Abstract
Aims: To investigate the relation between ambient temperature and serum potassium concentrations in samples from primary care.
Methods: Potassium concentrations were estimated on general practitioner and hospital ward samples taken over a two year period using serum obtained from gel separator samples. The number of samples analysed from general practice during each month varied from 5093 to 8978 (mean of 7068 samples/month).
Results: As the temperature fell in winter, the mean daily serum potassium concentration rose in samples from general practice, with the inverse occurring during the warmer summer months. This effect was restricted to samples coming from general practice, with inpatient samples seemingly unaffected.
Conclusions: These results indicate that exposure of the samples to variations in ambient temperature during their transport to the laboratory profoundly affects measured serum potassium concentrations.
Keywords: temperature, hyperkalaemia, potassium
Factitious hyperkalaemia is a problem that has dogged the estimation of serum potassium concentrations since laboratories first started measuring the analyte. It is known that a delay in centrifugation of blood samples leads to an artificially high potassium concentration in the serum or plasma because potassium leaks out of the blood cells and into the serum or plasma. The other main causes of factitious hyperkalaemia include haemolysis, thrombocytosis, “leaky cell syndrome” (secondary to chronic lymphocytic leukaemia or other idiopathic causes), other leucocytoses, and refrigeration of the whole blood sample before centrifugation.1,2 Even factors such as fist clenching before venepuncture can influence potassium concentrations.3 For a review on pre-analytical variables that affect laboratory results, see Narayanan and Guder.4 Clearly, steps must be taken at the pre-analytical stage to minimise the effects of such factors because failure to do so may mean that erroneous potassium concentrations are communicated to the requesting clinician, sometimes as a matter of urgency. Potentially dangerous patient treatment may well be initiated unless the clinician is aware of the possibility that the reported potassium concentration is artificially high.
“Even factors such as fist clenching before venepuncture can influence potassium concentrations”
The Portsmouth pathology service serves a population of 560 000 with general practitioner surgeries using our service being situated up to 22 miles from the laboratory. Most samples are transported to the laboratory in vans that visit the surgeries once or twice a day depending on the surgery size. A long standing problem is that of delay in serum separation causing spurious hyperkalaemia. As noted earlier, these results have to be telephoned to the requesting physician and the test is often repeated at extra expense and great inconvenience to the patient. This is a problem common to most laboratories, but despite recent improvements to our transport service, we thought that the incidence of this problem seemed to be increasing. Therefore, we looked retrospectively at the trends in the prevalence of hyperkalaemia, with unexpected results. Here, we present data indicating that the ambient temperature at which blood samples are transported from general practitioner surgeries has a profound but predictable effect on the measured serum potassium result, and that this effect is not seen in samples from inpatients, where the exposure to temperature variation is much reduced.
MATERIALS AND METHODS
Potassium concentrations were estimated on general practitioner and hospital ward samples taken over a two year period (1999–2000) using serum obtained from gel separator samples (Becton Dickinson, Cowley, Oxfordshire, UK). The number of samples analysed from general practice during each month varied from 5093 to 8978 (mean of 7068 samples/month).
These analyses were performed routinely on either a Technicon DAX 72 or Hitachi 911 analyser using an indirect ion selective electrode. The internal quality control for potassium estimation was satisfactory for both analysers and was consistent throughout the study period (within batch coefficient of variation (CV), < 2%; between batch CV, < 2%). The performance of both analysers in two national external quality assurance programmes was also satisfactory.
Temperature data were obtained from the department of geography automatic weather station at Portsmouth University. The roof top site is approximately 18 m above ground level and 1 km inland from the coast. Temperatures were recorded using a Campbell scientific HMP45C temperature probe every 10 seconds and averaged over each five minute period. The mean daily temperature was calculated using all the five minute averages (midnight to midnight Greenwich mean time).
Samples are transported in vans whose round trip from the hospital to pick up samples in different general practitioner surgeries does not exceed four hours, regardless of season. Clearly, most sample journey times will be shorter than this figure. These van interiors are heated but samples are not transported in insulated containers. After arrival in the laboratory, samples are centrifuged within 17 minutes on average.
