Abstract
Chemokines activate and recruit specific leukocyte subpopulations. We sought to determine whether neutrophil migration, which can contribute to the development of ischemia-reperfusion injury, correlates with lung allograft rejection. Orthotopic left lung allotransplantation was performed from Brown Norway (donor) to Fisher 344 (recipient) rats. Because the role of activated neutrophils in the development of allograft rejection is believed to be biphasic, we used specific CXC receptor inhibition with antileukinate in 2 dosing regimens. Recipients were allocated into 4 groups; A (early administration) received 2 doses of antileukinate (10.0 mg/kg) intramuscularly 24 h before and immediately after transplantation; B (continuous administration) continuously received antileukinate intraperitoneally (10.0 mg/kg/day) for 7 days after surgery. Groups A or B were compared with individual controls that received PBS alone. The progression of rejection was assessed radiographically. Histologic evaluation of allograft rejection based on pathologic rejection grade, performed on day 7, demonstrated significantly lower histologic rejection in group B compared with the control group (2.1 ± 1.0 vs. 3.3 ± 0.5; P = 0.018), whereas there was no significant difference in group A compared with the control group. There were no significant differences between the aeration scores of groups A or B compared with their control groups. Our data suggest that neutrophils may play a promoting role in the development of allograft rejection, and blockage of neutrophil migration may suppress acute lung allograft rejection.
INTRODUCTION
In the early 1980s, lung transplantation was rarely used, but in the last decade it has become a widely available therapeutic option. The recent evolution of preservation solutions (1,2) and other techniques for organ procurement (3) facilitate more remote organ donation and the increased capability to use marginal donor lungs; however, preservation-related organ injury is still an unavoidable and sometimes critical problem (4).
The preservation-related injury of the allograft, including ischemia-reperfusion injury imposed on lung allografts after transplantation, induces a substantial tissue inflammatory response resulting in a lung injury characterized by diffuse alveolar damage. Ischemia-reperfusion injury increases activation of vascular endothelial cells and releases a variety of chemoattractants, which help the activated neutrophils influx into the lung parenchyma with the coordination of adhesion molecules that are upregulated during ischemia-reperfusion injury (5). During this nonspecific insult, it has been thought that neutrophils play a key role through a number of mechanisms including release of reactive oxygen species and proteolytic enzymes (6).
Meanwhile, there have been several reports suggesting potent direct or indirect effects of activated neutrophils in the development of acute allograft rejection. Activated neutrophils release several factors such as CC chemokines that attract and activate antigen-presenting cells such as dendritic cells (DCs), thus influencing the priming of naive antigen-specific T cells (7,8). It was recently shown that the neutrophil itself can take up antigens and transcribe and express the genes encoding major histocompatibility complex (MHC) molecules as well as costimulatory molecules, thereby behaving as antigen presenting cells (9,10). Additionally, once neutrophils are exposed to inflammatory signals, activated neutrophils produce cytokines and chemokines to induce Th1 polarization of antigen-specific T lymphocytes that influence the T-cell immune response (11). These observations suggest a linkage between neutrophils and the development of acute cellular rejection. We speculated that suppression of neutrophil activation after allograft implantation might alleviate the molecular or cellular events in allograft rejection as well as ischemia-reperfusion injury.
Antileukinate is defined as a hexa-peptide, RRWWCR with acetylated amino-terminus and amidated carboxyl-terminus (Ac-RRWWCR-NH2), which is a potent inhibitor of interleukin-8 (IL-8)/CXCL8 and growth-related oncogene (GRO) α/CXCL1 binding to the receptors on activated neutrophils. It is known to specifically inhibit IL-8–induced neutrophil chemotaxis and enzyme release (12) and has been considered a potent neutrophil activation inhibitor with the capability to protect lung tissue from acute lung injury induced by drugs (13) or sepsis (14). These findings suggest that antileukinate or its derivatives might prove useful in lung allograft reperfusion injury caused by activated neutrophils due to the unrestrained action of CXC-chemokines. This study was designed to investigate whether suppression of neutrophil activation by this novel chemokine receptor antagonist affects the development and severity of ischemia-reperfusion injury following acute lung allograft rejection.
