Abstract
The application of immunohistology to the spectrum of plasma cell disorders has yet to be incorporated widely into routine haematology practice. This technique enables the direct visualisation of specific surface and cytoplasmic antigens in the context of the individual cell and the surrounding anatomical neighbourhood. This review outlines the role of bone marrow immunohistology in the laboratory evaluation of patients with suspected and established plasma cell neoplasms and its emerging role in understanding myeloma biology for possible future therapeutic application.
Keywords: myeloma, immunohistochemistry, CD138, bcl-2
Plasma cell malignancies are characterised by the clonal expansion of terminally differentiated B lymphoid cells that have undergone somatic hypermutation, usually resulting in the production of a monoclonal immunoglobulin protein. Diagnosis involves evaluation of the clinical burden of plasma cell infiltration, analysis of radiologically detectable bone lesions, electrophoretic determination of the monoclonal immunoglobulin, and assessment of plasma cells in the bone marrow or extramedullary tissue. The World Health Organisation (WHO) classification1 has categorised the diverse range of malignant immunosecretory disorders under the collective title of “plasma cell neoplasms”. The main subgroups include plasma cell myeloma, plasmacytoma, immunoglobulin deposition disease, osteosclerotic myeloma, and heavy chain disease. Recent technological advances in the detection of plasma cell pathology include magnetic resonance imaging of bone lesions, immunophenotype detection of aberrant surface antigens on plasma cells,2 molecular characterisation of clonally rearranged immunoglobulin genes,3 and an expanding spectrum of cytogenetic abnormalities enhanced by fluorescent in situ hybridisation technology.4 These exciting new diagnostic modalities will probably reshape the way we define plasma cell disorders as their clinical relevance becomes clearer. Morphological analysis of the bone marrow aspirate and trephine remains the “gold standard” for quantifying the volume of medullary plasma cell infiltration and assessing the degree of plasma cell dysplasia. This review will focus on the evolving use of immunohistology which, aided by an expanding repertoire of monoclonal antibodies, allows the anatomical and functional detail of plasma cells in the bone marrow trephine to be examined “in situ”. The clinical application of immunohistology in the diagnostic assessment, prognosis, monitoring, and biological evaluation of plasma cell neoplasms with emphasis of its additional value over other laboratory techniques is highlighted.
DIAGNOSIS OF PLASMA CELL NEOPLASMS
Reactive versus neoplastic plasma cell proliferation
There are many causes of reactive bone marrow plasmacytosis including infection, malignancy, inflammation, Castleman’s disease, iron deficiency, megaloblastic anaemia, haemolytic anaemia, diabetes mellitus, cirrhosis,5 and streptokinase treatment.6 In most cases the plasma cell infiltrate is less than 20% but it can sometimes reach 50%. Plasma cells can be found in normal bone marrow surrounding macrophages and capillaries. However, perivascular plasma cell collections are not always benign, and can be seen in 30% of patients with myeloma.7 Plasma cell aggregates in patients with reactive plasmacytosis are usually composed of less than 10 plasma cells.7 An immunohistological study determined the ratio of cytoplasmic κ to λ light chains in plasma cells to be 0.4–3.5 in reactive plasmacytosis, 0.2–3.0 in monoclonal gammopathy of unknown significance (MGUS), and < 0.2 or > 11.1 in multiple myeloma.8 Although the cytoplasmic light chain ratio was useful in distinguishing multiple myeloma from MGUS and reactive plasmacytosis, the last two diagnoses were not always differentiated using this method. Rare cases of monoclonal gammopathy associated with crystal storage histiocytosis (CSH) may present problems in the differential diagnosis between plasma cell neoplasms and other causes of CSH, such as Gaucher’s disease, rhabdomyoma, fibrosclerosis, and Weber-Christian disease. In plasma cell neoplasms associated with CSH, κ light chain inclusions are usually found, presumably as a result of lysosomal incorporation of secreted immunoglobulins.9 Bcl-2 has been studied as a marker to distinguish neoplastic gammopathies from reactive plasmacytosis. Staining for Bcl-2, although sensitive for detecting plasma cells, does not distinguish between benign and malignant plasmacytosis because it is often positive in both.10 Finally, in non-secretory plasma cell neoplasms, immunohistological detection of pathological plasma cell aggregates, atypia, and cytoplasmic light chain restriction may be fundamental in making a diagnosis.
MGUS versus multiple myeloma
In the presence of a monoclonal paraprotein, the WHO1 definition of MGUS is the presence of less than 10% plasma cells in the bone marrow with no lytic bone lesions, clinical, or biochemical abnormalities. Identification of plasma cells by subjective enumeration in the bone marrow aspirate is the standard practice for determining the percentage of plasma cells. A suboptimal blood dilute or dry tap aspirate will increase the likelihood of sampling error. The bone marrow aspirate should be viewed in conjunction with the trephine section to exclude the presence of localised plasma cell collections indicating more advanced disease.
