Abstract
Aims: To evaluate a recently developed digital slide and virtual microscope system, and to compare this method with optical microscopy on routine gastrointestinal biopsy specimens in both local and remote access modes.
Methods: A fully computer controlled commercial microscope was used. The scanning program included object detection, autofocus, and image compression algorithms. The overall hard disk space for a gastric biopsy was between 30 and 50 MB and the scanning time was between 20 and 40 minutes. Haematoxylin and eosin stained routine gastric (61) and colon (42) biopsy specimens were selected, scanned, and evaluated by two specialists on an optical (OM) and virtual microscope (VM).
Results: The overall concordance of VM and OM with the consensus diagnosis was 95.1% and 97%, respectively. Clinically important concordance was 96.1% and 98% for VM and OM, respectively. The two methods showed concordance in 92% of cases and clinically important concordance in 94.1% of cases. The reasons for discordance were image quality (one case), interpretation difference (three cases), and insufficient clinical information (three cases). Remote evaluation of the digital slides through the Internet has the advantages of the previously used static and dynamic telepathology methods.
Conclusions: Diagnostic concordance was found between OM and VM. The digital slide and the virtual microscope can be alternative techniques in the computerisation of the histology laboratory and in teleconsultation services after further evaluation of time and storage constraints.
Keywords: digital slide, digital microscope, gastric biopsy, section
As a result of the rapid developments in information technology, medical digital image analysis is becoming a widely accepted technique.1 Digital techniques and laboratories2 already exist for radiological applications. New stand alone automated microscope systems have been developed for histological and cytological applications in the past decade.
The automated screening and rescreening of cervical smears is now available for routine practice.3,4 In addition, several new semiautomatic microscopes have been developed to aid in quality control of the cytotechnologists’ work.5–8 These systems notify the observer of the scanned areas and images, and electronic recording of selected images is also available.
Automated histological analysis has been an important research tool for several years, but until recently it was not suitable for routine applications.9,10 However, now histological diagnoses can be supported by new electronic techniques, such as TV image cytometry and teleconsultation based on histological images rather than entire samples.11
“Automated histological analysis has been an important research tool for several years, but until recently it was not suitable for routine applications”
Telepathology services were built up during the past decade around two technology platforms. Dynamic telepathology uses remote controlled microscope systems with high throughput online image transport channels.12,13 This method has the advantage of entire slide access and lacks the error source of preselected microscopical frames, although the costs of setting up and running the system are relatively high. The use of static preselected images for teleconsultation needs much less hardware investment, but sampling errors can occur.14,15 Using the Internet as a telecommunication pathway for static images is a low cost widely available alternative.16,17
The applications of digital slides are considerable, as sources and targets of telepathology and automated histological analysis; however, attempts to prepare electronic or digital slides have been limited (R Ferreira et al. Digital dynamic telepathology—the virtual microscope. Proceedings of the 1997 AMIA Annual Fall Symposium, 1997).18,19 Only recently has the storage capacity and speed of personal computers become sufficient to handle the extremely large amount of image information stored on a slide. In addition, low cost, commercial motorised microscopes have recently been developed by several manufacturers.
Working on digital slides requires virtual or digital microscopy. Preliminary positive results on a limited number of mosaicked microscopic images have been reported recently.20
The aim of our present study was to evaluate our recently reported digital slide and microscopy system21 on routine gastrointestinal biopsy specimens. We compared the performance of optical and digital microscope evaluations in local and remote modes.
MATERIALS AND METHODS
Gastric routine biopsy specimen analysis
Biopsy specimens were placed in buffered formalin, routinely processed, and stained with haematoxylin and eosin. Altogether, 103 specimens were selected from the files of the first department of pathology. Single representative slides were evaluated from each case. Table 1 shows the distribution of the cases.
Table 1.