MATERIALS AND METHODS
Animals
Inbred, male, specific pathogen–free Brown Norway (BN, RT1n) and F344 (RT1lvl) rats, weighing 250 to 300 g, were purchased from Kyudo (Tosu, Japan) and SLC Japan (Shizuoka, Japan), respectively. Animals were cared for in compliance with the Principles of Laboratory Animal Care, formulated by the Institute of Laboratory Animal Resources, and the Guide for the Care and Use of Laboratory Animals, prepared by the National Academy of Sciences and published by the Institute for Laboratory Animal Research (National Academy Press, revised 1996).
Transplant Model
Rat left-lung transplantation was performed in a RT1 (MHC) incompatible donor-recipient combination from a BN donor into a F344 recipient. Complete graft rejection in this severely mismatched combination would be expected histologically and radiographically on postoperative day 7. The orthotopic left-lung transplantation procedure used in this study was a modification of the “cuff” technique for vascular anastomosis (15). In brief, the lung grafts were flushed and immersed in ice-cold normal saline solution before implantation. Cuffs with internal diameters of 1.65 and 2.20 mm for pulmonary arterial and venous anastomosis, respectively, were used. The left main bronchus was anastomosed using 8-0 prolene running sutures. In rats, radiographic assessment of a left-lung transplantation graft can be obscured by the right lung. Therefore, a postcaval lobectomy on the recipient’s right lung was performed immediately after implantation so as to facilitate radiographic assessment of the left-lung allograft. Rats with acute allograft dysfunction, as seen by an abnormal chest radiography immediately after implantation, were judged as technical failures and excluded from further analysis.
Agent
A hexa-peptide, Ac-RRWWCR-NH2 (antileukinate), was synthesized by the t-boc method and purified by SynPep (Dublin, CA, USA).
Experimental Groups
Recipient animals were divided into 4 groups (Table 1). Animals from group A (early administration; n = 6) received 0.5 mL PBS solution containing a 10.0 mg/kg concentration of antileukinate intramuscularly 24 h before (day –1) and immediately after (day 0) transplantation. The control group for group A (Cont-A) received 0.5 mL PBS in a similar fashion. Allograft recipients from group B (continuous administration; n = 8) were given continuous antileukinate administration intraperitoneally for 7 days after transplantation, starting immediately after surgery, using a micro-osmotic pump that was implanted into the peritoneal cavity 2 h before transplantation. Antileukinate was released into the recipient’s peritoneal cavity continuously at a dose of 10.0 mg/kg/d from day 0 to 7. Cont-B served as the control group for group B and received the same amount of PBS (without antileukinate) in the same manner.
Table 1.
Experimental groups.
| Groups | n | Antileukinate administration | TST, min | GIT, min |
|---|---|---|---|---|
| A | 6 | 2 doses of 10.0 mg/kg/d, bolus injection; im 24 h before and immediately after surgery | 140.3 ± 7.4 | 109.7 ± 3.6 |
| Cont-A | 6 | None* | 130.8 ± 7.9 | 104.0 ± 7.6 |
| B | 8 | Continuous, ip 10.0 mg/kg/d, days 0 to 7 | 140.6 ± 24.5 | 113.3 ± 24.0 |
| Cont-B | 8 | None* | 138.8 ± 17.6 | 107.3 ± 15.2 |
Each control group was given PBS in the same manner as its respective treated group. im, intramuscular; ip, intraperitoneal using micro-osmotic pumps; TST, total surgical time (from beginning of thoracotomy to end of surgery); GIT, graft ischemic time (from donor lung flush to reperfusion).