“The bone marrow aspirate count may underestimate the degree of bone marrow plasmacytosis in up to 30% of cases when compared with immunohistological examination”
Detection of small plasma cell collections on haematoxylin and eosin (H&E) stained sections can be challenging, even for the experienced observer. This is particularly so when the quality of the H&E sections is suboptimal. Immunohistology “lights up” interspersed and small collections of plasma cells, allowing a more straightforward assessment of plasma cell infiltration. The bone marrow aspirate count may underestimate the degree of bone marrow plasmacytosis in up to 30% of cases when compared with immunohistological examination.11 The natural history of MGUS is that 16% of patients progress to multiple myeloma at a rate of 0.8% each year.12 Knowing which patients are at risk for early progression to symptomatic myeloma would assist in appropriate management. An immunohistological study of 176 patients found that 40% of those with myeloma had less than 10% plasma cells on bone marrow aspirate.7 Immunohistology using cytoplasmic staining for κ and λ light chains revealed monotypic aggregates and homogenous nodules in 79% patients with less than 10% plasma cells, suggesting that these features were a better indicator of multiple myeloma than the absolute plasma cell count. Monotypic aggregates were defined as occupying at least one inter-fat marrow space, with more than 90% of the plasma cells expressing one type of immunoglobulin. Homogeneous nodules were plasma cell collections spanning more than half the diameter of a high power magnification field. In the same study, four of 30 patients with more than 10% plasma cells had no monotypic aggregates or plasma cell nodules. After a mean follow up of four years, all four patients remained clinically stable, indicating a “MGUS-like” disorder, despite the presence of more than 10% plasma cells on the aspirate at diagnosis.
Solitary plasmacytoma
A solitary plasmacytoma is diagnosed when there is no evidence of multifocal disease on bone marrow and radiological examination. Although radiotherapy may prevent local relapse in 90% of patients, half of those with solitary bone plasmacytoma progress to myeloma within three years, suggesting that subclinical myeloma was present at diagnosis.13 Patients at higher risk for early progression are those with persistent monoclonal protein, despite local treatment, and those with magnetic resonance imaging evidence of disease elsewhere.14 Immunohistology may detect small foci of plasma cells in the bone marrow distant from the sentinel plasmacytoma at initial presentation not obvious on H&E staining. The importance of such microscopic lesions in predicting earlier clinical progression to myeloma is not known. In the differential diagnosis between undifferentiated carcinoma and plasmacytoma, both may be positive for epithelial membrane antigen, vimentin, and cytokeratin and negative for CD45.15,16 The presence of aberrant myelomonocytic markers may also complicate the differential diagnosis. In such cases, the expression of CD138 and cytoplasmic immunoglobulins should be helpful in revealing a plasma cell origin.
Amyloidosis
The relevance of bone marrow plasmacytosis in amyloidosis is not always clear. Monoclonal protein in the serum or urine is found in 80–90% of patients with primary amyloidosis when assessed by immunofixation. Lambda light chains predominate in a 3 : 1 ratio over κ light chains, in contrast to a 2 : 1 κ to λ ratio in patients with MGUS and plasma cell myeloma.15 A spectral overlap between amyloidosis and myeloma is not uncommon, with 20% of each group having or eventually being diagnosed with the other disease.17 An arbitrary definition of amyloidosis with reactive plasmacytosis is given when there are less than 30% plasma cells in the bone marrow and no clinical hallmarks of myeloma, such as lytic bone lesions; otherwise, a diagnosis of amyloidosis with plasma cell myeloma should be considered. Immunohistology of the bone marrow revealing pathological plasma cell aggregates or abnormal cytological features may support the presence of an associated plasma cell myeloma with lesser degrees of plasmacytosis. Extensive bone marrow plasma cell infiltration in amyloidosis has been associated with a worse clinical outcome.18 Caution should be exercised in assessing cytoplasmic monotypic restriction in amyloid syndromes because of the occurrence of non-specific staining in amyloid tissues for immunoglobulin heavy and light chains. Immunohistology for Congo red staining in vascular structures will be positive in approximately 50% of patients with amyloidosis.19
Lymphoplasmacytic lymphoma
Histological evidence of bone marrow involvement occurs in 80% of patients with lymphoplasmacytic lymphoma or Waldenström’s macroglobulinaemia (WM) on initial bone marrow assessment.20 Using trephine section immunohistology, disease infiltration has been found in 96% of patients with WM.21 The involvement was diffuse, interstitial, nodular, and paratrabecular in 58%, 32%, 6%, and 4% of cases, respectively. All cases were CD20 positive and immunostaining was helpful in defining small aggregates not obvious on H&E staining. Patients with lymphoplasmacytic lymphoma may also express the multiple myeloma oncogene 1/interferon regulatory factor 4 (MUM1/IRF4) gene product. Exclusion of other B cell lymphomas such as small lymphocytic lymphoma/B cell lymphocytic leukaemia with plasmacytoid features, follicular lymphoma, and mantle cell lymphoma is aided by finding the small lymphocyte population negative for CD5, CD10, and cyclin D1. CD10 may be present, however, in advanced myeloma.22
Antibodies useful for plasma cell immunohistology
A panel of antibodies is recommended rather than reliance on a single antibody. The combination of antibodies to CD138 or VS38c, Bcl-2, CD79a, CD20, and, if optimised, immunoglobulin heavy and/or light chain enables assessment of malignant plasmacytosis in the bone marrow, taking into account occasional heterogeneity in tumour antigen expression. VS38c and CD138 are excellent antibodies for identifying plasma cells. Although we have no experience using anti-CD38 on paraffin wax embedded sections, there are reports of its use in frozen sections and fine needle aspirate material.23 Plasma cells are also stained well with anti Bcl-2, but the observer should note that normal lymphoid cells may also be positive. CD79a and CD20 staining may be helpful in some cases in which the neoplastic plasma cells are positive for these antigens but negative for other markers.