Diagnoses
| Organ | Diagnoses | No of cases |
| Gastric | Healthy | 5 |
| Chronic gastritis without intestinal metaplasia | 16 | |
| Chronic gastritis with intestinal metaplasia | 12 | |
| Chronic gastritis with atrophia | 3 | |
| Adenocarcinoma, well differentiated, intestinal type | 9 | |
| Adenocarcinoma, non-differentiated | 6 | |
| Sigillocellular carcinoma | 4 | |
| Anaplastic carcinoma | 2 | |
| Adenopapillar carcinoma | 1 | |
| Hyperplastic polyp | 1 | |
| MALT lymphoma | 2 | |
| Colon | Healthy | 5 |
| Colitis ulcerosa | 14 | |
| Crohn’s disease | 7 | |
| Chronic aspecific colitis | 4 | |
| Hyperplastic polyp | 2 | |
| Adenoma tubulare with severe dysplasia | 2 | |
| Adenoma tubulovillusom with severe dysplasia | 1 | |
| Adenoma papillare | 1 | |
| Adenoma tubulopapillare | 1 | |
| Adenoma villosum with dysplasia | 1 | |
| Adenocarcinoma | 4 |
MALT, mucosa associated lymphoid tissue.
The histological sections were evaluated in separate settings on a virtual microscope (VM) and an optical microscope (OM), without knowledge of the results of the previous analysis by two independent, board certified histologists.
First, the glass slides were assessed by optical microscopy. The paper copies of the clinical histories and the evaluation report were collected.
Over the next few weeks the slides were digitised; this was carried out in the digital microscopy laboratory. The scanning computer was also used as a digital slide server. The virtual microscopy evaluation was done on a separate local area network workstation in the pathology department with access to the slide server computer using the Internet.
Data analysis
At the end of the study, when all the optical and virtual microscopy results were available, the consensus data, concordance, and source of diagnostic discordance were determined.
Concordance was designated level “A”. Levels “B” and “C” were clinically unimportant and important discordance, respectively, as suggested by Weinberg et al.22
Those cases with discordant results were collected together with the optical and electronic data. The final diagnosis and definition of the source of the discordant data were agreed upon at a consensus session in the consultation room. Here, access to the digital slides was also available through a computer workstation and the Internet.
The reasons for discordance were classified by the consensus meeting as follows: inadequate image quality (class I), interpretation (class II), and insufficient clinical information (class III).
The significance of the concordance was determined using Kendall’s concordance coefficient determined by the Statistica program package (V.4.3, Statsoft, Tulsa, Oklahoma, USA).
Slide digitisation and the virtual microscope system
Hardware tools used
We used an Axioplan 2 MOT (Carl Zeiss, Jena, Germany) microscope. The microscope functions (objectives, stage, focus, illumination, and filters) can be controlled and changed through the RS232 interface from an application program. The mechanical accuracy of the motorised scanning stage for X/Y and Z directions was 0.5 μm.
We used the Grundig FAC 830 1/2 inch, one chip CCD camera with 752 × 581 (440 000) pixels. The integration time of the camera can be controlled through the computer interface RS485. The applied image digitisation card was the Screen Machine II from Fast Electronic Germany (Munich, Germany) with a resolution of 640 × 560 pixels and 64 K colour depth. The programs were running on computers with at least an Intel Pentium II 350 MHz processor, 128 MB RAM, and 2 GB hard disk.
Features of the digital slide scanning program
Scanning area determination was performed by upper right and lower left corner fixing.
Autoscanning was started after setting the stage at the zero position. During the scanning process autofocusing was done only at each third to fifth field of view. All the images were compressed in JPEG format and stored in the slide databank in the corresponding position. Autofocusing was done using Brenner’s algorithm.23 At ×40 magnification, 125 856 frames would need to be stored for the entire slide. However, a threshold filter was used to store only those frames with image content. In this way, only the area containing the biopsy was stored, amounting to 302 to 1334 frames for each slide.
First, the fields of view were mosaicked using mathematical algorithms24; however, the error between the required and real positions of the stage was found to be less than ± 0.5 μm. Based on this high precision no software mosaic alignment was used.
