Abstract
Reactive free radical and oxidant production leads to DNA damage during myocardial ischemia/reperfusion. Consequent overactivation of poly(ADP-ribose) polymerase (PARP) promotes cellular energy deficit and necrosis. We hypothesized that PARP is activated in circulating leukocytes in patients with myocardial infarction and reperfusion during primary percutaneous coronary intervention (PCI). In 15 patients with ST segment elevation acute myocardial infarction, before and after primary PCI and 24 and 96 h later, we determined serum hydrogen peroxide concentrations, plasma levels of the oxidative DNA adduct 8-hydroxy-2′-deoxyguanosine (8OHdG), tyrosine nitration, PARP activation, and translocation of apoptosis-inducing factor (AIF) in circulating leukocytes. Plasma 8OHdG levels and leukocyte tyrosine nitration were rapidly increased by PCI. Similarly, poly(ADP-ribose) content of the leukocytes increased in cells isolated just after PCI, indicating immediate PARP activation triggered by reperfusion of the myocardium. In contrast, serum hydrogen peroxide concentrations and the translocation of AIF gradually increased over time and were most pronounced at 96 h. Reperfusion-related oxidative/nitrosative stress triggers DNA damage, which leads to PARP activation in circulating leukocytes. Translocation of AIF and lipid peroxidation occurs at a later stage. These results represent the first direct demonstration of PARP activation in human myocardial infarction. Future work is required to test whether pharmacological inhibition of PARP may offer myocardial protection during primary PCI.
INTRODUCTION
Over the last 2 decades, coronary reperfusion therapy has been established for the management of acute ST-segment elevation myocardial infarction (STEMI) as an absolute prerequisite for the survival of ischemic myocardium (1). Paradoxically, reperfusion of ischemic areas, in particular the readmission of oxygen, may trigger tissue damage resulting in a spectrum of reperfusion-associated pathologies, collectively called “reperfusion injury” (2,3), with clinical manifestations including myocardial stunning, potentially lethal arrhythmias, and endothelial and/or microvascular dysfunction resulting in the no-reflow phenomenon.
Reintroduction of abundant oxygen at the onset of reperfusion evokes a burst of potent oxygen- and nitrogen-derived free radicals and is considered a fundamental element of reperfusion injury (4,5). Free radicals and oxidants trigger modifications in lipid membranes and proteins and produce DNA damage, culminating in loss of cellular integrity (6). Poly(ADP-ribose) polymerase-1 (PARP-1), activated by single-strand DNA breaks, emerged as a critical regulatory component of the immediate cellular response to DNA damage (7,8). In physiological conditions, PARP-1 is an abundant nuclear chromatin-bound DNA repair enzyme, catalyzing transfer of ADP-ribose moieties from NAD+ to acceptor DNA binding proteins (7,9). Under pathophysiological conditions, overactivation of PARP leads to consumption of the cellular NAD+ and ATP content, mitochondrial dysfunction, and ultimately, necrotic cell death (7); the enzyme plays a key role in myocardial reperfusion injury (10).
Hypoxia/reperfusion promote explicit PARP-1 activation, mitochondrial damage, and dysfunction in myocytes and in endothelial cells by decreasing the mitochondrial transmembrane potential (10–12). In oxidant-challenged myocytes, PARP also regulates the mitochondrial-to-nuclear translocation of cell death factors such as apoptosis-inducing factor (AIF) and cytochrome c (13). In vitro experimental animal studies using perfused heart systems demonstrated that PARP inhibitors improve myocardial contractility and preserve myocardial ATP and NAD+ pools during reperfusion (14–16). PARP inhibitors significantly diminish infarct size and reduce creatine phosphokinase levels and mortality in mouse, rat, pig, and rabbit models (10). Moreover, PARP inhibitors protect endothelial cell integrity, preserve endothelium-dependent relaxation, and down-regulate multiple pro-inflammatory genes during reperfusion (17,18). These observations were confirmed in vivo, by demonstrating that transgenic mice lacking the functional PARP-1 gene are resistant to myocardial infarction (19). Furthermore, activation of PARP and beneficial effects of PARP inhibition have been demonstrated after heart transplantation or cardiopulmonary bypass (20).
Recently it was reported in a rat model that free radical–mediated activation of PARP is not limited to the myocardium: in response to myocardial ischemia, in circulating leukocytes—similarly to myocytes—significant PARP activation occurs after reperfusion. This phenomenon—which is most likely caused by free radicals as the circulating cells pass through the reperfused myocardial tissue—has been proposed to serve as a potential marker of myocardial oxidative and peroxidative injury (21).