CD138 (syndecan-1) is a transmembrane heparan sulfate present on the surface membrane of 95% of plasma cells in paraffin wax sections. Other haemopoietic cells, endothelial cells, and lymphoplasmacytoid lymphomas are not stained with antibodies to CD138.24 Plasma cells may occasionally be negative in fibrotic areas because CD138 is shed from the surface membrane into the surrounding fibrotic matrix.25
VS38c is a mouse monoclonal antibody reactive against the intracellular protein p63 located within the rough endoplasmic reticulum. It stains normal and neoplastic plasma cells in paraffin wax embedded sections, in addition to lymphoplasmacytoid lymphomas, endothelial cells, and one third of diffuse large B cell lymphomas. The VS38c antibody also stains non-haemopoietic tumours.26
“Immunoglobulin heavy and light chain staining is useful for identifying plasma cells and for demonstrating the presence of clonal restriction”
The Bcl-2 protein is aberrantly expressed in patients with the t(14;18) translocation, which brings the bcl-2 gene under the influence of the immunoglobulin heavy chain gene promoter. Bcl-2 is an important protein inhibiting programmed cell death and overexpression is present in a wide variety of lymphomas other than follicular lymphoma, despite the absence of the bcl-2/IgH gene rearrangement.27 Both normal and malignant plasma cells exhibit cytoplasmic Bcl-2 staining. In a study of 49 patients with multiple myeloma, Bcl-2 positivity was present in 97%.10
Immunoglobulin heavy and light chain staining is useful for identifying plasma cells and for demonstrating the presence of clonal restriction. In MGUS, monotypic restriction may not always be obvious, particularly when the plasma cell burden is minimal. Optimising background staining can be complicated by the presence of extracellular light chains. Prolonged fixation, the decalcification process, and plasma cell dysplasia can result in false negative results.7
Cyclin D1 is a cell cycle regulator that is overexpressed in 24–40% of patients with myeloma, and positivity is associated with higher tumour grade and stage.28 Patients with myeloma who are positive for cyclin D1 have also been shown to have shorter survival.29 Cyclin D1 expression in myeloma has not been found to correlate with proliferative activity as assessed by Ki-67 staining.30
PROGNOSIS
Determining those patients with myeloma who will develop progressive disease is an important clinical issue. The β2 microglobulin and plasma cell labelling index remain important independent laboratory markers of prognosis.31 Immunohistological assessment of plasma cell differentiation, the volume of plasma cell infiltration, and the pattern of infiltration all have prognostic value.32 An increased volume of myeloma in the bone marrow trephine is associated with shorter survival.29 Furthermore, the pattern of infiltration has prognostic value, with interstitial infiltration associated with a median overall survival of 65 months, interstitial/nodular infiltration 40 months, diffuse involvement 26 months, and a fibrotic pattern 18 months.29 Another study confirmed the association of a diffuse plasma cell infiltrate with reduced survival, but fibrosis was not predictive of an adverse clinical outcome.7
In rare cases of IgD myeloma, which usually presents as heavy Bence-Jones proteinuria with minimal monoclonal serum paraprotein, intracytoplasmic detection of the heavy chain may indicate a condition with a median survival of only two years.33
Patients with myeloma who have CD86 positive plasma cells using flow cytometry have a worse prognosis than those negative for this antigen.34 Immunohistological staining for CD86 would highlight plasma cells positive for this adverse prognostic marker, but to our knowledge no study using this marker in paraffin wax embedded sections has been published. CD56 negativity has recently been reported in association with plasmablastic myeloma and more aggressive disease.35
ASSESSMENT OF MINIMAL RESIDUAL DISEASE
With median survival now approaching 50 months in allografted patients with myeloma36 and increased interest in the potential benefit of graft versus myeloma effect offered by non-myeloablative “mini-allografts”,37 more sensitive markers of minimal residual disease are required. The European Group for Blood and Marrow Transplantation defines complete remission (CR) as less than 5% plasma cells on morphological assessment of the bone marrow aspirate and trephine. The absence of serum and urine monoclonal paraprotein by immunofixation must also be sustained for at least six weeks.38 Although high dose treatment and stem cell transplantation for myeloma result in CR rates of 24–75%, over 90% of patients relapse, indicating the persistence of clinically relevant residual disease.39 Almost all patients with 13q abnormalities and myeloma will relapse.40 Molecular monitoring of immunoglobulin heavy chain gene rearrangements can detect residual disease in 83–90% of patients postautograft and 50% postallograft.41,42 Interphase fluorescent in situ hybridisation studies reveal a frequent persistence of clonal disease in patients deemed to be in morphological CR, despite high dose treatment.4 The quantity of aberrant plasma cells as determined by flow cytometry has been shown to have prognostic value after transplantation, with CD38/138/56 positive and CD19 negative plasma cells the most common myeloma immunophenotype.43 Such methods are dependent upon high quality aspirate samples, which may be challenging in the context of a hypocellular bone marrow after high dose treatment. Immunohistological assessment for minimal disease does not depend on aspirate quality and may be a useful addition to CR criteria for patients receiving high dose treatment for myeloma. The prognostic value of immunohistologically defined plasma cell aggregates on trephine biopsy and cytological atypia in patients with < 5% plasma cells on aspirate has not been reported.
