Abstract
Aims: To determine the relation between clinical outcome and tumour grade defined by a MIB-1 (Ki-67) score based grading system.
Method: The clinical and pathological features of 50 patients with myxoid liposarcoma were evaluated, and MIB-1 immunostaining was performed to grade these patients’ tumours. Univariate and multivariate analyses were conducted to evaluate survival. Clinical follow up details were available for all patients (median, 46.5 months; range, 9–408).
Results: Univariate analysis revealed that the tumour site (p < 0.05), round cell component content (p < 0.01), necrosis (p < 0.01), mitosis (p < 0.01), MIB-1 labelling index (p < 0.001), and tumour grade (p < 0.001) had a significant impact on overall survival. Multivariate analysis showed that, of the variables evaluated, the tumour grade defined by a MIB-1 score based grading system was the most significant adverse prognostic factor.
Conclusion: Tumour grade determined by the grading system using the MIB-1 score (MIB-1 system) is a very strong prognostic factor in patients with myxoid liposarcoma.
Keywords: MIB-1, myxoid liposarcoma, prognosis
Myxoid and round cell liposarcomas are regarded as belonging to a continuous histopathological spectrum characteristic of a chromosome translocation, t(12;16)(q13;p11), resulting in the fusion of the TLS and CHOP genes.1–5 These tumours show a variable clinical behaviour, with round cell liposarcomas being highly metastatic, poorly differentiated tumours, whereas myxoid liposarcomas are less metastatic, moderately and well differentiated tumours, and are associated with a more favourable survival rate.6–9 Diagnosis and, hence, prognostic predictions can be complicated by lesions that often contain admixed components with myxoid and round cell morphologies.
“No detailed study of the prognostic significance of tumour grades assigned by a grading system in patients with myxoid liposarcoma has been published”
Among several different grading schemes for patients with soft tissue sarcomas, a grading system based on three criteria: tumour differentiation/histological type, necrosis, and the MIB-1 (Ki-67) score has been proposed.10–12 Multivariate analysis showed that the tumour grade assigned using this system was the most significant independent prognostic factor in adult patients presenting with the main histological types of soft tissue sarcoma.10–12 However, to the best of our knowledge no detailed study of the prognostic significance of tumour grades assigned by a grading system in patients with myxoid liposarcoma has been published. The objective of our study was to determine the relation between clinical outcome and tumour grade defined by a MIB-1 score based grading system in patients with myxoid liposarcoma.
MATERIALS AND METHODS
Patients
We reviewed the cases of 50 patients with myxoid liposarcoma who were registered in the pathology files of the National Cancer Centre (NCC), Tokyo, Japan. The clinical details, including follow up information, were obtained by reviewing all the medical charts. Thirty three of the 50 patients were male and 17 were female. Their mean age at diagnosis was 47 years and ranged from 17 to 87 years. No patients were lost to follow up, which began on the date of primary surgery. The median duration of follow up was 46.5 months and ranged from 9 to 408 months. Overall survival was recorded as the time to death as a result of any cause.
Pathology review, grading, and p53 immunostaining
Histological slides of all the patients’ tumours were reviewed for diagnosis by an expert pathologist at the NCC who had developed the tumour grading system that we used. Whenever necessary, immunohistochemical staining was carried out to confirm the diagnosis or tumour type according to the classification system described by Enzinger and Weiss.1 Tumour specimens were immunostained with the MIB-1 antibody directed at Ki-67 (Dako, Glostrup, Denmark; diluted 1/100 and autoclaved) and the MIB-1 (Ki-67) labelling index (LI) was estimated by determining the percentage of Ki-67 positive cell nuclei in each 1000 tumour cells in the region of the tumour with the greatest density of Ki-67 staining viewed under a light microscope. A MIB-1 score based grading system (MIB-1 system) is a three grade system obtained by summing the tumour differentiation, tumour necrosis, and the MIB-1 scores, each of which was given a score of 0, 1, 2, or 3.10 The tumour differentiation score according to the histological type was modified slightly from the French system.12 Myxoid and round cell liposarcomas were assigned tumour differentiation scores of 2 and 3, respectively. Tumour necrosis was assessed as 0 for no necrosis on any slide, 1 for < 50% tumour necrosis, and 2 for > 50% tumour necrosis. The MIB-1 score was estimated by counting the percentage of MIB-1 positive cell nuclei in each 1000 tumour cells in the region of the tumour with the greatest density of staining, which in most instances corresponded to the area with the highest mitotic activity. Lesions with MIB-1 LIs of 0–9%, 10–29%, or > 30% were assigned MIB-1 scores of 1, 2, or 3, respectively. The three separate scores were added together to produce a combined grade: lesions with a total score of 2 or 3 were classified as grade 1, those that scored 4 or 5 were grade 2, and those that scored 6, 7, or 8 were grade 3. According to this MIB-1 system, myxoid liposarcomas were assigned grades 1–3. A mouse monoclonal antibody, clone DO7 (Dako; diluted 1/100, and autoclaved) was used to immunostain tumour specimens to detect the p53 epitope located between amino acids 19 and 26 of the wild-type and mutant human p53 proteins. Tumours in which > 10% of the cells had nuclei that were immunostained were considered to be p53 positive.