Whereas the above experimental data demonstrated the role of PARP in myocardial reperfusion injury in animal models, PARP activation in human myocardial infarction has not yet been studied. In the present study, circulating peripheral leukocytes were isolated from cardiovascular patients with STEMI to analyze coronary reperfusion-related pathways in humans. The objective of the study was to test whether direct DNA damage occurs during STEMI, as indicated by increased levels of serum 8OHdG (8-hydroxy-2′-deoxyguanosine). We also tested whether PARP-1 activity changes after the primary PCI-mediated coronary reperfusion in circulating human leukocytes. Moreover, we tested whether reperfusion may induce tyrosine nitration (a marker of nitrosative stress) and whether it promotes nuclear translocation of AIF, a downstream event of PARP-1 activation.
METHODS
Patient Population
We enrolled 15 cardiovascular patients with acute ST-segment elevation myocardial infarction referred to our institution for primary percutaneous coronary intervention between October 2004 and February 2005. Blood and leukocyte samples from age-matched patients with stable angina pectoris undergoing elective coronary angiography (n = 6) and elective percutaneous intervention (n = 9) were also collected and analyzed in parallel as negative controls. The study protocol was approved by the institutional and regional ethics review committee, and written informed consent was obtained from all participating patients before enrollment.
Blood Sampling and Preparation of Peripheral Leukocytes
Resting venous blood was taken into native and heparin- and EDTA-containing tubes from STEMI and elective PCI patients at 4 different time points: (1) before the angiography, (2) within 15 min after opening of the infarct-related or target coronary artery, (3) 24 ± 4 h after the PCI, and (4) 96 ± 4 h after the PCI. Peripheral leukocytes were prepared using Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) and the density gradient centrifugation method from heparinized blood (6 mL). After isolation (centrifugation at 400g for 30 min) and washing in PBS, a 100-mL aliquot of mononuclear cells was used to prepare peripheral smears for immunohistochemistry. Remaining leukocytes were pelleted and kept at −80 °C until Western blot analysis.
Hydrogen Peroxide and 8OHdG Level Measurements
Using the OxyStat assay (Biomedica Gruppe, Wien, Austria), we determined the total hydrogen peroxide concentration in patient EDTA plasma samples (detection limit 7 μmol/L). Serum levels of 8OHdG were measured using a competitive enzyme-linked immunosorbent assay based on a 8OHdG monoclonal antibody (Gentaur, Brussels, Belgium). Serum samples were purified using an ultrafilter according to the instructions of the manufacturer.
Poly(ADP-Ribose) Western Blot Analysis
Isolated peripheral mononuclear cell pellets were resuspended in SDS-containing loading buffer (20 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 100 mM β-mercaptoethanol, and 100 μg/mL bromophenol blue) to a concentration of 2.5 × 107 cells/mL. Twenty microliters of the sample was separated on a 4% to 20% SDS-polyacrylamide gel. Blots were probed with anti-PAR (poly-ADP-ribose) polyclonal antibody (EMD Biosciences) and with an HRP-conjugated secondary antibody. Bound enzyme was detected with the enhanced chemiluminescence system (ECL, Amersham). Densitometric analysis of Western blots was performed using AlphaImager (Alpha Innotech Corporation, San Leandro, CA, USA); arbitrary densitometric units were corrected to background intensity.
Immunohistochemistry
Anti-nitrotyrosine rabbit polyclonal antibody (Upstate Biotechnology, Lake Placid, NY, USA) (1:80, 4 °C, overnight) was used to stain 3-nitro-tyrosine, the marker of tyrosine nitration. Poly(ADP-ribose) detection was performed using the mouse monoclonal anti-PAR antibody (Tulip Biolabs, West Point, PA, USA) (1:100, 4 °C, overnight) after antigen retrieval (0.1 M citrate buffer, pH 3, cooked in microwave oven for 15 min). Anti-AIF rabbit polyclonal antibody (Chemicon International, Temecula, CA, USA) (1:100, 4 °C, overnight) was used to label AIF. A specific labeling was avoided by incubating the smears in 15% normal goat/horse serum for 1 h at room temperature. Secondary labeling was achieved using biotinylated anti-mouse horse or anti-rabbit goat antibody (Vector Laboratories, Burlingame, CA, USA) (30 min, room temperature). Horseradish peroxidase–conjugated avidin (30 min, room temperature) and diaminobenzidine (6 min, room temperature) was used to visualize the labeling (Vector Laboratories). Smears were counter-stained with hematoxylin.
To determine the number of NT- and AIF-positive cells, at least 300 cells were counted on each smear. Semiquantitative PAR-positivity score was established from 1 to 10 and used to score the slides by an investigator who was blinded to the identity of the individual slides: score 1, no staining; 2, light cytoplasmic staining; 3, strong cytoplasmic staining; 4, cytoplasmic staining with a few positive nuclei; 5, approximately 50% of the nuclei positive; 6, approximately 75% of the nuclei positive; 7, general nuclear staining with a few negative cells; 8, all nuclei positive; 9, strong nuclear staining in all cells; 10, very strong general nuclear staining in all cells.