Implantation of Micro-osmotic Pumps for Groups B and Cont-B
Micro-osmotic pumps (model 2001; Alzet, distributed by Charles River Laboratories, Sulzfeld, Germany) were used for intraperitoneal delivery of antileukinate. Pumps were preincubated for 4 h at 37°C in sterile saline according to the manufacturer’s instructions and subsequently filled with 200 μL antileukinate solution (104.2 μg/μL in PBS, pH 7.4). After rats were anesthetized, a ~1-cm median laparotomy was performed and the pump was implanted in the abdominal cavity.
Radiographic Evaluation
Transplanted lungs were serially assessed every other day by chest roentgenogram under spontaneous ventilation with halothane for sedation. Each chest roentgenogram was graded according to a previously published aeration score (AS) (16) (0 for an opaque lung, up to 6 for a normal-appearing lung) by a blinded observer.
Histological Evaluation
Allograft lungs were harvested on day 7 after transplantation. Acute rejection was evaluated histologically and assigned a rejection score (RS) based on the clinical international working formulation (IWF): grade 0, no significant abnormality; grade 1, scattered infrequent perivascular mononuclear infiltration; grade 2, frequent perivascular mononuclear infiltration; grade 3, extension of mononuclear infiltration into alveolar septum/space; and grade 4, diffuse perivascular, interstitial, and airspace infiltration of mononuclear cells. The number of neutrophils was counted in a blinded fashion in 10 random high-power fields (hpf) per slide at ×1000 magnification.
Statistical Analysis
The data were expressed as mean ± standard deviation. Analysis of intergroup differences by radiographic and histological scores was performed using the Mann-Whitney U test. Differences were considered significant if the P value was less than 0.05.
RESULTS
All animals survived the transplantation procedure. There were no significant differences in total surgical time (time from beginning of recipient’s thoracotomy to the end of surgery) and graft ischemic time (time from donor lung perfusion to its reperfusion when implantation was completed) in the 4 groups (Table 1).
Radiographic Findings
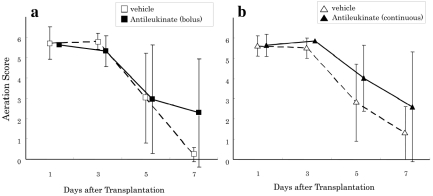
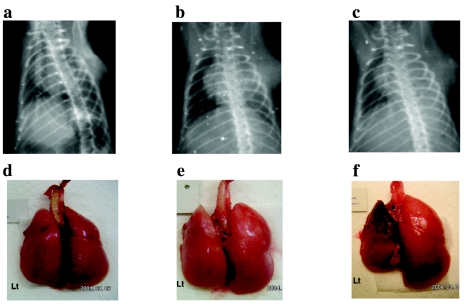
Allograft recipients from all 4 groups demonstrated excellent radiographic findings with no sign of rejection until day 3. On day 5 and thereafter, there was a progressive decrease in aeration score in all 4 groups with progression of rejection. No significant differences were observed between groups A and Cont-A or B and Cont-B at any time (Figure 1). On day 7 after transplantation, whereas the aeration scores for groups A and B were not significantly different, the aeration scores of the antileukinate-treated groups tended to be better than the corresponding controls (AS on day 7, group B and Cont-B: 2.6 ± 2.1 vs. 1.6 ± 1.3; P = 0.16). Representative chest radiographs from groups A, B, and Cont-B are shown in Figure 2 (upper panel). The AS values for each of the 3 radiographs were 6, 5, and 1, respectively.
Figure 1.
Radiographic aeration score (0 = opaque to 6 = normal-appearing lung). (a) ▪ and □ demonstrate group A (antileukinate early administration) and Cont-A (vehicle), respectively. There are no significant differences at each time point. (b) ▴ and ▵ demonstrate group B (antileukinate continuous) and Cont-B (vehicle), respectively. There are no significant differences at each time point. The value was expressed as mean ± standard deviation.
