Abstract
A rare case of intracranial metastatic amelanotic melanoma with cyst is presented. The patient was a 51 year old woman with a malignant melanoma arising on her right chest. Two years after a wide excision, skin and brain metastasis occurred. Brain magnetic resonance images demonstrated a tumour with a cyst in the left occipital lobe. Because the tumour showed low intensity on T1 weighted images and high intensity on T2 weighted images, the metastatic melanoma was identified as an amelanotic melanoma. Intracranial amelanotic melanoma is very rare, and there have been few reports of melanoma with cyst.
Keywords: amelanotic melanoma, cyst, intracranial metastasis, melanoma
A rare case of intracranial metastatic melanoma is presented. Brain magnetic resonance (MR) images demonstrated a melanoma with cyst in the left occipital lobe. There have been few reports of malignant melanoma with cyst. MR images also indicated that the metastatic melanoma was amelanotic. This report discusses the characteristics of the MR images in this patient, and the mechanisms of discolouration and cystic formation in malignant melanoma.
CASE REPORT
A 55 year old woman presented with a black nodule on her right chest that had been increasing in size for two years. Initial diagnosis was a skin tumour suspected of being a malignant melanoma. The nodule was flat and irregular in shape, and 1.5 × 1.3 × 0.2 cm in size. It was black with brown shades, and bleeding was seen from the ulcer in the centre of the tumour. No inflammation, itchiness, or altered sensations were recognised. Laboratory data were normal, and serum 5-S-cysteinyl dopa was 4.5 nmol/litre.
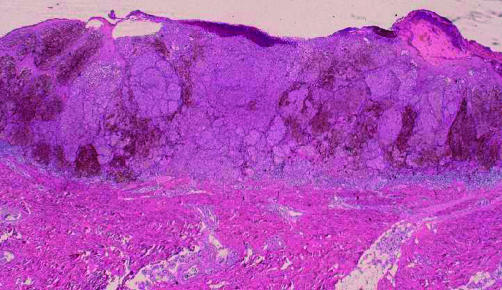
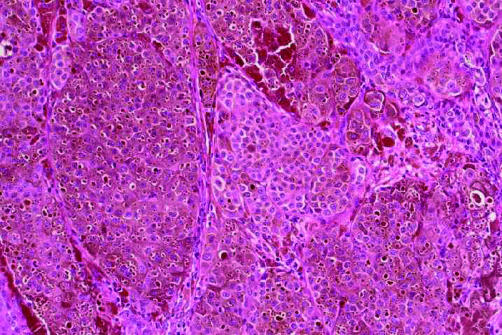
An excisional biopsy with a 5 mm normal skin margin was performed to decide the specific treatment plan (fig 1). Pathological findings indicated a superficial spreading melanoma, and the tumour cells had clearly invaded between the collagen bundles of the reticular dermis with nest formation (figs 2 and 3). The Clark level was IV and Breslow’s tumour thickness was 2.3 mm. TNM staging was UICC (Union Internationale Contre Cancer) pT3aN0M0, stage II and AJCC (American Joint Committee on Cancer) T3bN0M0, stage IIb.
Figure 1.