Statistical analysis
The following parameters were considered for their prognostic value: age at presentation, sex, anatomical site, tumour size, surgical margin, tumour depth, the percentage of round cell (RC) components, MIB-1 score, and assigned tumour grade. Univariate analysis was performed by comparing Kaplan-Meier survival curves and carrying out log rank tests. The relative risk (RR) of each variable subjected to multivariate analysis was estimated using a Cox proportional hazards model. All analyses were conducted using SPSS software (version 11.0J; SPSS, Chicago, Illinois, USA). Differences and correlations with a p value < 0.05 derived from the two tailed test were considered to be significant.
RESULTS
Patients and tumour characteristics
The mean age at the time of presentation was 47 years (range, 17–87), and 25 patients were under 47 years of age (table 1). The tumours were located on the lower extremities in 42 patients and the trunk in eight. The mean tumour size was 9.6 cm and 24 tumours were greater than 10 cm. Forty three tumours were situated deeply, and seven were superficial. The surgical procedures performed consisted of wide excision, amputation, or disarticulation with adequate surgical margins in 34 patients, and marginal or intralesional excision with marginal or inadequate margins in 16. Additional treatment included chemotherapy in eight patients, radiotherapy in 12, and both in 10. Metastases occurred in 14 of the 50 patients and their locations were the peritoneal cavity in eight patients, soft tissue in eight, retroperitoneum in three, lung in three, and bone, liver, and mediastinum in two. Eighteen patients developed local recurrences.
Table 1.
Patient and tumour characteristics, and univariate analyses of survival in 50 cases of myxoid liposarcoma
| Variable | No of cases (%) | Per cent 5 year survival rate (%) | Logrank p value |
| Age (years) | |||
| ⩽47 | 25 | 87.4 | |
| >47 | 25 | 65.1 | 0.51 |
| Sex | |||
| Female | 17 | 87.1 | |
| Male | 33 | 71.8 | 0.23 |
| Site | |||
| Trunk | 8 | 100 | |
| Lower extremities | 42 | 71.7 | 0.04 |
| Surgical margin | |||
| Inadequate | 16 | 79.4 | |
| Adequate | 34 | 75.6 | 0.32 |
| Depth | |||
| Superficial | 7 | 100 | |
| Deep | 43 | 72.6 | 0.10 |
| Size (cm) | |||
| ⩽10 | 26 | 84.2 | |
| >10 | 24 | 68.0 | 0.30 |
| Necrosis | |||
| Absent | 36 | 85.5 | |
| Present | 14 | 51.4 | <0.01 |
| Mitosis | |||
| 0–9/10 HPF | 33 | 93.4 | |
| 10–19/10 HPF | 6 | 20.0 | |
| >20/10 HPF | 11 | 60.6 | <0.01 |
| MIB-1 labelling index (%) | |||
| 0–9 | 29 | 100 | |
| 10–29 | 9 | 43.8 | |
| >30 | 12 | 44.0 | <0.001 |
| Grade | |||
| 1 | 30 | 100 | |
| 2 or 3 | 20 | 41.4 | <0.001 |
HPF, high power field.