Figure 2.
Chest radiographic findings and macroscopic view of the allografts. Upper panel: Chest x-ray of allograft recipients on day 7 after transplantation. (a) Group A (antileukinate early administration) (AS = 6). (b) Group B (antileukinate continuous administration) (AS = 5). (c) Cont-B allograft recipient (AS = 1). Lower panel: Gross anatomy of group A allograft (d), group B allograft (e), and Cont-B allograft (f).
Macroscopic Findings of the Allograft
The allograft lungs from both Cont-A and Cont-B showed significant scattered hemorrhage, massive atelectasis, and complete consolidation without aeration compared with the native lungs. In contrast, the allografts from groups A or B demonstrated macroscopically adequate ventilation, akin to the contralateral native right lungs. Gross images of the representative allografts from groups A, B, and Cont-B on day 7 after transplantation are shown in Figure 2 (lower panel).
Microscopic Finding and Rejection Score
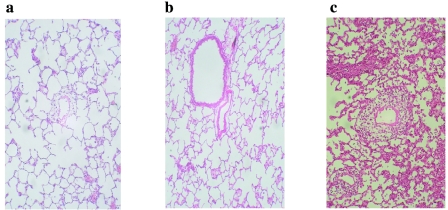
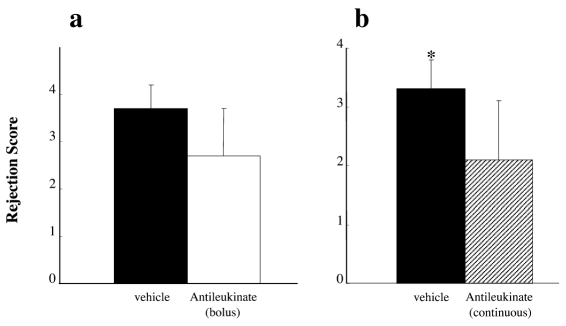
Allografts from groups Cont-A and Cont-B showed diffuse perivascular, interstitial, and airspace infiltration of mononuclear cells and were judged as the destructive phase of acute rejection (RS 4). In contrast, histology of allografts in groups A and B demonstrated only minimal perivascular infiltration of mononuclear cells around the pulmonary artery, indicating slight acute rejection (RS 1). Microscopic findings of the representative allografts from groups A, B, and Cont-B on day 7 are presented in Figure 3. The histologic rejection score, evaluated by means of IWF score on day 7, demonstrated significantly less rejection in group B compared with Cont-B (2.1 ± 1.0 vs. 3.3 ± 0.5; P = 0.018); however, there was no significant difference between groups A and Cont-A (2.7 ± 1.0 vs. 3.7 ± 0.5; P = 0.078) (Figure 4).
Figure 3.
Representative photomicrographs of lung allografts at day 7 after transplantation (H&E, magnification ×200). (a) Group A allograft: severe leukocyte infiltration and cuffing formation in the perivascular space (RS = 3). (b) Group B allograft, (c) Cont-B allograft: weak rejection; perivascular infiltrates present without extension into alveoli (RS = 1).
Figure 4.
Histologic rejection score of lung allografts. (a) Group A (antileukinate early administration, white bar) and Cont-A (vehicle, black bar). (b) Group B (antileukinate continuous administration, shadow bar) and Cont-B (vehicle, black bar). There is significant difference in histologic rejection score between B and Cont-B group (2.1 ± 1.0 vs. 3.3 ± 0.5, P = 0.018). The value is expressed as mean ± standard deviation. *Significant difference (P = 0.018) in comparison with vehicle group.