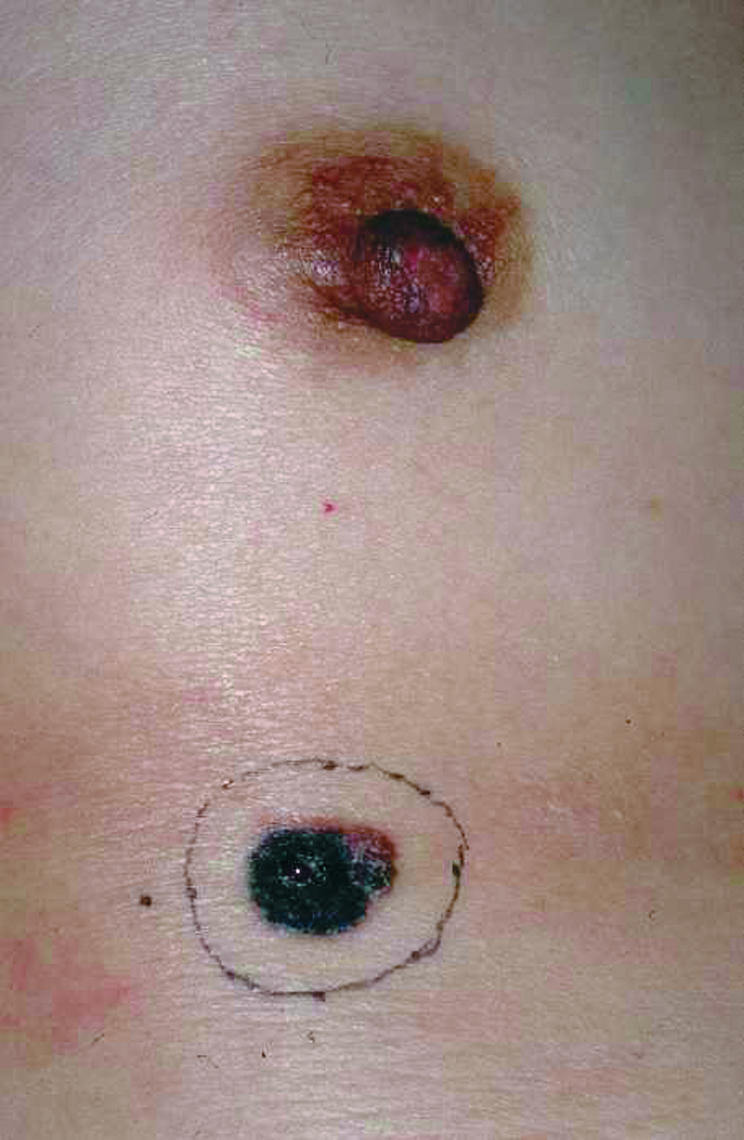
Preoperative view: the nodule was flat, irregular in shape, and 1.5 × 1.3 × 2 cm in size. An excisional biopsy with a 5 mm normal skin margin was performed to decide on the specific treatment plan.
Figure 2.
Pathological findings: the tumour cells had clearly invaded between the collagen bundles of the reticular dermis with nest formation (haematoxylin and eosin stain; original magnification, ×2).
Figure 3.
Pathological findings (haematoxylin and eosin stain; original magnification, ×20).
Two weeks after the excisional biopsy, a wide excision with a 2.5 cm normal skin margin from the previous incision line including the fascia was performed. A right axillary lymph node dissection was also performed. The skin damage was covered with a free split thickness skin graft from the right anterior thigh. After the operation, TNM staging was UICC pT3aN0M0, stage II and AJCC T3bN0M0, stage IIb. Two weeks after the wide excision, DAV (dacarbazine, nimustine, and vincristine) treatment was given for a total of five courses.
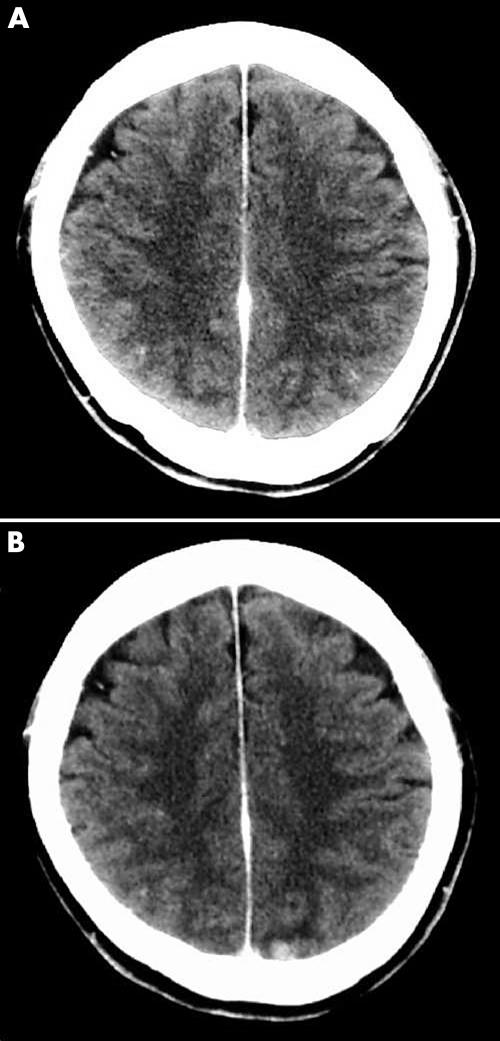
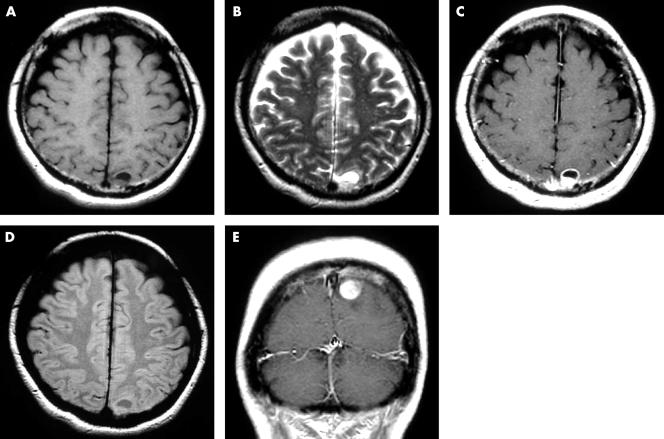
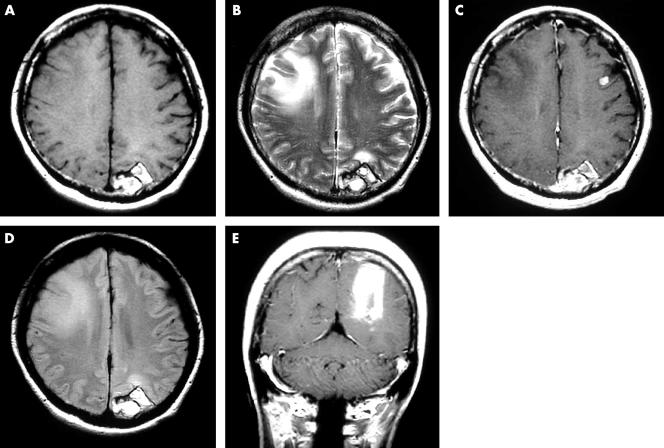
Two years after the operation, two subcutaneous tumours were found around the primary site. Excisional biopsy results showed them to be metastatic malignant melanomas: one on the left chest 13 cm from the primary site, and the other on the right chest 3 cm above the primary site. An immediate local injection of interferon β was given. However, skin metastasis developed throughout the body, and the patient also complained of homonymous haemianopia. Brain computed tomography scanning did not reveal the cause of the homonymous haemianopia, but the images showed a high density area in the left occipital lobe (fig 4). Brain MR scanning was then performed, and the MR images showed a tumour with