Histological features, grades, and p53 status
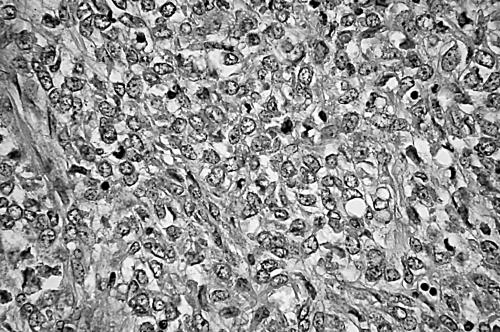
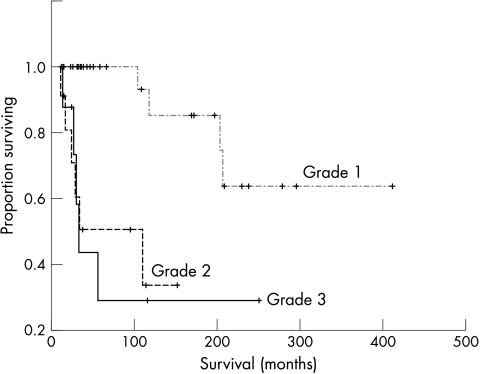
All the tumours exhibited a prominent plexiform or arborising vascular pattern within an abundant myxoid matrix. The extent of cellularity ranged from slight to moderate and the lesions were composed of uniform, small, round to oval shaped, primitive non-lipogenic mesenchymal cells and a variable number of small lipoblasts. Nuclear pleomorphism was not a prominent feature. The proportions of the tumours containing RC components were determined by microscopic observation: 15 tumours contained 0–5% RC components (fig 1), six contained 5–25% RC components, eight contained 25–50% RC components (fig 2), 10 contained 50–75% RC components, and 11 contained > 75% RC components (fig 3). These last tumours were defined as purely round cell liposarcomas. The round cells were often distributed around the septa, blood vessels, or at the tumour edges. Tumour necrosis was present in less than 50% of the area in 14 patients. Tumour necrosis was found only in tumours with > 25% RC components (p < 0.001). The mitotic counts were 0–9/10 high power fields (HPF) in 33 patients, 10–19/10 HPF in six, and > 20/10 HPF in 11. Mitotic figures were more common in the areas containing round cells. The MIB-1 LIs ranged from 1% to 90%. Based on the MIB-1 LI, 33, eight, and 12 of the tumours were assigned MIB-1 scores of 1, 2, and 3, respectively. According to the MIB-1-based grading system, 30 tumours were grade 1, nine were grade 2, and 11 were grade 3. Five tumours showed nuclear p53 immunoreactivity in > 10% of the tumour cells. Four of the 11 grade 3 tumours overexpressed p53, whereas only one of the 30 grade 1 tumours was p53 positive.
Figure 1.
A myxoid liposarcoma containing 0–5% round cell components consisting of uniform round to oval shaped primitive non-lipogenic mesenchymal cells and small signet ring lipoblasts in a prominent myxoid stroma.
Figure 2.
A myxoid liposarcoma containing 25–75% round cell components and signet ring lipoblasts with multivacuolated cytoplasm.
Figure 3.
A myxoid liposarcoma containing > 75% round cell components characterised by solid sheets of primitive round cells with a high nuclear to cytoplasmic ratio and conspicuous nucleoli, with no intervening myxoid stroma.
Prognostic analysis
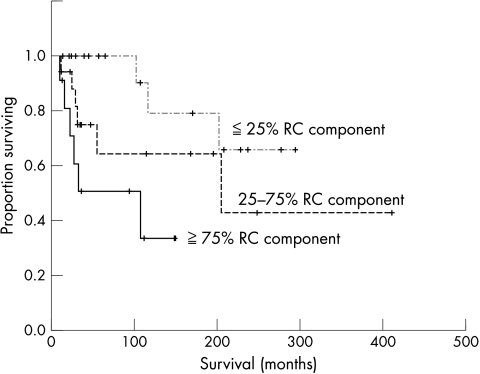
At the last follow up, 15 of the 50 patients had died and five were alive with metastatic disease, with five year and 10 year overall survival rates of 80.5% and 72.4%, respectively. The univariate analysis (table 1) showed that the tumour site (p < 0.05), RC component content (p < 0.01; fig 4), necrosis (p < 0.01), mitosis (p < 0.01), MIB-1 LI (p < 0.001), and tumour grade (p < 0.001; fig 5) had a significant impact on overall survival. Table 2 summarises the relation between RC component content and clinical outcome. The patients with tumours with < 25% RC components had a five year survival rate of 100% and a 10 year survival rate of 78.8%, significantly better than was seen in patients with tumours with > 25% RC components, who had a five year survival rate of 59.7% and a 10 year survival rate of 53.1% (p < 0.05). Using 75% RC component as the cutoff point, this value was also significant (p < 0.01). The patients with tumours with < 75% RC components had a five year survival rate of 83.4% and a 10 year survival rate of 72.2%, which was significantly better than was seen in patients with tumours with > 75% RC components, who had a 5 year survival rate of 50.5% and a 10 year survival rate of 33.7%. Univariate analysis showed no significant impact of surgical margins (p = 0.32) or additional treatment (p = 0.53) on outcome. No significant correlation was found between metastasis and tumour grade (p = 0.07). Three of five patients with tumours that overexpressed p53 had died. However, univariate analysis revealed no significant association between p53 status and survival (p = 0.20). Multivariate analysis adjusting for RC component content, necrosis, mitosis, MIB-1 LI, and tumour grade was conducted. Of these variables, the tumour grade was the most significant adverse prognostic factor, with an RR of 7.7 (95% confidence interval, 2.4 to 25.1; p < 0.001).
Figure 4.
Kaplan-Meier survival curve according to round cell (RC) component for 50 patients with myxoid liposarcoma.
Figure 5.
Kaplan-Meier survival curve according to tumour grade for 50 patients with myxoid liposarcoma.
Table 2.