Our “action limit” for raised potassium concentration is 6.0 mmol/litre and these are scrutinised by senior clinical staff before phoning, if deemed appropriate.
RESULTS
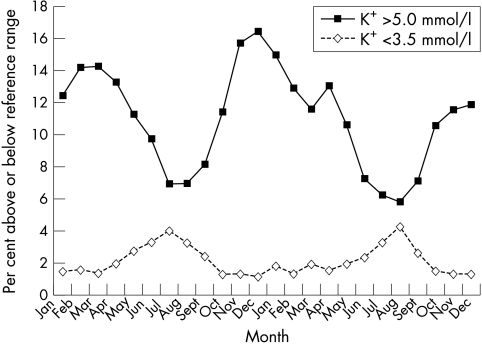
Figure 1 shows the percentage of potassium results that are above and below the reference range (3.5–5.0 mmol/litre). During the colder winter months, 15–16% of general practitioner samples fell above the reference range and were therefore classified as hyperkalaemic. During the summer months, however, the number of samples above the reference range fell to 6–7%. Similarly, when considering those estimations below the lower end of the reference range, during the colder parts of the year, only 1–2% of samples were estimated to have potassium concentrations below the reference range. In the summer months when temperatures rise, this figure increased to 4%; that is, as the temperature rose, the number of patients with hypokalaemia also appeared to rise.
Figure 1.
Potassium concentrations above and below the reference range.
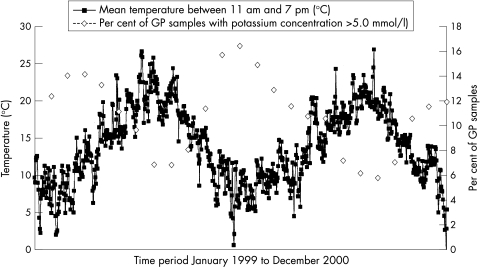
Figure 2 shows the percentage of serum potassium concentrations for all general practitioner samples above 5.00 mmol/litre throughout the 1999–2000 study period, along with mean “day time” temperatures for the same period. The figure demonstrates a close relation between the two variables, showing that as the ambient temperature fell, the potassium concentrations in the general practitioner samples rose, with the reverse occurring during the warmer summer months; that is, the warmer the month, the lower the mean potassium concentration in general practitioner samples.
Figure 2.
Comparison of mean daily temperature between 11.00 and 19.00 hours and potassium concentrations of > 5.0 mmol/litre in general practitioner (GP) samples.
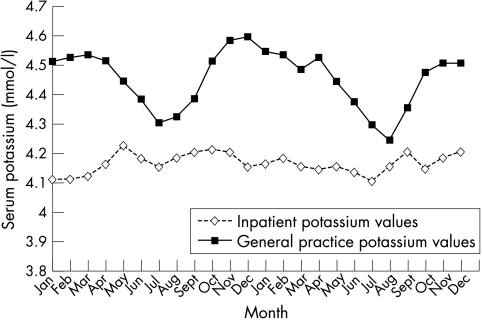
Figure 3 shows the mean potassium concentrations in samples from both general practice and inpatient samples taken over the same period. The inpatient samples will be exposed to smaller temperature fluctuations than the general practitioner samples. These results show that the mean potassium concentrations from ward samples are relatively constant throughout the year, whereas the seasonal peaks and troughs in potassium concentrations in the general practitioner samples are obvious.
Figure 3.
Mean potassium concentrations from inpatients and general practice 1999–2000.
The Pearson product moment correlation coefficient using mean monthly temperatures and potassium concentrations from general practice samples is −0.9148, thus confirming this inverse relation. The only disruption to the smooth annual progression is from March to April 2000 when mean potassium concentrations rose. Mean monthly temperatures for March and April were 9.24°C and 9.37°C, respectively. Mean daytime maximum temperatures were marginally higher in March than in April. It is unusual for the difference to be so small, and the warm March followed by the relatively cold April appears to be reflected in the potassium data.