a cyst in the same lesion (fig 5). The tumour showed low intensity in T1 weighted images (T1WI) and high intensity in T2 weighted images (T2WI). The cyst wall was shown as a ring enhancement in enhanced T1WI. The contents of the cyst showed intermediate intensity in fluid attenuated inversion recovery (FLAIR) images. The clinical course indicated that this tumour was a metastatic melanoma, but the images did not conform with those in normal malignant melanoma, which usually show high intensity in T1WI and low intensity in T2WI. The MR images indicated a tumour suspected of being an amelanotic melanoma. There is a possibility that what looked like a cyst because of the ring enhancement image was in fact central necrosis in the tumour. However, the FLAIR images of the contents showed intermediate intensity, suggesting a very low concentration of fluid, which was unlikely to be necrosed tissue.
Figure 4.
(A) A brain computed tomography (CT) image at one year after a wide excision. There are no specific findings. (B) A brain CT image at two years after a wide excision. The image shows a high density area in the left occipital lobe.
Figure 5.
Brain magnetic resonance images at two years after a wide excision. (A) T1 weighted image (T1WI): axial view; (B) T2 weighted image (T2WI): axial view; (C) enhanced T1WI: axial view; (D) fluid attenuated inversion recovery: axial view; (E) enhanced T1WI: coronal view.
One month later, the patient complained of an acute headache, and MR scanning was performed. The images showed a cerebral haemorrhage around the tumour (fig 6). After the haemorrhage was absorbed, γ knife irradiation was performed. Serum 5-S-CD at this time was 8.7 nmol/litre. Conservative treatment was carried out, but the metastasis spread throughout the patient’s body, including the lungs and liver, over the following year, and she eventually died.
Figure 6.