Features of the virtual microscope program
Slide selection
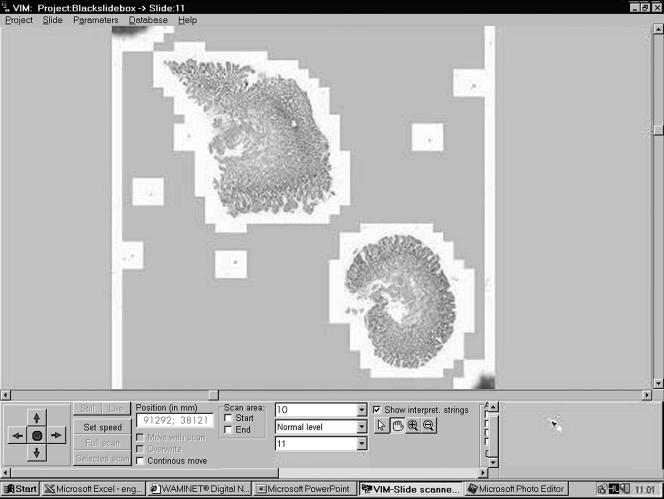
After scanning, the slides were stored in subdirectories called projects for a higher ordering. After selection an electronically minimised slide image is shown on the screen (fig 1).
Figure 1.
The user interface of the virtual microscope program. The artificial magnification (×10) of a scanned gastric biopsy section is shown on the screen. Note the moving arrows in the left bottom corner and the + and – labels in the middle. These can be used for discrete step magnification and the reduction of a specified area of the slide. On the right bottom of the screen the scanned area of the slide is highlighted.
Slide orientation map
This map represents the whole slide, where one pixel on the screen corresponds to one field of view. The recorded segments are labelled with white pixels on a grey background.
Applicable electronic magnifications
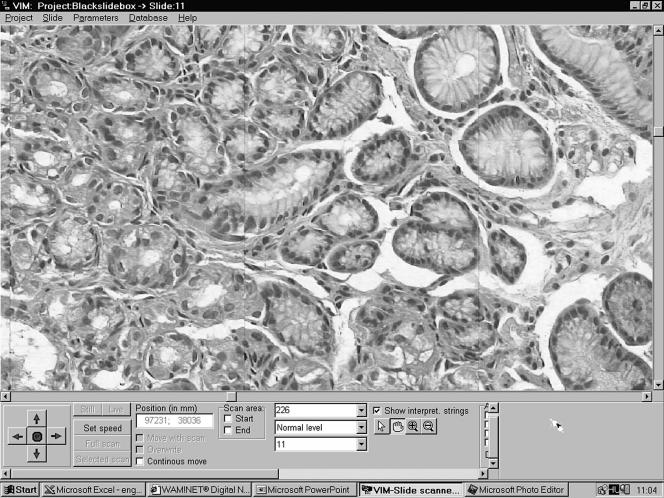
After finding the area of interest, the user has several options for magnification of the selected segments. The prepared magnification steps of ×100 and ×200 or special “+” and “−” mouse arrows can be used to change the magnification (figs 1–3).
Figure 2.
An intermediate magnification of a scanned gastric biopsy specimen (original magnification, ×200).
Figure 3.
The real world scanning magnification (original magnification, ×400) on the virtual microscope. The borders between the mosaicked microscopic field of views can also be detected.
Moving and scrolling the slide
If the interested area is not on the screen at the required magnification, then the moving arrows (up, down, left, and right) can be used to move the slide in any direction.
Labelling of interesting frames for re-evaluation, consultation, and reporting
Up to 10 coloured labels can be placed on the digital slide in the software. This option can also be used for reconsultation by experts via the local area network or in specific cases via the Internet.
Internet access
Every virtual microscopy workstation can be a slide server too. On the remote workstation the slide server’s IP address has to be defined. After connecting to the server computer, a list of available slides and the information about these slides is transported to the client workstation. After selecting a slide, its electronically compressed low resolution image is transferred to the workstation. Every image that is transported during the evaluation will be stored on the local machine for safety reasons.
RESULTS
Concordance
Discordance was found in eight cases (7.8%) between the VM or OM results and the consensus diagnosis. In five cases the OM and in three cases the VM yielded the correct diagnosis.
There was concordance in 95 (92.2%) of the 103 cases, and clinically important concordance in 97 cases (94.1%). OM yielded higher (100 of 103; 97%) concordance with the consensus results than did VM (98 of 103; 95.1%). The highest degree of concordance for clinically important diagnosis was also seen for OM (101 of 103; 98%) (table 2).