Statistical Analysis
Results are expressed as mean ± SEM and SD by box plots. Data were not normally distributed; therefore, nonparametric statistic tests were performed using Statistica 6.0 software (Stat Soft, Tulsa, OK, USA). Mann-Whitney U test was conducted to investigate the association between independent parameters of different patient groups. To analyze dependent variables, the Wilcoxon matched-pairs test was used. P values less than 0.05 were considered significant.
RESULTS
Patient Demographics
Detailed patient demographics, clinical parameters, and angiography and laboratory test results are summarized in Tables 1 and 2. The enrolled STEMI patients were predominantly men, with multiple cardiovascular risk factors in their medical history. All of them had permanent chest pain (maximum 12 h) and showed ST elevation (≥2 mm) in at least 2 consecutive ECG leads on admission. In each case, coronary angiography revealed subtotal or total coronary artery occlusion, and successful recanalization was confirmed by high TIMI flow rate values after PCI. Definitive acute myocardial damage was demonstrated by elevated CK/CK MB values.
Table 1.
Patient baseline characteristics.
| Stable angina pectoris
|
|||
|---|---|---|---|
| Acute ST-segment elevation myocardial infarction | Coronarography | Elective PCI | |
| n | 15 | 6 | 9 |
| Age, y | 68.13 ± 2.93 | 57.0 ± 2.47 | 61.22 ± 3.6 |
| Sex, M/F | 9/6 | 2/3 | 5/4 |
| Body mass index, kg/m2 | 28.11 ± 0.86 | 30.83 ± 1.32 | 30.3 ± 1.28 |
| Risk factors | |||
| Family history of IHD | 6 (40) | 4 (80) | 4 (66) |
| History of tobacco use | 7 (46.7) | 5 (100) | 9 (100) |
| Hypertension | 15 (100) | 4 (80) | 7 (77) |
| Diabetes mellitus | 5 (33) | 2 (40) | 4 (44) |
| Previous evidence of IHD | 7 (46.7) | 5 (100) | 9 (100) |
| Angina 1–6 days before admission | 4 (26.7) | 4 (80) | 6 (66) |
| Previous PCI | 0 | 1 (20) | 8 (88) |
| Diagnosis on admission | |||
| Stable angina pectoris | — | 5 (100) | 9 (100) |
| Acute myocardial infarction | — | — | |
| Inferior | 3 (20) | ||
| Infero-posterior | 6 (40) | ||
| Anterior | 2 (13.4) | ||
| Extensive anterior | 3 (20) | ||
| Posterior | 1 (6.7) | ||
| Time from event to balloona | |||
| <3 h | 3 (20) | ||
| 3–6 h | 8 (53.3) | ||
| >6 h | 4 (26.6) | ||
| Coronarography results | |||
| LM stenosis/occlusion | 3/0 | 1/0 | 1/0 |
| LAD stenosis/occlusion | 9/4 | 3/1 | 7/1 |
| RCA stenosis/occlusion | 2/10 | 2/2 | 6/0 |
| LCx stenosis/occlusion | 8/1 | 2/0 | 2/0 |
| R. diagonalis stenosis/occlusion | 6/0 | 1/0 | 2/0 |
| RRV stenosis/occlusion | 1/1 | 0/0 | 0/0 |
| OM stenosis/occlusion | 6/0 | 0/0 | 1/0 |
| Number of coronary stenoses per patient | 2.27 ± 0.32 | 1.8 ± 0.58 | 2.11 ± 0.2 |
| Number of coronary occlusions per patient | 1.06 ± 0.12 | 0.6 ± 0.24 | 0.11 |
| Number of implanted stents per patient | 1.53 ± 0.27 | — | 0.88 ± 0.26 |
| TIMI flow grade of the target vessel after PCI | 2.77 ± 0.12 | — | 2.75 ± 0.16 |
| Complications | |||
| Atrialflutter/fibrillation | 2 (13.3) | — | — |
| Ventricular tachycardia/fibrillation | 4 (26.6) | — | — |
| AV block | 3 (20) | — | — |
| Pacemaker therapy | 2 (13.3) | — | — |
| Cardiogenic shock | 3 (20) | — | — |
| IABP | 2 (13.3) | — | — |
| Gastrointestinal/femoral bleeding | 4 (26.6) | — | — |
| Exithus lethalis | 2 (13.3) | — | — |
Values are mean ± SEM or n (%). Percentages are compared to the entire patient population.
Time between the onset of persisting chest pain and the beginning of the 0PCI. IHD indicates ischemic heart disease; PCI, percutaneous coronary intervention; LM, left main coronary artery; LAD, left anterior descending artery; RCA, right coronary artery; LCx, left circumflex artery; RRV, right retroventricularis; OM, obtuse marginal; TIMI, thrombolysis in myocardial infarction; IAB, intra-aortic balloon pump.
Table 2.
