin associated gene A
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
HBD, human β defensin
HD, human defensin
NFκB, nuclear factor κB
NSAID, non-steroidal anti-inflammatory drug
PAI, pathogenicity island
RT-PCR, reverse transcription polymerase chain reaction
TBS, Tris buffered saline
REFERENCES
- 1.Crabtree JE, Wyatt JI, Trejdosiewicz LK, et al. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol 1994;47:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu S, Ramanujam KS, Wong A, et al. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology 1999;116:1319–29. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T, Lehrer RI. Defensins. Curr Opin Immunol 1994;6:584–9. [DOI] [PubMed] [Google Scholar]
- 4.Ouellette AJ, Hsieh MM, Nosek MT, et al. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect Immun 1994;62:5040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selsted ME, Miller SI, Henschen AH, et al. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol 1992;118:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehrer RI, Barton A, Daher KA, et al. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest 1989;84:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagan BL, Selsted ME, Ganz T, et al. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A 1990;87:210–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem 1992;267:23216–25. [PubMed] [Google Scholar]
- 9.Jones DE, Bevins CL. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett 1993;315:187–92. [DOI] [PubMed] [Google Scholar]
- 10.Bevins C, Porter EM, Ganz T. Defensins and innate host defence of the gastrointestinal tract. Gut 1999;45:911–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett 1996;396:319–22. [DOI] [PubMed] [Google Scholar]
- 12.Salzman NH, Polin RA, Harris MC, et al. Enteric defensin expression in necrotizing enterocolitis. Pediatr Res 1998;44:20–6. [DOI] [PubMed] [Google Scholar]
- 13.Harder J, Bartels J, Christophers E, et al. A peptide antibiotic from human skin. Nature 1997;387:861. [DOI] [PubMed] [Google Scholar]
- 14.Harder J, Bartels J, Christophers E, et al. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 2001;276:5707–13. [DOI] [PubMed] [Google Scholar]
- 15.Wada A, Mori T, Oishi K, et al. Induction of human β-defensin-2 mRNA expression by Helicobacter pylori in human gastric cell line MKN45 cells on cag pathogenicity island. Biochem Biophys Res Commun 1999;263:770–4. [DOI] [PubMed] [Google Scholar]
- 16.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney system. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 17.Porter EM, Liu L, Oren A, et al. Localization of human intestinal defensin 5 in Paneth cell granules. Infect Immun 1997;65:2389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valore EV, Park CH, Quayle AJ, et al. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest 1998;101:1633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frye M, Bargon J, Lembcke B, et al. Differential expression of human α- and β-defensins mRNA in gastrointestinal epithelia. Eur J Clin Invest 2000;30:695–701. [DOI] [PubMed] [Google Scholar]
- 20.Wehkamp J, Schwind B, Herrlinger KR, et al. Innate immunity and colonic inflammation: enhanced expression of epithelial alpha-defensins. Dig Dis Sci 2002;47:1349–55. [DOI] [PubMed] [Google Scholar]
- 21.Cunliffe RN, Rose FRAJ, Keyte J, et al. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 2001;48:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wehkamp J, Fellermann K, Herrlinger KR, et al. Human beta-defensin 2 but not beta-defensin 1 is expressed preferentially in colonic mucosa of inflammatory bowel disease. Eur J Gastroenterol Hepatol 2002;14:745–52. [DOI] [PubMed] [Google Scholar]
- 23.Hamanaka Y, Nakashima M, Wada A, et al. Expression of human beta-defensin 2 (hBD-2) in Helicobacter pylori induced gastritis: antibacterial effect of hBD-2 against Helicobacter pylori. Gut 2001;49:481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Neil DA, Cole SP, Martin-Porter E, et al. Regulation of human beta-defensins by gastric epithelial cells in response to infection with Helicobacter pylori or stimulation with interleukin-1. Infect Immun 2000;68:5412–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harder J, Meyer-Hoffert U, Teran LM, et al. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol 2000;22:714–21. [DOI] [PubMed] [Google Scholar]
- 26.O’Neil DA, Porter EM, Elewaut D, et al. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol 1999;163:6718–24. [PubMed] [Google Scholar]
- 27.Liu L, Wang L, Jia HP, et al. Structure and mapping of the human beta-defensin HBD-2 gene and its expression at sites of inflammation. Gene 1998;222:237–44. [DOI] [PubMed] [Google Scholar]
- 28.Keates S, Hitti YS, Upton M, et al. Helicobacter pylori infection activates NF-κB in gastric epithelial cells. Gastroenterology 1997;113:1099–109. [DOI] [PubMed] [Google Scholar]
- 29.Wada A, Ogushi K, Kimura T, et al. Helicobacter pylori-mediated transcriptional regulation of the human β-defensin 2 gene requires NF-κB. Cell Microbiol 2001;3:115–23. [DOI] [PubMed] [Google Scholar]