Brain magnetic resonance images at two years and one month after a wide excision. (A) T1 weighted image (T1WI): axial view; (B) T2 weighted image (T2WI): axial view; (C) enhanced T1WI: axial view; (D) fluid attenuated inversion recovery: axial view; (E) enhanced T1WI: coronal view.
DISCUSSION
One of the important characteristics of malignant melanoma is the high rate of skin or subcutaneous metastasis, which can be further subdivided into satellite metastasis and in-transit metastasis. Satellite metastasis refers to skin metastasis within 2 cm of the primary site, and in-transit metastasis to that from 2 cm to the regional lymph nodes. Both are lymphogenous metastases.
In the case presented here, subcutaneous metastasis occurred at two sites two years after a wide excision. One was the left chest, 13 cm from the primary site; this appeared to be a haematogenous metastasis, because the metastasis occurred across the centre line of the chest. The other was on the right chest, 3 cm above the primary site; this appeared to be a lymphogenous in-transit metastasis. Intracranial metastasis also occurred at almost the same time as the subcutaneous metastasis. Published reports on the organic compatibility of metastatic melanoma have indicated that the relative frequency is regional lymph nodes > lung > liver > skin or subcutaneous metastasis > brain.1
“There have been few previous reports of intracranial amelanotic melanomas”
In MR images, intracranial malignant melanomas generally show intermediate to high intensity in T1WI, and low to intermediate intensity in T2WI. On the other hand, amelanotic melanomas show low to intermediate intensity in T1WI and intermediate to high intensity in T2WI.2–4 The factors influencing MR images of melanomas are free radicals and chelate metals in the melanin.2–4 Melanomas easily lead to intratumour haemorrhages, and these haemorrhages also show characteristic images. After bleeding, it becomes impossible to distinguish between melanotic melanomas and amelanotic melanomas. In our patient, it was clear from the clinical course that the intracranial tumour was a metastatic melanoma, and we considered the tumour to be an amelanotic melanoma. In general, it is not rare for a melanotic melanoma to change into an amelanotic melanoma in the course of metastasis. However, there have been few previous reports of intracranial amelanotic melanomas.2–4 Again, the clinical course in this patient made it clear that the brain tumour was a metastatic melanoma. If a brain tumour is a primary brain amelanotic melanoma, or a metastatic amelanotic melanoma of unknown origin, it will be a problem for neurosurgeons and radiology doctors.
It is also a problem when discolouration occurs, as in this case. The possibilities are: (1) amelanotic tumour cells metastasised into the intracranium and multiplied or (2) melanotic tumour cells metastasised into the intracranium and multiplied with discolouration.
The conceivable mechanisms of discolouration are: (1) the melanotic cells change into amelanotic cells or (2) the tumour cells disappear.
Discolouration means that the tumour cells have changed into poorly differentiated cells that do not generate melanin and whose neoantigen concentration is low.5 One report describes the correlation between discolouration and malignant progression5: the disappearance of tumour cells (mechanism number 2 above) seems to occur as the result of an immune response including a lymphocyte attack. However, in this mechanism, only partial discolouration would occur, so it seems that in addition to this process, poorly differentiated cells that do not generate melanin escape lymphocyte attack and multiply, leading to complete discolouration.
Whether a malignant melanoma is melanotic or amelanotic cannot always be diagnosed from MR images. There are almost no variations in findings on intracranial melanomas in published reports2–4: melanotic melanomas show high intensity in T1WI and low intensity in T2WI, and amelanotic melanomas show low intensity in T1WI and high intensity in T2WI. However, there are variations in reports of MR findings on skin melanomas, so MR images cannot be completely relied upon. This difference between intracranial melanomas and skin melanomas is presumably a result of moisture content: MR findings are highly influenced by moisture content. The moisture content of intracranial melanomas is thought to be much less variable than that of skin melanomas, which are always contiguous with air.
Because of this, MR images are mainly of use in the non-invasive diagnosis of malignant skin melanomas, and the discrimination between melanotic and amelanotic melanomas is not so reliable. MR images are also important in regions where excisional biopsy is difficult, such as the finger tip and subungual region. It goes without saying that MR is important in the diagnosis of melanomas in metastatic sites.