Laboratory results in the STEMI group
| Pre-PCI | Post-PCI | After 24 h | After 96 h | |
|---|---|---|---|---|
| CK, U/L | 230.6 ± 64.9 | 2152.8 ± 914.3 | 854.2 ± 194.42 | 275.5 ± 58.7 |
| CKMB, U/L | 53.3 ± 17.1 | 254.4 ± 108.7 | 121.1 ± 35.46 | 33.25 ± 6.3 |
| LDH, U/L | 366.1 ± 35.8 | — | 1339.21 ± 207.2 | 1703 ± 488.6 |
| AST, U/L | 40.13 ± 8.29 | — | 174.1 ± 35.84 | 157.63 ± 91.53 |
| ALT, U/L | 27.2 ± 5.51 | — | 48.07 ± 9.61 | 56.18 ± 16.03 |
Values are mean ± SEM. CK indicates creatine kinase; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Determination of the Oxidative Imbalance: Plasma Total Peroxide Concentration
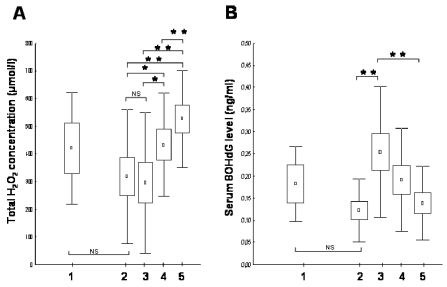
Myocardial reperfusion-related oxidative/peroxidative stress is known to lead to cellular damage by lipid peroxidation (10). Therefore, total plasma hydrogen peroxide concentration as an established marker of oxidative injury and lipid peroxidation was determined. As shown in Figure 1A, total hydrogen peroxide concentration was not affected by coronary reperfusion directly; pre-and post-PCI values were not different statistically. A significant increase was observed in total peroxide levels 24 and 96 h after the acute cardiovascular event. Baseline values in patients with stable angina pectoris and acute MI were not different. Similar to the primary PCI group, in control stable angina patients hydrogen peroxide concentrations were identical before and after coronarography (420 ± 90 vs. 431 ± 104 μmol/L, respectively, n = 5, data not shown).
Figure 1.
Determination of the oxidative imbalance in patients with stable angina pectoris and acute ST-segment elevation myocardial infarction. (A) Total plasma hydrogen peroxide concentration measurements. (B) Serum 8OHdG level measurements. Results are expressed as mean (represented by squares) ± SEM (represented by boxes) and ± SD (represented by bars). Lane 1 indicates peroxide levels in stable angina patients; lanes 2–5 show peroxide concentration in patients with acute myocardial infarction before coronarography (lane 2), just after the successful primary PCI (lane 3), 24 ± 4 h after reperfusion of the ischemic myocardium (lane 4), and 96 ± 4 h after PCI (lane 5). Primary PCI itself did not affect total peroxide levels; gradual increase of hydrogen peroxide concentration was observed at 24- and 96-h time points after myocardial infarction. In contrast, serum 8OHdG levels showed a significant, rapid increase after the primary PCI, and were normalized by 96 h. *P < 0.05, **P < 0.005; NS, nonsignificant.
Verification of PCI-Related DNA Damage: Serum 8OHdG Level Measurements
DNA is a major target of constant oxidative damage from endogenous oxidants (4). In contrast to peroxide levels, primary PCI, representing a successful myocardial reperfusion, led to an explicit, rapid 8OHdG level increase (P < 0.005), indicating systemic, immediate, reperfusion-related DNA damage in the STEMI patients in response to PCI (Figure 1B). Furthermore, unlike peroxide concentrations, 8OHdG levels were normalized within 96 h. Serum 8OHdG concentrations in control stable angina patients were not different statistically from pre-PCI STEMI values.
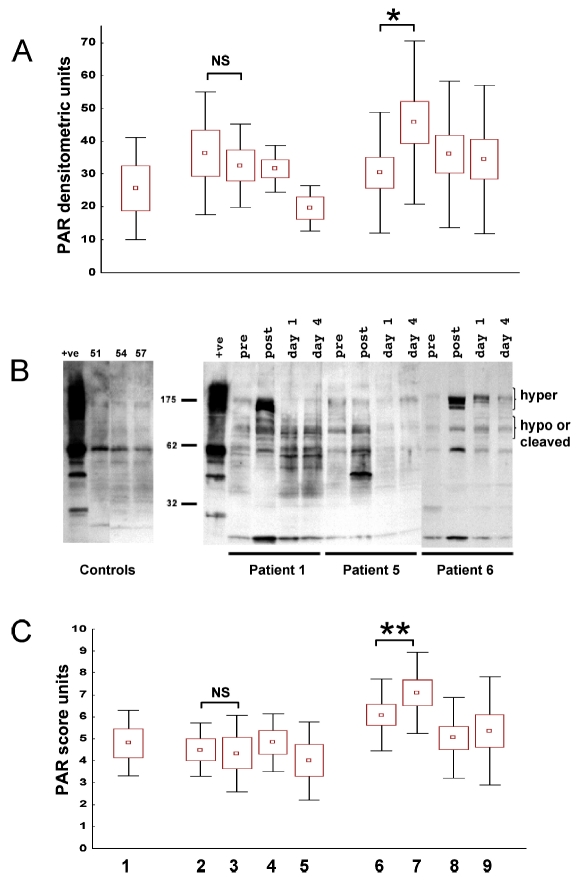
Analysis of PARP-1 Activation in Circulating Leukocytes
Immunohistochemistry and densitometry analysis of the buffy-coat cell pellet Western blots (n = 15) confirmed a significant immediate PARP-1 activation in circulating leukocytes due to myocardial reperfusion achieved by primary PCI (Figure 2A,C). Similar to the kinetics of 8OhdG serum levels, the increase of PARP-1 activity in the isolated leukocytes occurred rapidly after myocardial reperfusion and decreased over time. Importantly, in patients undergoing elective percutaneous intervention (n = 9), no PARP-1 activation occurred after the PCI. These results indicate that significant myocardial ischemia is required before reperfusion to induce PARP-1 activation. Baseline initial PARP-1 activity in stable angina and STEMI patients was comparable.