Effects of Antileukinate on the Allograft
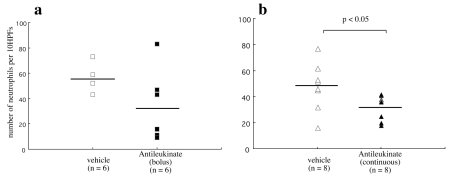
To investigate whether antileukinate acts on the allograft, we blindly counted the number of neutrophils in the graft (Figure 5). The neutrophil count was significantly decreased to 32.1 ± 9.6 per 10 hpf in group B compared with 47.9 ± 18.4 per 10 hpf in Cont-B (P = 0.046); however, there was no significant difference between group A and Cont-A (34.8 ± 28.7 vs. 55.7 ± 10.0 per 10 hpf; P = 0.093).
Figure 5.
Number of neutrophils infiltrated into lung grafts. (a) Group A (antileukinate early administration, ▪) and Cont-A (vehicle, □). (b) Group B (antileukinate continuous administration, ▴) and Cont-B (vehicle, ▵). The values are shown with the corresponding means. There is a significant difference in the number of neutrophils that have infiltrated the lung between B and Cont-B.
DISCUSSION
Acute allograft rejection is basically primed by alloantigen-specific T cells that respond to specific donor antigen and infiltrate into the graft, where they are activated to exhibit immune functions that mediate cellular immunologic reactions. However, the immunologic processes that initiate allograft rejection of a solid organ allograft are complex, involving the interplay of cells, antigens, and cytokines or chemokines.
Meanwhile, many reports have suggested that preservation-related injury including ischemia-reperfusion injury causes not only early organ dysfunction after transplantation, but also increased frequency or severity of acute allograft rejection (17,18). Local production of cytokines in the allograft such as IL-1 and IL-2, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) is increased during ischemia-reperfusion injury, and such mediators play an important role in initiating increased T lymphocyte and natural killer cell cytotoxicity (19–22). Furthermore, these cytokines are known to be important regulators of MHC antigen expression and allograft immunogenicity. In addition, intracellular adhesion molecule expression has been shown to be upregulated during reperfusion, with increased adhesion and migration of alloreactive lymphocytes that trigger the allograft rejection process (23–26).
The neutrophil, which has been studied mainly in relation to ischemia-reper-fusion injury such as the source of reactive oxygen intermediates, plays a crucial role in ischemia-reperfusion injury development. Neutrophils are among the earliest leukocytes to traffic into inflammatory sites and are potent amplifiers of early inflammation in the allograft after organ transplantation. Reperfusion of ischemic tissues quickly induces production of neutrophil chemoattractants, such as GRO-α or macrophage inflammatory protein (MIP)-2, which enhance neutrophil infiltration into the parenchyma of transplanted tissues. In addition to chemokines, neutrophil trafficking into parenchymal tissues can be directed by many other mediators, such as C5a (27).
The important role of neutrophils and their potential effects in the development of the cellular immunologic reaction are well recognized. However, the role of neutrophils on macrophage recruitment and activation (28) and the direct and indirect effects of neutrophils on adaptive T-cell responses have only recently been recognized (8). Activated neutrophils stimulate antigen-presenting cells such as DCs by producing CC chemokine ligands CCL3 (MIP-1α), CCL4 (MIP1-β), CCL5 (RANTES), and CCL20 (MIP-3α), which chemoattract immature DCs (29). Neutrophils can induce DC maturation as indicated by upregulating key costimulatory molecules, including CD40, CD80, and CD86 (30). In addition, it is reported that neutrophils behave as antigen-presenting cells by antigen uptake, transcribing and expressing the genes encoding MHC class I and class II molecules, and inducing T-cell–dependent immune responses (31). Furthermore, neutrophils are endowed with cross-presentation ability, as they can process exogenous antigens via an alternate MHC class I pathway and subsequently stimulate CD8+ T cells. Once exposed to inflammatory signals, activated neutrophils produce cytokines and chemokines that influence T-cell differentiation. In several models, neutrophils recruited after infection indeed produced IFN-γ, TNF-α, and IL-12 and induced Th1 polarization of antigen-specific T lymphocytes that influence T-cell immune responses (11,32–35). Those findings suggest that neutrophils influence both the “induction” phase, when alloantigen is recognized by the recipient immune system, and the “effector” phase, when specific T-cell proliferation is accelerated (29).