Some definitions of amelanotic melanomas are as follows: (1) the tumour is amelanotic in clinical appearance; (2) the tumour is amelanotic clinically and there is no melanin pathologically; and (3) only premelanosomes can be detected under a light microscope, and no melanin under an electron microscope.
In the case reported here, we could not ascertain that the intracranial tumour was an amelanotic melanoma from the MR images alone using these definitions, but the images were highly suggestive of such.
A cyst was also evident in MR images in this case. There have been few reports of cystic metastatic melanoma.6–10 A brain abscess could also be considered from the ring enhancement on enhanced T1WI, but such a diagnosis was belied by the clinical course. This region would be haemorrhaged presently, and no infection was recognised. We can consider four explanations: (1) the cyst resulted from necrosis of the central portion of the tumour; (2) the melanoma had the ability to form cysts; (3) cerebrospinal fluid flow was prevented by the tumour, and the cyst developed as the result of cerebrospinal fluid pooling; or (4) the cyst already existed before lodgement of the metastatic melanoma.
The contents of the cyst appeared to be of very low concentration in the MR images, especially in the FLAIR image. Thus, the first explanation above is impossible, whereas the second is possible, because there have been a few reports of metastatic cystic melanomas on the liver and brain; the third explanation is also possible but the fourth is impossible, because the cyst was not recognised in the earlier computed tomography images.
An extremely rare case of intracranial metastatic amelanotic melanoma with cyst is presented. The primary tumour was on the right chest, and subcutaneous metastasis occurred in two sites two years after a wide excision. The metastasis was impossible to prevent, because one of the subcutaneous metastasis sites was haematogenous. Chemotherapy was of limited effect. Intracranial metastasis subsequently occurred, and the intracranial metastatic melanoma appeared to be amelanotic and cystic in MR images. The conceivable mechanisms of discolouration and cystic formation are reported.
Abbreviations
AJCC, American Joint Committee on Cancer
FLAIR, fluid attenuated inversion recovery
MR, magnetic resonance
T1WI, T1 weighted images
T2WI, T2 weighted images
UICC, Union Internationale Contre Cancer
REFERENCES
- 1.Patel JK, Didolkar MS, Pickren JW, et al. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg 1978;135:807–10. [DOI] [PubMed] [Google Scholar]
- 2.Isiklar I, Leeds NE, Fuller GN, et al. Intracranial metastatic melanoma: correlation between MR imaging characteristics and melanin content. Am J Roentgenol 1995;165:1503–12. [DOI] [PubMed] [Google Scholar]
- 3.Vanzieleghem BD, Lemmerling MM, Van Coster RN. Neurocutaneous melanosis presenting with intracranial amelanotic melanoma. Am J Neuroradiol 1999;20:457–60. [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi I, Sugimoto S, Nunomura M, et al. A case of cystic metastatic intracranial amelanotic melanoma—analysis of findings in CT and MRI. No to Shinkei 1990;42:1031–4. [PubMed] [Google Scholar]
- 5.Hahimoto A, Aoki M, Kawana S. Two cases of amelanotic malignant melanoma. Nishinihon Journal of Dermatology 2002;64:45–7. [Google Scholar]
- 6.Dublin AB, Norman D. Fluid—fluid level in cystic cerebral metastatic melanoma. J Comput Assist Tomogr 1979;3:650–2. [DOI] [PubMed] [Google Scholar]
- 7.Tipton A, Goldman SM, Fishman EK, et al. Cystic renal melanoma: CT/ultrasound correlation. Urol Radiol 1987;9:39–41. [DOI] [PubMed] [Google Scholar]
- 8.Borup K, Rasmussen KL, Schierup L, et al. Amelanotic malignant melanoma arising in an ovarian cyst. Acta Obstet Gynecol Scand 1992;71:242–4. [DOI] [PubMed] [Google Scholar]
- 9.Nambu Y, Fujii S, Konishi I, et al. Primary ovarian malignant amelanotic melanoma arising in cystic teratoma. Gynecol Oncol 1990;37:138–42. [DOI] [PubMed] [Google Scholar]
- 10.Saeki T, Mandai K, Yamagami K, et al. A metastatic liver tumor with a cystic melanoma cell proliferation—report of an autopsied case. Japanese Journal of Cancer Research and Clinical Oncology 1993;39:1063–7. [Google Scholar]