TUMOUR CELL BIOLOGY
Plasma cell proliferation status
The plasma cell labelling index using flow cytometry is an established method for determining plasma cell proliferation status.44 The MIB1 antibody can be used to detect Ki-67 in plasma cells on paraffin wax embedded sections. Normal plasma cells are usually negative with this antibody.45 Ki-67 expression in myeloma is associated with higher β2 microglobulin, advanced or relapsed disease, and plasmablastic morphology.46 Double immunostaining for Ki-67 and Bcl-2 showed that most proliferating plasma cells (Ki-67+) had weak Bcl-2 expression.47
Angiogenesis
Increased bone marrow microvessel density in myeloma is associated with a higher plasma cell labelling index and poor prognosis.48 Thalidomide has proved beneficial in treating patients with myeloma who are resistant to standard chemotherapy. The antiangiogenic activity of this drug led to investigations into the measurement of microvessel density in the bone marrow as a potential predictor of response. However, a recent study failed to find an association between microvessel density and response to thalidomide.49 Another vascular marker, aquaporin 1 (the erythrocyte water channel), has been reported to stain more immature vessels and to be associated with more active myeloma.50 With continuing research into the potential role of antiangiogenic treatment against myeloma, immunohistological assessment of bone marrow vascularity will remain an important investigative tool.
Tumour resistance
The lack of significant improvement in the five year overall survival rate in patients with myeloma over the past 20 years is in part related to resistance to conventional treatment.51 P glycoprotein (PGP) is associated with anthracycline drug resistance in acute myeloid leukaemia. In patients with myeloma, PGP expression on malignant plasma cells also predicts drug resistance and suggests that such patients should be considered for novel treatments.52 Major vault protein, which is another drug efflux pump, has also been studied immunohistochemically in myeloma. The protein was present in 74% of patients with myeloma but did not impact on overall survival.53 A small study found strong plasma cell Bcl-2 expression in myeloma to be associated with tumour resistance to interferon treatment but not treatment with melphalan/prednisolone.54 The antiapoptotic factors Bcl-2, Bcl-XL, and Mcl-1 are commonly expressed in myeloma cells. Mcl-1, which is upregulated by interleukin 6, has been shown to be the dominant player when myeloma cells overexpressing these factors are challenged with antisense treatment.55
Myeloma specific antigens
Myeloma specific antigen targets would be extremely useful in the diagnosis and follow up of patients with plasma cell neoplasms. Cytoplasmic immunoglobulin restriction is useful at diagnosis, but is more difficult to interpret when the plasma cell burden is low. Some emerging tumour specific antigens include the cancer-testis antigen sperm protein 17, detectable in 26% of patients with myeloma,56 and the MUC-1 antigen.57 These antigens are used as targets for cytotoxic T cell immunotherapy. Immunohistology could identify myeloma cells positive for these aberrant proteins and allow minimal residual disease monitoring after treatment. Pathological plasma cells were found to be CD56 positive and CD19 negative in 62% of cases using a flow cytometric technique.2 Immunohistology could similarly utilise CD56 as a tumour specific plasma cell marker. An antibody recognising the MUM1/IRF4 protein has been reported in association with the t(6;14)(p25;q32) rearrangement. A confounding problem, however, is that IRF4 is expressed in late germinal centre B cells that have undergone somatic hypermutation and is strongly expressed in all elements showing plasma cell differentiation.58 The t(4;14)(p16.3;q32) translocation, which occurs in 15% of multiple myeloma tumours,59 results in enhanced expression of the fibroblast growth factor 3 receptor and multiple myeloma SET domain. Immunohistochemical labelling of this protein and others as a consequence of translocations in myeloma opens an exciting door for the future use of immunohistology in the diagnosis and monitoring of patients receiving specifically targeted myeloma treatment.