Table 2.
Concordance between optical microscopy, virtual microscopy, and the consensus diagnosis
| Concordance (all types) | Concordance (clinically important) | |
| OM v VM | 92% (95/103) | 94% (97/103) |
| OM v Con | 97% (100/103) | 98% (101/103) |
| VM v Con | 95% (98/103) | 96% (99/103) |
Con, consensus; OM, optical microscopy; VM, virtual microscopy.
Reasons for diagnostic discordance
Because the entire specimen was digitised there was no sampling error. However, the other common sources of error in telepathology—low image quality (one of eight), interpretation (four of eight), and insufficient clinical information (three of eight) were seen.
The effects of poor image quality were seen in only one case, although this was a clinically important one. In this case, the VM diagnosis was ulceration ventriculi and the OM diagnosis was ulcerated well differentiated adenocarcinoma ventriculi. The correct diagnosis was achieved by evaluating multiple focus levels in OM, an option that is not currently available in VM (table 3).
Table 3.
Reasons for diagnostic discordance
| Level of discordance | Reason for discordance | VM diagnosis | OM diagnosis | Review consensus diagnosis |
| B | II | Chronic gastritis | Acute and chronic gastritis | VM |
| C | III | Gastric adenoma | Gastric hyperplastic polyp | VM |
| C | I | Ulceration ventriculi | Ulcerated, well differentiated adenocarcinoma | OM |
| C | II | Chronic atrophic gastritis | Chronic gastritis with low grade dysplasia | OM |
| B | II | Hyperplastic polyp | Hyperplastic mucosa | OM |
| C | II | Mild colitis | Normal colon | OM |
| C | III | Colitis ulcerosa with dysplasia | Colitis ulcerosa | VM |
| C | III | Adenoma papillare | Hyperplastic polyp | OM |
Discordance: B, clinically not important; C, clinically important. Reason for discordance: I, image quality; II, interpretation; III, insufficient clinical information.
OM, optical microscopy; VM, virtual microscopy.
Technical and practical data regarding the application of VM
The hard disk volume of a microscopic field of view is between 60 and 100 KB after JPEG compression. The overall hard disk space required for a gastric biopsy is between 30 and 50 MB and the scanning time is between 20 to 40 minutes, depending on the number and area of sections on a slide.
Evaluating the specimen on the monitor is more comfortable for the histologist, more reproducible, and more easily documented when compared with the optical method (table 4).
Table 4.
Comparison between optical and virtual microscope evaluation methods
| Microscopy feature | Optical evaluation | Electronic evaluation |
| Speed | 4–5 minutes | 2–3 minutes |
| Costs | Every user, high quality microscope | One high quality scanning microscope, image server, PC |
| Working conditions | Only limited number of slides can be evaluated because of fatigue | Normal monitor work |
| Consultation | With slide transportation or with image transfer, through ISDN, telephone line | Internet Remote access to the image server through local area network or internet |
| Quality control | With special additional hardware | By recording of evaluated image segments |
Remote access via the Internet was relatively fast. The first minimised image was uploaded in real time. As the user switched over to high magnification the single fields of view were seen on the screen in seconds (figs 2,3).
DISCUSSION
In our study, we evaluated the technical feasibility and diagnostic concordance of a digital histological section evaluation system and compared it with the routine OM procedure.
Low cost computational techniques were used. The scanning speed was too slow for routine applications, but there are free resources available for increasing scanning speed by several magnitudes. The digitisation card should be changed to a higher resolution and speed (×10). The computer should also be updated (×5). In addition, an automated microscope could be considered.
Image quality should be further enhanced using a digital one chip, analogue three chip camera instead of the analogue, one chip camera used at present. In this case, an increase in diagnostic accuracy would be expected.
However, these developments and results show that in the near future we can have microscope free virtual microscopy workstations with the functions of the optical microscope. One advantage would be a more comfortable working environment for histologists. In addition, it would also support quality control techniques and consultation with remote experts. The first application of digital slides and virtual microscopy should be telepathology consultation. However, later this technology could be used in the routine histology evaluation process, as was done in our study.