These observations and suggestions caused us to ascertain the linkage between neutrophils and the development of acute cellular rejection. Thus, we prepared these 2 experimental settings to investigate the effect of neutrophil suppression, through either the induction or effector phases, on the development of allograft rejection. In addition, antileukinate is a hexapeptide and would be expected to be cleared rapidly from the bloodstream. Part of the reason for the disappearance from the plasma is binding to the CXC receptors on the neutrophils. Neutrophils in the circulation are short lived (8–20 h), after which they undergo spontaneous apoptosis and are cleared (36). For these reasons, we have used 2 different administration regimen. In the A group, recipients were given an antileukinate bolus to suppress neutrophil activation during the induction phase. In contrast, animals from group B were administered antileukinate continuously to investigate the effect of neutrophils in the effector phase.
All left-lung allografts were well-aerated during the first 3 days after transplantation. Then, acute rejection was detected by means of decreased AS in all 4 groups. The data from chest radiographs on the basis of semiquantitative aeration score demonstrated no significant difference between group A andCont-A or B and Cont-B during the entire observation period. However, on day 5 and thereafter, the aeration score progressively decreased in the Cont-B, whereas group B showed less of a decrease in the aeration score. Significant alleviation of acute allograft rejection in group B versus its control was confirmed histologically by the rejection score based on the IWF scoring system. However, there was no significant difference in the degree of acute rejection in both radiographic and histologic findings against its control in group A, which received antileukinate only on the induction phase after transplantation. Interestingly, consistent with its CXC receptor inhibitory activity, continuous administration of antileukinate resulted in significantly fewer neutrophils within the graft. Whereas the trend was similar in the early antileukinate administration groups, it did not reach the level of significance.
These findings suggest that antileukinate may effectively suppress the progression of rejection during the effector phase. The mechanism of action of antileukinate on the suppression of acute lung allograft rejection was not fully confirmed in this experimental setting. However, it is thought that neutrophil suppression in the induction phase only is not sufficient to suppress the rejection process. Long-term suppression of neutrophil activation might be necessary to suppress the neutrophil-dependent allograft rejection process.
Although antileukinate had only a partial beneficial effect in this experiment, inhibition of neutrophil activation may contribute to preventing allograft rejection. We could expect better results using antileukinate efficiently in combination with other immunosuppressive drugs.
In conclusion, our data suggest that neutrophils may promote the development of allograft rejection and that inhibition of neutrophil activation and migration may suppress acute rat lung allograft rejection.
ACKNOWLEDGMENTS
This work was funded in part by the Foundation for Medical, Care, Education Research (T. Shiraishi) and the National Institutes of Health GM60197 and HL081655 (E.J.M.).