Figure 2.
Rapid activation of PARP-1 in peripheral leukocytes induced after recanalization of the infarct-related coronary artery by primary PCI. (A) Densitometry analysis of PAR Western blots performed on patient leukocytes. (C) Immunohistochemical PAR-score analysis of patient leukocytes. In both figures, lane 1 indicates densitometry units (A) or PAR-score values (C) in stable angina patients before coronarography; lanes 2–5 show PAR content in control patients with elective PCI before coronarography (lane 2), immediately after the successful PCI (lane 3), 24 ± 4 h after (lane 4), and 96 ± 4 h after PCI (lane 5); lanes 6–9 indicate PAR content in patients with acute myocardial infarction before coronarography (lane 6), immediately after the successful PCI (lane 7), 24 ± 4 h after reperfusion of the ischemic myocardium (lane 8), and 96 ± 4 h after PCI (lane 9). Primary PCI leads to a significant increase in leukocyte cellular PAR content, reflecting rapid activation of PARP during reperfusion. A gradual decrease of PARP activity can be observed at 24- and 96-h time points after myocardial infarction. PARP activity is not affected during elective PCI. Results are expressed as mean (represented by squares) ± SEM (represented by boxes) and ± SD (represented by bars). (B) Representative examples of PAR Western blots from 3 stable angina patient leukocytes as controls (first panel) and 3 STEMI patients (second panel). Time points are indicated. Commercially available PARP enzyme served as a positive control. *P < 0.05, NS, nonsignificant.
Immunohistochemical Studies of Nitrotyrosine Production and Translocation of AIF
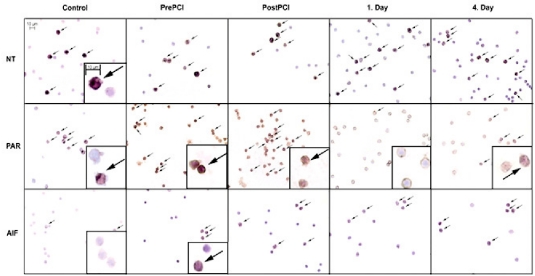
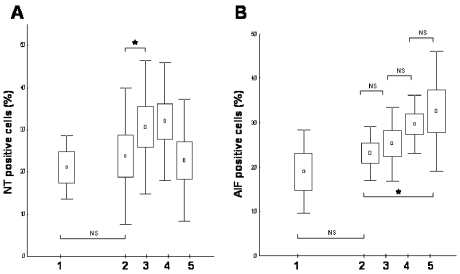
Immunohistochemical staining demonstrated that tyrosine nitration of the isolated cells significantly increased after PCI, compared with pre-PCI values (Figures 3 and 4A). Again, when nitrotyrosine-positive cells were counted, rapid kinetics were observed: tyrosine nitration was maximal just after PCI and decreased by 96 h (Figure 4A). The number of nitrotyrosine-positive cells did not differ between control and STEMI patients prior to surgery (Figure 4A).
Figure 3.
Immunohistochemical analysis of tyrosine nitration, PAR content, and AIF translocation. Representative examples indicating gradual increase of the NT positive cell numbers (arrows) in peripheral leukocyte preparations after STEMI. The second row demonstrates increased PAR content in leukocytes immediately after the primary PCI. In the third row, AIF staining was performed and positive cells are depicted by arrows; AIF translocation increased by 96 h. Leukocytes from a stable angina patient are shown as negative controls in the first column.
Figure 4.
Tyrosine nitration (A) and AIF translocation (B) determined by immunohistochemistry scores. After staining, NT-positive cells were counted in peripheral leukocyte smears. Results are expressed as mean (represented by squares) ± SEM (represented by boxes) and ± SD (represented by bars). Lane 1 indicates control samples from stable angina patients; lanes 2–5 show NT-positive cell counts or AIF translocation–positive cell counts in patients with acute myocardial infarction before coronarography (lane 2), immediately after the successful primary PCI (lane 3), 24 ± 4 h after reperfusion of the ischemic myocardium (lane 4), and 96 ± 4 h after PCI (lane 5). Primary PCI induced an immediate increase in tyrosine nitration, whereas a gradual increase of AIF translocation was observed at 24- and 96-h time points after reoxygenation of the ischemic myocardium. *P < 0.05; NS, nonsignificant.