Remote evaluation of the slides through the Internet has the advantages of the previously used static or dynamic telepathology methods (the entire slide is available at high magnification, microscope and remote assistance free evaluation).25
We found a higher concordance with optical microscopy than previous static image based telepathology studies.13–15 This might be explained by the fact that this technology eliminates sampling error.
Time constraints and fatigue were not evaluated. We are planning to assess these factors in a larger interlaboratory study.
“In the near future we can have microscope free virtual microscopy workstations with the functions of the optical microscope”
The reasons for discordance yielded new information for future developments. In selected cases several focus planes are required for correct diagnosis. The virtual microscope cannot be used for the analysis of underlying cells. However, with further technical and mathematical developments several focus levels can be recorded, stored, and evaluated by the virtual microscope.
In his 1996 review article, O’Brien stated that the computerisation of the histology laboratory was desirable but that it would be far in the future.26 More, recently, Leong and McGee27 stated that complete automated slide digitisation has influence at all levels of clinical practice and education. They emphasised the importance of dedicated software technology. Recently, the usefulness of digital slides and virtual microscopy for quality assurance and teaching applications has been demonstrated.28,29
Our results show that digital slides and virtual microscopy technology can be used in selected cases for telepathology consultation. After the definition of the time and storage requirements the routine use of this technique could also be considered.
Take home messages.
We found diagnostic concordance between optical microscopy and virtual microscopy on gastrointestinal biopsy specimens
Virtual microscopy has the advantage of providing a more comfortable working environment for histologists
Virtual microscopy also supports quality control techniques and consultation with remote experts
Initially, digital slides and virtual microscopy should be used in telepathology consultation
After further evaluation of time and storage constraints this technology might be useful in routine histology
Abbreviations
OM, optical microscopy
VM, virtual microscopy
REFERENCES
- 1.Shotton DM. Robert Feulgen prize lecture 1995. Electronic light microscopy: present capabilities and future prospects. Histochem Cell Biol 1995;104:97–137. [DOI] [PubMed] [Google Scholar]
- 2.Dooley RL, Engel C, Muller ME. Automated scanning and digitizing of roentgenographs for documentation and research. Clin Orthop 1997;274:113–19. [PubMed] [Google Scholar]
- 3.Mango LJ, Ivasauskas EZ. Computer assisted cervical cytology using the PAPNET testing. In: Wied GL, Keebler CM, Rosenthal DL, et al, eds. Compendium on quality assurance, proficiency testing and workload limitations on clinical cytology. Chicago, USA: Tutorials of Cytology, 1995:155–67.
- 4.Hailey DM, Lea R. Prospects for newer technologies in cervical cancer screening programmes. J Qual Clin Pract 1995;15:139–45. [PubMed] [Google Scholar]
- 5.Baker RW, Wadsworth J, Brugal G, et al. An evaluation of “rapid review” as a method of quality control of cervical smears using the AxioHOME microscope. Cytopathology 1998;8:85–95. [DOI] [PubMed] [Google Scholar]
- 6.Grohs DH, Dadeshidze VV, Domanik RA, et al. Utility of the TracCell system in mapping Papanicolaou-stained cytologic material. Acta Cytol 1997;41:144–52. [DOI] [PubMed] [Google Scholar]
- 7.Anderson TL, Nelson AC. Quality control and proficiency testing of cytological smear screening: an integrated approach using automation. In: Wied GL, Keebler CM, Rosenthal DL, et al, eds. Compendium on quality assurance, proficiency testing and workload limitations on clinical cytology. Chicago, USA: Tutorials of Cytology, 1995:83–287.