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Fukuse T, Hirata T, Ueda M, Hitomi S, Wada H. Effects of Euro-Collins, University of Wisconsin, and new extracellular-type trehalase-containing Kyoto solutions in an ex vivo rat lung preservation model. Transplantation. 1996;62:1212–7. doi: 10.1097/00007890-199611150-00004. [DOI] [PubMed] [Google Scholar]
- 2.Wittwer T, et al. Experimental lung transplantation: impact of preservation solution and route of delivery. J Heart Lung Transplant. 2005;24:1081–90. doi: 10.1016/j.healun.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Ardehali A, Laks H, Russell H, Levine M, Shpiner R, Lackey S, Ross D. Modified reperfusion and ischemia-reperfusion injury in human lung transplantation. J Thorac Cardiovasc Surg. 2003;126:1929–34. doi: 10.1016/s0022-5223(03)00976-0. [DOI] [PubMed] [Google Scholar]
- 4.Meyers BF, et al. Primary graft dysfunction and other selected complications of lung transplantation: a single-center experience of 983 patients. J Thorac Cardiovasc Surg. 2005;129:1421–9. doi: 10.1016/j.jtcvs.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Naka Y, Toda K, Kayano K, Oz MC, Pinsky DJ. Failure to express the P-selectin gene or P-selectin blockade confers early pulmonary protection after lung ischemia or transplantation. Proc Natl Acad Sci U S A. 1997;94:757–61. doi: 10.1073/pnas.94.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiraishi T, et al. Free radical-mediated tissue injury in acute lung allograft rejection and the effect of superoxide dismutase. Ann Thorac Surg. 1997;64:821–5. doi: 10.1016/s0003-4975(97)00754-6. [DOI] [PubMed] [Google Scholar]
- 7.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 8.Chertov O, Yang D, Howard OM, Oppenheim JJ. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol Rev. 2000;177:68–78. doi: 10.1034/j.1600-065x.2000.17702.x. [DOI] [PubMed] [Google Scholar]
- 9.Reali E, et al. Polymorphonuclear neutrophils pulsed with synthetic peptides efficiently activated memory cytotoxic T lymphocytes. J Leukoc Biol. 1996;60:207–13. doi: 10.1002/jlb.60.2.207. [DOI] [PubMed] [Google Scholar]
- 10.Potter NS, Harding CV. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J Immunol. 2001;167:2538–46. doi: 10.4049/jimmunol.167.5.2538. [DOI] [PubMed] [Google Scholar]
- 11.Graf MR, Prins RM, Merchant RE. IL-6 secretion by a rat T9 glioma clone induces a neutrophil-dependent antitumor response with resultant cellular antiglioma immunity. J Immunol. 2001;166:121–9. doi: 10.4049/jimmunol.166.1.121. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi S, Kurdowska A, Miller EJ, Albright ME, Girten BE, Cohen AB. Synthetic hexa- and heptapeptides that inhibit IL-8 from binding to and activating human blood neutrophils. J Immunol. 1995;154:814–24. [PubMed] [Google Scholar]
- 13.Hayashi S, Yatsunami J, Fukuno Y, Kawashima M, Miller EJ. Antileukinate, a hexapeptide inhibitor of CXC-chemokine receptor, suppresses bleomycin-induced acute lung injury in mice. Lung. 2003;180:339–48. doi: 10.1007/s00408-002-0106-7. [DOI] [PubMed] [Google Scholar]
- 14.Lin X, et al. alpha-Chemokine receptor blockade reduces high mobility group box 1 (HMGB1) protein induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;I289:L583–90. doi: 10.1152/ajplung.00091.2005. [DOI] [PubMed] [Google Scholar]
- 15.Mizuta T, Kawaguchi AT, Nakahara K, Kawashima Y. Simplified rat lung transplantation using cuff technique. J Thorac Cardiovasc Surg. 1989;97:578–81. [PubMed] [Google Scholar]
- 16.Prop J, Wagenaar-Hilbers JP, Petersen AH, Wildenuur CR. Diagnosis of rejection in rat lung allografts by bronchoalveolar lavage. Transplant Proc. 1987;19:3779–80. [PubMed] [Google Scholar]
- 17.Ettinger SL, McLoughlin MG, Scudamore CH, Miller RR, Keown PA. Effects of recovery from ischemic injury on class I and class II MHC antigen. Transplantation. 1990;49:641–3. [PubMed] [Google Scholar]