“The unique combination of anatomical, antigenic, functional, and molecular information provided by immunohistology will ensure an expanding role in the investigation and management of patients with plasma cell neoplasms”
TECHNICAL CONSIDERATIONS
An adequate biopsy sample of at least 15 × 3 mm is preferred for histological analysis. At our institution, B5 fixative is used. The specimens are decalcified in aqueous hydrogen chloride (RDO solution-Lomb) and dewaxed 2 μm thick sections obtained. Epitope retrieval is by microwave heating and immunostaining is performed using the DakoCytomation LSAB II system on a DakoCytomation autostainer (DakoCytomation, Australia). Table 1 lists the monoclonal antibodies used. As discussed earlier, an antibody panel rather than reliance on a single antibody has the advantage of detecting plasma cells that fail to stain optimally with one particular antibody. Automated staining can enhance throughput in busy pathology services.
Table 1.
Technical details of antibodies used for immunohistology of plasma cell disorders in our laboratory
| Antibody to | Source | Clone |
| BCL-2 | DakoCytomation | 124 |
| Plasma cell | DakoCytomation | VS38c |
| CD138 | DakoCytomation | MI15 |
| κ Light chain | DakoCytomation | R10-21-F3 |
| λ Light chain | DakoCytomation | N10/2 |
| CD79a | DakoCytomation | JCB117 |
| CD20 | DakoCytomation | L26 |
Take home messages.
Immunohistology has an important role in the diagnosis and management of patients with suspected and established plasma cell neoplasms
It also provides a wealth of information regarding the biology of myeloma and related disorders in the context of preserved anatomical architecture
When assessing immunostained bone marrow biopsies, we use the following definitions for describing the pattern of plasma cell infiltration.
Interstitial: interspersed plasma cells with no focal collections (fig 1).
Microaggregates: collections of 10 to 30 plasma cells often occupying inter-fat spaces (fig 2A,B).
Nodular: focal, well defined collections of more than 30 plasma cells (fig 2C).
Diffuse: sheets of plasma cells with replacement of the fat spaces.
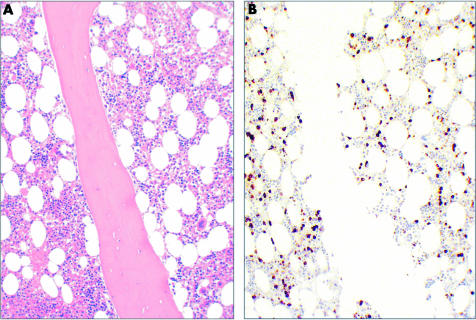
Figure 1.
(A) Haematoxylin and eosin staining and (B) immunohistochemical staining for Bcl-2 showing an interstitial plasma cell infiltrate in a patient with monoclonal gammopathy of undetermined significance (original magnification, ×100).
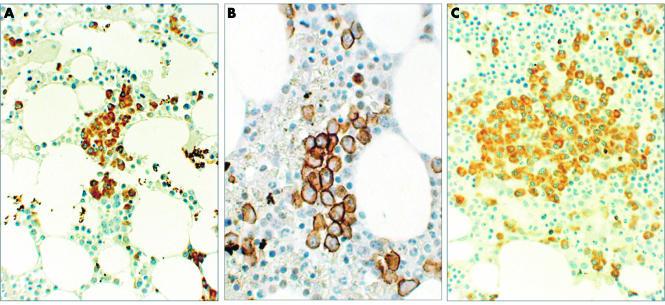
Figure 2.
(A) Staining for Bcl-2 showing a plasma cell microaggregate in a patient with indolent multiple myeloma (original magnification, ×200); (B) staining for CD138 showing plasma cell infiltration in a patient with early relapse of myeloma post-transplant (original magnification, ×400); (C) staining for VS38c showing a plasma cell nodule in a patient with multiple myeloma (original magnification, × 200).
SUMMARY AND CONCLUSIONS
The plasma cell dyscrasias cover a broad spectrum of clinical disorders presenting many diagnostic and therapeutic challenges for the treating clinician. Immunohistology is useful in the assessment of “benign” versus malignant plasma cell disorders and the distinction of MGUS from early myeloma. Immunohistology can provide information about the spatial characteristics of plasma cell involvement, with greater distinction from background haemopoietic elements than standard H&E sections. This may have particular value in the monitoring of low amounts of disease after high dose treatment or in bone marrow assessment before autologous stem cell collection. The increasing availability of tumour specific antibodies suitable for paraffin wax section immunostaining will enhance the accuracy of diagnosis and residual disease monitoring. Immunohistological analysis of multidrug resistance expression, microvessel density, and antiapoptotic factors will provide important information regarding the biology of myeloma when new treatments are considered. The unique combination of anatomical, antigenic, functional, and molecular information provided by immunohistology will ensure its expanding role in the investigation and management of patients with plasma cell neoplasms.