In contrast to these parameters, translocation of AIF to the nuclei showed a gradual tendency to increase (Figure 3), a difference that became significant compared with the pre-PCI values by day 4 (23 ± 2% vs. 33 ± 5% positive cells, P < 0.05, n = 8, Figure 4B).
DISCUSSION
Despite the fact that myocardial reperfusion therapy is often called a double-edged sword (2), the aim of the clinician is to reperfuse the ischemic myocardium as soon as possible to recover contractile function and to avoid irreversible myocardial damage. Previous studies have demonstrated oxidative DNA injury and consequent PARP-1 activation in cardiomyocytes, endothelial cells, and circulating peripheral leukocytes in animal models of acute myocardial infarction (for review, see Jagtap and Szabo [22]). The current study investigated multiple aspects of human myocardial ischemia/reperfusion-related pathologies by analyzing serum, plasma, and isolated peripheral leukocyte samples from cardiovascular patients with acute ST-segment elevation myocardial infarction and successful primary PCI. Our results provide evidence for (1) general oxidative/peroxidative imbalance (elevated total plasma peroxide concentration and augmented nitrotyrosine production), (2) direct, PCI-generated DNA damage (evidenced by increased levels of serum 8OHdG), (3) rapid post-PCI activation of PARP-1 in circulating human peripheral leukocytes (shown by immunohistochemistry and Western blotting), and (4) translocation of AIF from mitochondria to nuclei (which may be a downstream signaling event triggered by PARP-1 activation). These results provide the first clinical evidence for PARP activation in patients with myocardial infarction and are consistent with the concept that local myocardial hypoxia/reperfusion triggered by percutaneous interventions in acute myocardial infarction is able to trigger systemic oxidative responses in humans.
Although the pathomechanism of reperfusion injury has been extensively studied, relatively limited therapeutic applications have been developed to date (6,23). So far, therapeutic attempts to prevent reperfusion injury are limited to relatively small-scale studies testing the administration of vitamin E (24), calcium antagonists (25), early use of ACE inhibitors, sulfhydryl-rich reagents such as N-acetylcysteine, magnesium, and various free radical scavengers (26). However, accelerated myocyte necrosis and destruction related to reoxygenation of the ischemic myocardium continues to represent a clinically relevant question. It is generally accepted that the combination of the reopening of the coronary artery with therapeutic approaches that protect the reperfused myocardium may improve the outcome of the PCI. As demonstrated by analysis of circulating leukocytes passing through the reperfused myocardium, consequent PARP-1 activation with abrupt kinetics also occurs. Although the specific cell types were not identified with flow cytometry, it is well known from the literature that neutrophils do not contain the PARP enzyme (7). Therefore, the cells likely to be responsible for the observed increase in PARP activity are lymphocytes and/or monocytes. Taken together with previous data from experimental myocardial ischemia models (reviewed in Jagtap and Szabo [22]), one can speculate that, as with leukocytes, myocyte PARP activation is likely to develop in human reperfusion injury, leading to myocyte necrotic cell death.
The present observations may also have direct therapeutic implications. Potent novel PARP inhibitors have been developed in recent years, and these agents were shown to be beneficial in in vitro and in vivo ischemia/reperfusion models as attenuating reperfusion injury by acting at several levels (prevention of energetic failure, inflammatory mediator production, neutrophil infiltration, and endothelial dysfunction) (22,27,28). Therefore, in theory—as is supported by our human data confirming rapid PARP activation due to primary PCI—PARP inhibition before the planned reperfusion might provide multiple benefits, most importantly, myocyte salvage and therefore improved survival (22). In this context, analysis of PARP activity in human circulating leukocytes during PARP inhibitor treatment might serve as a useful marker and provide evidence for the ability of PARP inhibitors to block the activation of the target enzyme in clinical trials in vivo.
In the present study, circulating leukocytes were used to analyze oxidative imbalance. Nevertheless, other circulating cells such as circulating endothelial cells may also be investigated in future studies. These measurements might confirm involvement of oxidative/nitrosative stress pathways in such processes as pathophysiological endothelial cell function and detachment from the vessel wall and may explore the role of PARP-1 in endothelial dysfunction related to reperfusion.
Besides acute myocardial infarction, various degrees of myocardial reperfusion injury may occur under such common clinical conditions as elective percutaneous coronary interventions. Our data indicate that in the case of elective PCI without complications, PARP-1 activation did not develop in circulating leukocytes. It would be important to test whether in the case of periprocedural myocardial necrosis—which is a negative prognostic factor in patients with elective PCI (29–31)—the oxidative/nitrosative balance triggers the PARP-1 pathway.