- 8.Grohs DH, Gombrich PP, Domanik RA. AccuMed International, Inc. Meeting the challenges in cervical cancer screening: the AcCell Series 2000 automated slide handling and data management system. Acta Cytol 1996;40:26–30. [DOI] [PubMed] [Google Scholar]
- 9.Ong SH, Jin XC, Sinniah R. Image analysis of tissue sections. Comput Biol Med 1996;26:269–79. [DOI] [PubMed] [Google Scholar]
- 10.Belhomme P, Elmoataz A, Herlin P, et al. Generalised region growing operator with optimal scanning: application to segmentation of breast cancer images. J Microsc 1996;886:41–50. [DOI] [PubMed] [Google Scholar]
- 11.Dictor M. The surgical pathologist in a client/server computer network: work support, quality assurance, and the graphical user interface. Mod Pathol 1997;10:259–66. [PubMed] [Google Scholar]
- 12.Dunn BE, Almagro UA, Choi H, et al. Dynamic-robotic telepathology: Department of Veteran Affairs feasibility. Hum Pathol 1997;28:8–12. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein RS, Battacharayya AK, Graham AR, et al. Telepathology a ten-year progress report. Hum Pathol 1997;28:1–7. [DOI] [PubMed] [Google Scholar]
- 14.Cross SS, Burton JL, Dube AK, et al. Offline telepathology diagnosis of colorectal polyps: a study of interobserver agreement and comparison with glass slide diagnoses. J Clin Pathol 2002;55:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tucker JH, Busch C, Spatz A, et al. An experimental inter-expert telepathology network using static imaging. J Clin Pathol 2002;52:761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montironi R, Thompson D, Scarpelli M, et al. Transcontinental communication and quantitative digital histopathology via the Internet: with special reference to prostate neoplasia. J Clin Pathol 2002;55:305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gombas P, Szende B, Stotz G. Support by telecommunications in diagnostic pathology. Experience with the first telepathology system in Hungary. Orv Hetil 1996;137:2299–303. [PubMed] [Google Scholar]
- 18.Burns BF. Creating low power photomicrographs using a 35 mm digital slide scanner. Am J Surg Pathol 1997;21:865–6. [DOI] [PubMed] [Google Scholar]
- 19.Demichelis F, Barbareschi M, Dalla Palma P, et al. The virtual case: a new method to completely digitize cytological and histological slides. Virchows Arch 2002;441:159–64. [DOI] [PubMed] [Google Scholar]
- 20.Singson RPC, Natarajan S, Greenson JK, et al. Virtual microscopy and the Internet as telepathology consultation tools. A study of gastrointestinal biopsy specimens. Am J Pathol 1999;111:792–5. [DOI] [PubMed] [Google Scholar]
- 21.Molnar B, Papik K, Tagscherer A, et al. Three-dimensional reconstruction and analysis of gastric malignancies by electronic slides of consecutive sections and virtual microscopy. Proceedings of the International Society for Optical Engineering 1999;3605:190–200. [Google Scholar]
- 22.Weinberg DS, Allaert FA, Dusserre P, et al. Telepathology diagnosis by means of digital still images: an international validation study. Hum Pathol 1996;27:111–18. [DOI] [PubMed] [Google Scholar]
- 23.Firestone L, Cook K, Culp K, et al. Comparison of autofocus methods for automated microscopy. Cytometry 1991;12:195–206. [DOI] [PubMed] [Google Scholar]
- 24.Ott SR. Acquisition of high-resolution digital images in video microscopy: automated image mosaicking on a desktop microcomputer. Microsc Res Tech 1997;38:335–43. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg DS. How is telepathology being used to improve patient care. Clin Chem 1996;42:831–5. [PubMed] [Google Scholar]
- 26.O’Brien MJ, Sotnikov AV. Digital imaging in anatomic pathology. Am J Clin Pathol 1996;106:25–32. [PubMed] [Google Scholar]
- 27.Leong FJWM, McGee O’D. Automated complete slide digitization: a medium for simultaneous viewing by multiple pathologists. J Pathol 2001;195:508–14. [DOI] [PubMed] [Google Scholar]
- 28.Demichelis F, Della Mea V, Forti S, et al. Digital storage of glass slides for quality assurance in histopathology and cytopathology. J Telemed Telecare 2002;8:138–42 [DOI] [PubMed] [Google Scholar]
- 29.Hediger PM, Dee F, Consoer D, et al. Integrated approach to teaching and testing in histology with real and virtual imaging. Anat Rec 2002;269:107–19. [DOI] [PubMed] [Google Scholar]