Acknowledgments
Dr A Wei is supported in part by grant 7015-02 from the Leukemia-Lymphoma Society. We are grateful to F Feleppa for carrying out the immunohistological staining on our cases. DakoCytomation were approached and agreed to pay for the cost of printing colour images after the manuscript had been accepted for publication.
Abbreviations
CR, complete remission
CSH, crystal storage histiocytosis
H&E, haematoxylin and eosin
MGUS, monoclonal gammopathy of undetermined significance
MUM1/IRF4, multiple myeloma oncogene 1/interferon regulatory factor 4
PGP, P glycoprotein
WHO, World Health Organisation
WM, Waldenström’s macroglobulinaemia
REFERENCES
- 1.Jaffe ES, Harris NL, Stein H, et al, eds. World Health Organisation classification of tumours. Pathology and genetics of tumours of haemopoeitic and lymphoid tissues. IARC Press: Lyon, 2001.
- 2.Almeida J, Orfao A, Ocqueteau M, et al. High-sensitive immunophenotyping and DNA ploidy studies for the investigation of minimal residual disease in multiple myeloma. Br J Haematol 1999;107:121–31. [DOI] [PubMed] [Google Scholar]
- 3.Maes B, Achten R, Demunter A, et al. Evaluation of B cell lymphoid infiltrates in bone marrow biopsies by morphology, immunohistochemistry, and molecular analysis. J Clin Pathol 2000;53:835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genevieve F, Zandecki M, Lai JL, et al. Evaluation of minimal residual disease by interphase FISH in multiple myeloma: does complete remission exist? Leukemia 1999;13:641–4. [DOI] [PubMed] [Google Scholar]
- 5.Bain B, Clark D, Lampert I. Bone marrow pathology, 2nd ed. Oxford: Blackwell Science Ltd, 1996.
- 6.Gorden L, Smith C, Graber SE. Marked plasmacytosis and immunoglobulin abnormalities following infusion of streptokinase. Am J Med Sci 1999;301:186–9. [DOI] [PubMed] [Google Scholar]
- 7.Sukpanichnant S, Cousar JB, Leelasiri A, et al. Diagnostic criteria and histologic grading in multiple myeloma: histologic and immunohistologic analysis of 176 cases with clinical correlation. Hum Pathol 1994;25:308–18. [DOI] [PubMed] [Google Scholar]
- 8.Eckert F, Schmid L, Kradolfer D, et al. Bone-marrow plasmacytosis—an immunohistological study. Blut 1986;53:11–19. [DOI] [PubMed] [Google Scholar]
- 9.Lebeau A, Zeindl-Eberhart E, Müller E, et al. Generalized crystal-storing histiocytosis associated with monoclonal gammopathy: molecular analysis of a disorder with rapid clinical course and review of the literature. Blood 2002;100:1817–27. [PubMed] [Google Scholar]
- 10.Miguel-Garcia A, Orero T, Matutes E, et al. Bcl-2 expression in plasma cells from neoplastic gammopathies and reactive plasmacytosis: a comparative study. Haematologica 1998;83:298–304. [PubMed] [Google Scholar]
- 11.Pileri S, Poggi S, Baglioni P, et al. Histology and immunohistology of bone marrow biopsy in multiple myeloma. Eur J Haematol Suppl 1989;51:52–9. [DOI] [PubMed] [Google Scholar]
- 12.Kyle RA. “Benign” monoclonal gammopathy—after 20 to 35 years of follow-up. Mayo Clin Proc 1993;68:26–36. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, Moulopoulos LA, Maniatis A, et al. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood 2000;96:2037–44. [PubMed] [Google Scholar]
- 14.Liebross RH, Ha CS, Cox JD, et al. Solitary bone plasmacytoma: outcome and prognostic factors following radiotherapy. Int J Radiat Oncol Biol Phys 1998;41:1063–7. [DOI] [PubMed] [Google Scholar]
- 15.Beschorner R, Horny HP, Petruch UR, et al. Frequent expression of haemopoietic and non-haemopoietic antigens by reactive plasma cells: an immunohistochemical study using formalin-fixed, paraffin-embedded tissue. Histol Histopathol 1999;14:805–12. [DOI] [PubMed] [Google Scholar]
- 16.Boo K, Cheng S. A morphological and immunohistochemical study of plasma cell proliferative lesions. Malays J Pathol 1992;14:45–8. [PubMed] [Google Scholar]
- 17.Gertz MA, Lacy MQ, Dispenzieri A. Amyloidosis: recognition, confirmation, prognosis, and therapy. Mayo Clin Proc 1999;74:490–4. [DOI] [PubMed] [Google Scholar]
- 18.Wu SS, Brady K, Anderson JJ, et al. The predictive value of bone marrow morphologic characteristics and immunostaining in primary (AL) amyloidosis. Am J Clin Pathol 1991;96:95–9. [DOI] [PubMed] [Google Scholar]