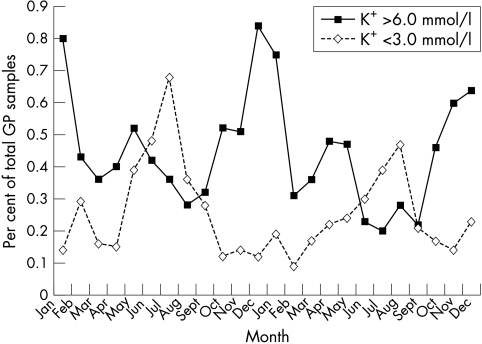
Figure 4 shows the relation between the season and potassium concentrations that might be deemed “clinically relevant” (values > 6.0 mmol/litre and < 3.0 mmol/litre) for general practitioner samples. To negate the effect of variable sample numbers from general practice throughout the year, we plotted the percentage of general practitioner samples in which potassium concentrations fell above or below these limits. The graph is very similar to those in figs 1–3, indicating that, in addition to those falling outside the reference range, clinically relevant results also vary according to the season, and we telephone more raised potassium concentrations in winter and more low potassium concentrations in summer.
Figure 4.
General practitioner (GP) samples with a potassium concentration > 6.0 mmol/litre or < 3 mmol/litre as a percentage of the total number of GP samples.
DISCUSSION
These data demonstrate the relation between ambient temperature and serum potassium concentrations in samples from general practice: as the temperature falls in winter, the measured mean daily serum potassium concentration rises in these samples. This effect is restricted to samples coming from general practice, with inpatient samples seemingly unaffected, thus confirming our initial suspicion about an increased incidence of hyperkalaemia that was apparent in the winter of 2000.
Raised potassium concentrations are a major cause of concern for general practitioners, who frequently get phone calls from laboratory staff advising them of a “high serum potassium” concentration. Often, it is not possible to calculate the time between blood collection and centrifugation and the general practitioner then has to decide whether to pursue the result with enthusiasm or, as sometimes happens, ignore it completely. The former involves re-bleeding the patient and ensuring that the sample is transported to the laboratory quickly: the patient is inconvenienced and the laboratory has another sample to process. However, ignoring it risks missing a potentially life threatening genuine hyperkalaemia. Vaidya et al suggest that a “race to the lab” may be the only way to solve the problem of pseudohyperkalaemia.5
The number of potassium estimations above the reference range was 15–17% in winter compared with 5–6% in the summer. This suggests to us that summer time potassium concentrations may not be artificially low as suggested by Seamark and colleagues6 and Ulahannan et al,7 but may reflect values closer to the true potassium concentrations. This is supported by the fact that in samples from inpatients, which are not subjected to the same temperature variation, the potassium results remain fairly constant throughout the year.
“We are considering placing samples in insulated containers to see whether this affects potassium concentration”
Our transport department reports no difference in the journey times in the winter compared with the summer. Hampshire gets very little snow and this would be the major influence on journey times in winter in other parts of the country. Factors such as roadworks (a constant feature), increased “cross channel” and ordinary tourist traffic in the city (occurs in summer only), and school holidays (fewer cars on the roads) will probably affect transport times to a greater extent than climate/season. However, the effects of these factors tend to even themselves out over the year. Sample storage before collection by the vans might have been relevant, but even if samples were stored in a general practitioner surgery refrigerator before collection, this would not explain the pronounced seasonal differences reported here. We recommend that these samples should be stored at room temperature in the general practitioner surgery before collection, thus the only time that samples are consistently exposed to low temperatures will be during transport, and this must be a factor causing this seasonal variation. The average time delay from sample arrival in the laboratory and centrifugation is 17 minutes so the maximum time that the sample remains “in transit” is around four hours and 17 minutes, and this remains constant throughout the year. Longer journey times in winter will not therefore explain this seasonal pseudohyperkalaemia. It can only be a combination of low ambient temperature and constant journey time, and we are considering placing samples in insulated containers to see whether this affects potassium concentration. Other laboratories may wish to review their transport systems because these findings may not be applicable to laboratories whose van routes are shorter and whose samples are therefore not exposed to low temperatures for even a relatively short time.
Take home messages.