What, then, is the molecular trigger of PARP activation in human myocardial infarction? Based on animal studies, oxidative and nitrosative stress (namely, hydrogen peroxide, peroxynitrite, and hydroxyl radical) are pathophysiologically relevant triggers of PARP activation (32). In the present study, tyrosine nitration (a relatively specific marker of peroxynitrite production) but not hydrogen peroxide levels showed a close correlation with the degree of PARP activation, possibly implicating the role of reactive nitrogen species, such as peroxynitrite. We must point out, nevertheless, that the likely trigger of PARP activation is within the reperfused myocardial tissue, and peripheral blood parameters may not necessarily correlate with the changes within the myocardial tissue itself.
It is interesting to note that the current study demonstrated that AIF translocation occurs 4 days after PCI during myocardial infarction. The translocation of AIF can be triggered by multiple factors, one of them being PARP activation. It is unlikely that PARP is the only contributor of AIF translocation in the current study, as the time course of PAR staining and AIF translocation are quite different.
Although our observations provide evidence for primary PCI-related oxidative injury and subsequent DNA damage–induced PARP-1 activation in a human cardiovascular patient cohort, our case numbers are limited. A subsequent large-scale study would be needed to link and correlate these biochemical markers to patient outcome and clinical parameters, such as major cardiac events or cardiovascular death. Analysis of 8OHdG or PARP-1 activity levels in context to serum troponin, CK MB values, or ejection fraction would be particularly important. Also, in future clinical studies with PARP inhibitors, peripheral leukocyte PAR content or PAR immunostaining may serve as useful sentinel markers for the efficacy of the PARP inhibitor to block its enzymatic target in vivo. In future studies, using antioxidants or PARP inhibitors, one may also be able to assess whether oxidant stress contributes to AIF translocation, whether there are alterations in the viability and lifetime of peripheral blood cells, and whether these changes are related to oxidant stress and PARP activation.
In conclusion, our data provide evidence for PARP activation for the first time in humans suffering from myocardial infarction. In the present population of cardiovascular patients with ST-segment elevation myocardial infarction, primary percutaneous intervention is accompanied by significant systemic DNA damage, PARP-1 activation, and consequent AIF translocation. PARP activation in circulating cells may serve as a sentinel pharmacodynamic marker in ongoing or future clinical trials (22) utilizing PARP inhibitors.
ACKNOWLEDGMENTS
The study was supported by research grants from the Hungarian Science Foundation (OTKA T042605, F046711, and K49488), the Hungarian Ministry of Health (ETT 086/2003, ETT 583/2003), and from the National Institutes of Health (RO1 GM60915 to CS). E.T.-Z. is a recipient of the “Bolyai” Scholarship Grant of the Hungarian Academy of Sciences. E.H. was supported by the Hungarian National Eötvös Fellowship.
Footnotes
Online address: http://www.molmed.org
E.T.-Z. and E.H. contributed equally to this work.
REFERENCES
- 1.DeWood MA, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303:897–902. doi: 10.1056/NEJM198010163031601. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E, Kloner RA. Myocardial reperfusion. A double-edged sword? J Clin Invest. 1985;76:1713–9. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179–90. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Piper HM, Meuter K, Schafer C. Cellular mechanism of ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:644–8. doi: 10.1016/s0003-4975(02)04686-6. [DOI] [PubMed] [Google Scholar]
- 5.Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988;263:1353–7. [PubMed] [Google Scholar]
- 6.Maxwell SRJ, Lip GYH. Reperfusion injury: a review of the pathophysiology, clinical manifestation and therapeutic options. Int J Cardiol. 1997;58:95–117. doi: 10.1016/s0167-5273(96)02854-9. [DOI] [PubMed] [Google Scholar]
- 7.Virág L, Szabó C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 8.Nguewa PA, et al. Poly(ADP-ribose) polymerases: Homology, structural domains and functions. Novel therapeutical applications. Prog Biophys Mol Biol. 2005;88:143–72. doi: 10.1016/j.pbiomolbio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Graziani G, Szabó C. Clinical perspectives of PARP inhibitors. Pharmacol Res. 2005;52:109–18. doi: 10.1016/j.phrs.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Szabó C. Cardioprotective effects of poly(ADP-ribose) polymerase inhibition. Pharmacol Res. 2005;52:34–43. doi: 10.1016/j.phrs.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Gilad E, et al. Protection by inhibition of poly(ADP ribose) synthetase against oxidant injury in cardiac myoblasts in vitro. J Mol Cell Cardiol. 1997;29:2585–97. doi: 10.1006/jmcc.1997.0496. [DOI] [PubMed] [Google Scholar]