- 19.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol 1995;32:45–59. [PubMed] [Google Scholar]
- 20.Bartl R, Frisch B, Mahl G, et al. Bone marrow histology in Waldenstrom’s macroglobulinaemia. Clinical relevance of subtype recognition. Scand J Haematol 1983;31:359–75. [DOI] [PubMed] [Google Scholar]
- 21.Owen R, Barrans S, Richards S, et al. Waldenström macroglobulinemia development of diagnostic criteria and identification of prognostic factors. Am J Clin Pathol 2001;116:420–8. [DOI] [PubMed] [Google Scholar]
- 22.San Miguel JF, Gonzalez M, Gascon A, et al. Immunophenotypic heterogeneity of multiple myeloma: influence on the biology and clinical course of the disease. Castellano-Leones (Spain) cooperative group for the study of monoclonal gammopathies. Br J Haematol 1991;77:185–90. [DOI] [PubMed] [Google Scholar]
- 23.Tani E, Santos GC, Svedmyr E, et al. Fine-needle aspiration cytology and immunocytochemistry of soft-tissue extramedullary plasma-cell neoplasms. Diagn Cytopathol 1999;20:120–4. [DOI] [PubMed] [Google Scholar]
- 24.Chilosi M, Adami F, Lestani M, et al. CD138/syndecan-1: a useful immunohistochemical marker of normal and neoplastic plasma cells on routine trephine bone marrow biopsies. Mod Pathol 1999;12:1101–6. [PubMed] [Google Scholar]
- 25.Bayer-Garner IB, Sanderson RD, Dhodapkar MV, et al. Syndecan-1 (cd138) immunoreactivity in bone marrow biopsies of multiple myeloma: shed syndecan-1 accumulates in fibrotic regions. Mod Pathol 2001;14:1052–8. [DOI] [PubMed] [Google Scholar]
- 26.Turley H, Jones M, Erber W, et al. VS38: a new monoclonal antibody for detecting plasma cell differentiation in routine sections. J Clin Pathol 1998;47:418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papakonstantinou G, Verbeke C, Hastka J, et al. Bcl-2 expression in non-Hodgkin’s lymphomas is not associated with bcl-2 gene rearrangements. Br J Haematol 2001;113:383–90. [DOI] [PubMed] [Google Scholar]
- 28.Athanasiou E, Kaloutsi V, Kotoula V, et al. Cyclin D1 overexpression in multiple myeloma. A morphologic, immunohistochemical, and in situ hybridization study of 71 paraffin-embedded bone marrow biopsy specimens. Am J Clin Pathol 2001;116:535–42. [DOI] [PubMed] [Google Scholar]
- 29.Sonoki T, Hata H , Kuribayashi N, et al. Expression of PRAD1/cyclin D1 plasma cell malignancy: incidence and prognostic aspects. Br J Haematol 1999;104:614–17. [DOI] [PubMed] [Google Scholar]
- 30.Wilson CS, Butch AW, Lai R, et al. Cyclin D1 and E2F-1 immunoreactivity in bone marrow biopsy specimens of multiple myeloma: relationship to proliferative activity, cytogenetic abnormalities and DNA ploidy. Br J Haematol 2001;112:776–82. [DOI] [PubMed] [Google Scholar]
- 31.Rajkumar SV, Greipp PR. Prognostic factors in multiple myeloma. Hematol Oncol Clin North Am 1999;13:1295–314. [DOI] [PubMed] [Google Scholar]
- 32.Sailer M, Vykoupil KF, Peest D, et al. Prognostic relevance of a histologic classification system applied in bone marrow biopsies from patients with multiple myeloma: a histopathological evaluation of biopsies from 153 untreated patients. Eur J Haematol 1995;54:137–46. [DOI] [PubMed] [Google Scholar]
- 33.Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am 1999;13:1259–72. [DOI] [PubMed] [Google Scholar]
- 34.Pope B, Brown RD, Gibson J, et al. B7-2-positive myeloma: incidence, clinical characteristics, prognostic significance, and implications for tumor immunotherapy. Blood 2000;96:1274–9. [PubMed] [Google Scholar]
- 35.Naohi S, Akihiro T, Kazuyuki S, et al. Clinicopathological and prognostic characteristics of CD56-negative multiple myeloma. Br J Haematol 2002;117:882. [DOI] [PubMed] [Google Scholar]
- 36.Gahrton G, Svensson H, Cavo M, et al. The European group for blood and marrow transplantation. Progress in allogenic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983–93 and 1994–8 at European group for blood and marrow transplantation centres. Br J Haematol 2001;113:209–16. [DOI] [PubMed] [Google Scholar]
- 37.Alyea E, Weller E, Schlossman R, et al. T-cell-depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood 2001;98:934–9. [DOI] [PubMed] [Google Scholar]
- 38.Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma subcommittee of the EBMT. European group for blood and marrow transplant. Br J Haematol 1998;102:1115–23. [DOI] [PubMed] [Google Scholar]