- 18.Howard TK, Klintmalm GBG, Cofer JB, Husberg BS, Goldstein RM, Gonwa TA. The influence of preservation injury on rejection in the hepatic transplantation recipient. Transplantation. 1990;49:103–7. doi: 10.1097/00007890-199001000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Welsh RM. Induction of alloreactive cytotoxic T cells by acute virus infection of mice. J Immunol. 1986;136:1186–93. [PubMed] [Google Scholar]
- 20.Chang SC, Hsu HK, Perng RP, Shiao GM, Lin CY. Increased expression of MHC class II antigens in rejecting canine lung allograft. Transplantation. 1990;49:1158–63. doi: 10.1097/00007890-199006000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Rao PN, et al. Reperfusion injury following cold ischemia activates rat liver Kuppfer cells. Transplant Proc. 1991;23:666–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Adoumie R, et al. Early cellular events in the lung allograft. Ann Thorac Surg. 1992;54:1071–7. doi: 10.1016/0003-4975(92)90072-c. [DOI] [PubMed] [Google Scholar]
- 23.Horgan MJ, Ge M, Gu J, Tothlein R, Malik AB. Role of ICAM-1 in neutrophil mediated lung vascular injury following occlusion and reperfusion. Am J Physiol. 1991;261:H1578–84. doi: 10.1152/ajpheart.1991.261.5.H1578. [DOI] [PubMed] [Google Scholar]
- 24.Fuggle SV, Sanderson JB, Gray DWR, Richardson A, Morris PJ. Variation in expression of endothelial adhesion molecules in pretransplant and transplanted kidneys: correlation with intra-graft events. Transplantation. 1993;55:117–23. doi: 10.1097/00007890-199301000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Morita K, Miura M, Paolone DR, Engeman TM, Kapoor A, Remick DG, Fairchaild RL. Early chemokine cascades in murine cardiac grafts regulate T cell recruitment and progression of acute allograft rejection. J Immunol. 2001;167:2979–84. doi: 10.4049/jimmunol.167.5.2979. [DOI] [PubMed] [Google Scholar]
- 26.Pelletier RP, Morgan CJ, Sedmak DD, Miyake K, Kincade PW, Ferguson RM, Orosz CG. Analysis of inflammatory endothelial changes, including VCAM-1 expression, in murine cardiac grafts. Transplantation. 1993;55:315–20. doi: 10.1097/00007890-199302000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin WM, Larsen CP, Fairchild RL. Innate immune responses to transplants: a significant variable with cadaver donors. Immunity. 2001;14:369–76. doi: 10.1016/s1074-7613(01)00117-0. [DOI] [PubMed] [Google Scholar]
- 28.Maus U, et al. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med. 2002;166:268–73. doi: 10.1164/rccm.2112012. [DOI] [PubMed] [Google Scholar]
- 29.Buonocore S, Surquin M, Moine AL, Abramowicz D, Flamand V, Goldman M. Amplification of T-cell responses by neutrophils: relevance to allograft immunity. Immunol Lett. 2004;94:163–6. doi: 10.1016/j.imlet.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol. 2003;171:6052–8. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- 31.Ashtekar AR, Saha B. Poly’s plea: membership to the club of APCs. Trends Immunol. 2003;24:485–90. doi: 10.1016/s1471-4906(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 32.Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–21. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Watanabe T, Watanabe H, Sendo F. Neutrophil depletion exacerbates experimental Chagas’ disease in BALB/c, but protects C57BL/6 mice through modulating the Th1/Th2 dichotomy in different directions. Eur J Immunol. 2001;31:265–75. doi: 10.1002/1521-4141(200101)31:1<265::AID-IMMU265>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Zhang Z, Sendo F. Neutrophils play a critical role in the pathogenesis of experimental cerebral malaria. Clin Exp Immunol. 2000;120:125–33. doi: 10.1046/j.1365-2249.2000.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tateda K, et al. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol. 2001;166:3355–61. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 36.Hu M, Miller EJ, Lin X, Simms HH. Transmigration across a lung epithelial monolayer delays apoptosis of polymorphonuclear leukocytes. Surgery. 2004;135:87–98. doi: 10.1016/s0039-6060(03)00347-7. [DOI] [PubMed] [Google Scholar]