- 12.Kirkland JB. Lipid peroxidation, protein thiol oxidation and DNA damage hydrogen-peroxide-induced injury to endothelial cells: role of activation of poly(ADP-ribose) polymerase. Biochim Biophys Acta. 1994;1092:319–25. doi: 10.1016/s0167-4889(97)90007-0. [DOI] [PubMed] [Google Scholar]
- 13.Chen M, et al. Mitochondrial-to-nuclear translocation of apoptosis-inducing factor in cardiac myocytes during oxidant stress: potential role of poly(ADP-ribose) polymerase-1. Cardiovasc Res. 2004;63:682–8. doi: 10.1016/j.cardiores.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Zingarelli B, Cuzzocrea S, Zsengeller Z, Salzman AL, Szabo C. Protection against myocardial ischemia and reperfusion injury by 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase. Cardiovasc Res. 1997;36:205–15. doi: 10.1016/s0008-6363(97)00137-5. [DOI] [PubMed] [Google Scholar]
- 15.Halmosi R, et al. Effect of poly(ADP-ribose) polymerase inhibitors on the ischemia-reperfusion-induced oxidative cell damage and mitochondrial metabolism in Langendorff heart perfusion system. Mol Pharmacol. 2001;59:1497–505. doi: 10.1124/mol.59.6.1497. [DOI] [PubMed] [Google Scholar]
- 16.Liaudet L, et al. Suppression of poly(ADP-ribose) polymerase activation by 3-aminobenzamide in a rat model of myocardial infarction: long-term morphological and functional consequences. Br J Pharmacol. 2001;133:1424–30. doi: 10.1038/sj.bjp.0704185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrillo A, et al. Transcription regulation of TNF-alpha-early response genes by poly(ADP-ribose) polymerase-1 in murine heart endothelial cells. Nucleic Acids Res. 2004;32:757–66. doi: 10.1093/nar/gkh239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piconi L, et al. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly(ADP-ribose)polymerase. J Thromb Haemost. 2004;2:1453–9. doi: 10.1111/j.1538-7836.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 19.Zingarelli B, Salzman AL, Szabo C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res. 1998;83:85–94. doi: 10.1161/01.res.83.1.85. [DOI] [PubMed] [Google Scholar]
- 20.Khan TA, et al. Poly(ADP-ribose) polymerase inhibition improves postischemic myocardial function after cardioplegia-cardiopulmonary bypass. J Am Coll Surg. 2003;197:270–7. doi: 10.1016/S1072-7515(03)00538-6. [DOI] [PubMed] [Google Scholar]
- 21.Murthy KG, et al. Activation of poly(ADP-ribose) polymerase in circulating leukocytes during myocardial infarction. Shock. 2004;21:230–4. doi: 10.1097/01.shk.0000110621.42625.10. [DOI] [PubMed] [Google Scholar]
- 22.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 23.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 24.Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–6. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 25.Chouairi S, Carrie D, Puel J. Myocardial protection with calcium-channel blockers during ischaemia and reperfusion by PTCA. Eur Heart J. 1995;16(Suppl H):3–8. doi: 10.1093/eurheartj/16.suppl_h.3. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari R, Pepi P, Ferrari F, Nesta F, Benigno M, Visioli O. Metabolic derangement in ischemic heart disease and its therapeutic control. Am J Cardiol. 1998;82:2K–13K. doi: 10.1016/s0002-9149(98)00531-1. [DOI] [PubMed] [Google Scholar]
- 27.Thiemermann C, et al. Inhibition of the activity of poly(ADP ribose) synthetase reduces ischemia-reperfusion injury in the heart and skeletal muscle. Proc Natl Acad Sci U S A. 1997;94:679–83. doi: 10.1073/pnas.94.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Zingarelli B, Szabo C. Effect of genetic disruption of poly(ADP-ribose) synthetase on delayed production of inflammatory mediators and delayed necrosis during myocardial ischemia-reperfusion injury. Shock. 2000;13:60–6. doi: 10.1097/00024382-200013010-00011. [DOI] [PubMed] [Google Scholar]
- 29.Califf RM, et al. Myonecrosis after revascularization procedures. J Am Coll Cardiol. 1998;31:241–51. doi: 10.1016/s0735-1097(97)00506-8. [DOI] [PubMed] [Google Scholar]
- 30.Narins CR, Miller DP, Califf RM, Topol EJ. The relationship between periprocedural myocardial infarction and subsequent target vessel revascularization following percutaneous coronary revascularization: insights from the EPIC trial. Evaluation of IIb/IIIa platelet receptor antagonist 7E3 in Preventing Ischemic Complications. J Am Coll Cardiol. 1999;33:647–53. doi: 10.1016/s0735-1097(98)00620-2. [DOI] [PubMed] [Google Scholar]
- 31.Akkerhuis KM, et al. Minor myocardial damage and prognosis: are spontaneous and percutaneous coronary intervention-related events different? Circulation. 2002;105:554–6. doi: 10.1161/hc0502.104278. [DOI] [PubMed] [Google Scholar]
- 32.Virag L, Szabo E, Gergely P, Szabo C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett. 2003;140–141:113–24. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]