- 39.The UK Myeloma Forum Guidelines Working Group. Diagnosis and management of multiple myeloma. Br J Haematol 2001;115:522–40. [DOI] [PubMed] [Google Scholar]
- 40.Desikan R, Barlogie B, Sawyer J, et al. Results of high-dose therapy for 1000 patients with multiple myeloma: durable complete remissions and superior survival in the absence of chromosome 13 abnormalities. Blood 2000;95:4008–10. [PubMed] [Google Scholar]
- 41.Corradini P, Voena C, Tarella C, et al. Molecular and clinical remissions in multiple myeloma: role of autologous and allogeneic transplantation of hematopoietic cells. J Clin Oncol 1999;17:208–15. [DOI] [PubMed] [Google Scholar]
- 42.Martinelli G, Terragna C, Zamagni E, et al. Molecular remission after allogeneic or autologous transplantation of hematopoietic stem cells for multiple myeloma. J Clin Oncol 2000;18:2273–81. [DOI] [PubMed] [Google Scholar]
- 43.Rawstron AC, Davies FE, DasGupta R, et al. Flow cytometric disease monitoring in multiple myeloma: the relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood 2002;9:3095–100. [DOI] [PubMed] [Google Scholar]
- 44.Pope B, Brown R, Gibson J, et al. The bone marrow plasma cell labeling index by flow cytometry. Cytometry 1999;38:286–92. [DOI] [PubMed] [Google Scholar]
- 45.Pellegrini W, Facchetti F, Marocolo D, et al. Assessment of cell proliferation in normal and pathological bone marrow biopsies: a study using double sequential immunophenotyping on paraffin sections. Histopathology 1995;5:397–405. [DOI] [PubMed] [Google Scholar]
- 46.Drach J, Gattringer C, Glassl H, et al. The biological and clinical significance of the KI-67 growth fraction in multiple myeloma. Hematol Oncol 1992;10:125–34. [DOI] [PubMed] [Google Scholar]
- 47.Miguel-Garcia A, Orero T, Matutes E, et al. Bcl-2 expression in plasma cells from neoplastic gammopathies and reactive plasmacytosis: a comparative study. Haematologica 1998;83:298–304. [PubMed] [Google Scholar]
- 48.Vacca A, Frigeri A, Ribatti D, et al. Microvessel overexpression of aquaporin 1 parallels bone marrow angiogenesis in patients with active multiple myeloma. Br J Haematol 2001;113:415–21. [DOI] [PubMed] [Google Scholar]
- 49.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 1999;341:1565–71. [DOI] [PubMed] [Google Scholar]
- 50.Vacca A, Ribatti D, Roccaro AM, et al. Bone marrow angiogenesis and plasma cell angiogenic and invasive potential in patients with active multiple myeloma. Acta Haematol 2001;106:162–9. [DOI] [PubMed] [Google Scholar]
- 51.Bergsagel PL, Kuehl WM. Multiple myeloma—molecular pathogenesis of multiple myeloma. Education program book of the American Society of Haematology 2001:157–77. [DOI] [PubMed]
- 52.Dalton WS. Detection of multidrug resistance gene expression in multiple myeloma. Leukemia 1997;11:1166–9. [DOI] [PubMed] [Google Scholar]
- 53.Rimsza LM, Campbell K, Dalton WS The major vault protein (MVP), a new multidrug resistance associated protein, is frequently expressed in multiple myeloma. Leuk Lymphoma 1999;34:315–24. [DOI] [PubMed] [Google Scholar]
- 54.Sangfelt O, Osterborg A, Grander D, et al. Response to interferon therapy in patients with multiple myeloma correlates with expression of the Bcl-2 oncoprotein. Int J Cancer 1995;63:190–2. [DOI] [PubMed] [Google Scholar]
- 55.Derenne S, Monia B, Dean N, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bl-xl is an essential survival protein of human myeloma cells. Blood 2002;100:194–9. [DOI] [PubMed] [Google Scholar]
- 56.Lim SH, Wang Z, Chiriva-Internati M, et al. Sperm protein 17 is a novel cancer-testis antigen in multiple myeloma. Blood 2001;97:1508–10. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi T, Makiguchi Y, Hinoda Y, et al. Expression of MUC1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient. J Immunol 1994;153:2102–9. [PubMed] [Google Scholar]
- 58.Falini B, Fizzotti M, Pucciarini A, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood 2000;95:2084–92. [PubMed] [Google Scholar]
- 59.Avet-Loiseau H, Li JY, Facon T, et al. High incidence of translocations t(11;14)(q13;q32) and t(4;14)(p16;q32) in patients with plasma cell malignancies. Cancer Res 1998;58:5640–5. [PubMed] [Google Scholar]