Mean daily serum potassium concentrations in samples from general practice rise as the temperature falls in winter, and the inverse occurs during the warmer summer months
Inpatient samples are not affected, indicating that exposure of the samples to variations in ambient temperature during their transport to the laboratory profoundly affects measured serum potassium concentrations
Because potassium leaks from blood cells into the serum/plasma at lower temperatures, the lower potassium results obtained on days when the ambient temperature is high are probably closer to the true potassium concentrations
Laboratories may wish to review their transport systems
This problem is so serious that some authors have advocated the placing of centrifuges in general practitioner surgeries.8 Our laboratory serves a total of 99 general practitioner practices, and with centrifuges costing over £600 each, this would represent a considerable outlay in terms of revenue and servicing costs, staff time, and health and safety issues. Ideally though, blood samples for measurement of potassium should be separated within one hour of venepuncture. The longer the plasma or serum is left in contact with the blood cells, the less likely the measured potassium will reflect the true in vivo concentration. Most potassium ions are intracellular, and the cell membrane is freely permeable to these ions. The concentration differential between plasma and cells can only be maintained as long as the enzyme systems involved in the sodium pump continue to function, and a source of energy in the form of glucose is available. Low temperatures inhibit these enzymes, and it is well known that serum/plasma potassium concentration rises rapidly in refrigerated whole blood. However, our observations show that even relatively small variations in the temperature to which blood is exposed before separation can significantly affect the measured potassium concentration.
It would seem logical to assume that the lower potassium results obtained on days when the ambient temperature is high would be closer to the true potassium concentrations. However, other investigators have concluded that higher ambient temperatures can lead to spuriously low potassium results. Masters and colleagues9 found a mean fall of 0.22 mmol/litre in lithium heparin blood samples after four hours at 37°C. Seamark and colleagues6 investigated the effect of higher ambient temperatures on blood samples during transport to the laboratory and found a significant lowering of potassium concentrations with lithium heparin tubes, but not with plain gel separation tubes. Hartland and Neary10 showed that plasma samples are the preferred medium for the estimation of potassium concentrations, but that this was not always suitable for other commonly requested analytes, and that the Becton Dickinson tube they studied was associated with the least variable potassium release. Further investigations are required to find the cause of these differences between the two types of tube. Meanwhile, readers should be aware that when Becton Dickinson gel separator tubes are used for the collection of samples for “urea and electrolytes”, the incidence of pseudohyperkalaemia will be higher during the colder months in samples from general practice and that patients with true hypokalaemia may be missed during the winter.
REFERENCES
- 1.Narayanan S. The preanalytic phase—an important component of laboratory medicine. Am J Clin Pathol 2000;113:429–52. [DOI] [PubMed] [Google Scholar]
- 2.Skinner SL, Adelaide MB. A cause of erroneous potassium levels. Lancet 1961;1:478–80. [Google Scholar]
- 3.Don BR, Sebastian A, Cheitlin M, et al. Pseudo hyperkalemia caused by fist clenching during phlebotomy. N Engl J Med 1990;322:1290–92. [DOI] [PubMed] [Google Scholar]
- 4.Narayanan S, Guder WG. Preanalytical variables and their influence on the quality of laboratory results. Electronic Journal of the International Federation of Clinical Chemistry 2001;13(http://www.ifcc.org/ejifcc/vol13no1/1301200107.htm). [PMC free article] [PubMed]
- 5.Vaidya B, Chan K, Drury J, et al. A race to the lab. Lancet 2002;359:848. [DOI] [PubMed] [Google Scholar]
- 6.Seamark D, Backhouse S, Barber P, et al. Transport and temperature effects on measurement of serum and plasma potassium. J R Soc Med 1999;92:339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulahannan TJ, McVittie J, Keenan J. Ambient temperatures and potassium concentrations. Lancet 1998;352:1680–1. [DOI] [PubMed] [Google Scholar]
- 8.Masters PW, Ismail AA. Minimising factitious hyperkalaemia: samples should be centrifuged after collection in general practices. BMJ 1997;315:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masters PW, Lawson N, Marenah CB, et al. High ambient temperature: a spurious cause of hypokalaemia. BMJ 1996;312:1652–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartland AJ, Neary RH. Serum potassium is unreliable as an estimate of in vivo plasma potassium. Clin Chem 1999;45:1091–2. [PubMed] [Google Scholar]