The relation between round cell (RC) component content and clinical outcome in 50 cases of myxoid liposarcoma
| RC component content (%) | No of cases (%) | DOD (%) | Survival duration (months) | Per cent 5 year survival rate (%) | Per cent 10 year survival rate (%) |
| 0–5 | 15 (30) | 1 (6.7) | 261 (29) | 100.0 | 83.3 |
| 5–25 | 6 (12) | 2 (33) | 181 (19) | 100.0 | 75.0 |
| 25–50 | 8 (16) | 3 (37.5) | 141 (47) | 75.0 | 50.0 |
| 50–75 | 10 (20) | 3 (30) | 231 (70) | 72.9 | 36.5 |
| 75–100 | 11 (22) | 6 (54.5) | 98 (19) | 50.5 | 33.7 |
Survival duration is given as mean (SD).
DOD, number of patients who died of disease.
DISCUSSION
In our current study, we have documented the prognostic significance of the tumour grade determined by a MIB-1 score based grading system in patients with myxoid liposarcoma. Univariate analysis revealed that the tumour site, RC component content, necrosis, mitosis, MIB-1 LI, and tumour grade had a significant impact on overall survival, in accordance with previous studies.13–15 Multivariate analysis showed that, of these variables, the tumour grade determined by the grading system was the most important adverse prognostic factor.
The prognostic importance of the RC component has been acknowledged in previous studies. Patients (n = 5) with > 5% RC components in their initial tumours had a significantly higher incidence of metastasis or death from disease than those (n = 7) with < 5% RC components, despite the small sample size.13 In a study of 24 patients, > 25% RC components was significantly associated with adverse survival.14 Our patients with < 25% RC components had significantly better five year and 10 year survival rates than did those with > 25% RC components. A significant difference was also found when the cutoff value of RC components was set at 75%, which was in accordance with the results of previous studies.14,15 However, the correlation between the proportion of RC components and the clinical outcome may depend on the difficulty in measuring the RC components or transitional areas. There was no significant difference between the risk of an adverse outcome in patients with myxoid and transitional areas without RC components and those with myxoid areas alone.14
“The tumour site, round cell component content, necrosis, mitosis, MIB-1 labelling index, and tumour grade had a significant impact on overall survival”
It has been reported that the degree of necrosis correlates with the clinical outcome.16–18 Spontaneous tumour necrosis identified in four of 95 patients with myxoid liposarcoma correlated with an increased risk of metastases and death.13 In our study, the presence of necrosis in 14 patients was also associated with, and was a significant predictor of, poor outcome. Fewer workers have analysed p53 immunoreactivity and examined the relation between p53 overexpression and clinical outcome.19,20 In the study by Antonescu et al, 12 of 71 myxoid liposarcomas examined overexpressed p53, which was associated with an adverse prognosis on multivariate analysis (RR = 3.2; p < 0.05).15 In our present study, three of five patients whose tumours overexpressed p53 had died by the time of the final follow up. However, no significant association was found between p53 status and survival (p = 0.20). Overexpression of p53 in myxoid liposarcomas seems to be uncommon, and further studies are needed to confirm this. Liposarcomas are often large and a large tumour size is associated with a poor prognosis.21,22 In a previous study, the size of myxoid liposarcomas did not have a significant effect on overall survival.13 Nevertheless, several variables were analysed along with tumour size in our study, the results of which may support this concept. Metastases occurred in 14 patients; however, there was no significant correlation between metastasis and tumour grade.
Take home messages.
Multivariate analysis showed that the tumour grade determined by the MIB-1 score based grading system (MIB-1 system) is the most important adverse prognostic factor in patients with myxoid liposarcoma
This grading system is based on three variables: tumour differentiation/histological type, necrosis, and the MIB-1 (Ki-67) score
In conclusion, multivariate analysis of our results suggests that the tumour grade determined by the MIB-1 score based grading system (MIB-1 system) is the most important adverse prognostic factor in patients with myxoid liposarcoma.
Abbreviations
HPF, high power field
LI, labelling index
NCC, National Cancer Centre
RC, round cell
RR, relative risk
REFERENCES
- 1.Enzinger FM, Weiss SW. Soft tissue tumors, 4th ed. St Louis: Mosby Year Book, 2001.
- 2.Knight JC, Renwick PJ, Cin PD, et al. Translocation t(12;16)(q13;p11) in myxoid and round cell liposarcoma: molecular and cytogenetic analysis. Cancer Res 1995;55:24–7. [PubMed] [Google Scholar]
- 3.Panagopoulos I, Mandadhl N, Ron D, et al. Characterization of the CHOP breakpoints and fusion transcripts in myxoid liposarcomas with the 12;16 translocation. Cancer Res 1994;54:6500–3. [PubMed] [Google Scholar]
- 4.Kuroda M, Ishida T, Horiuchi H, et al. Chimeric TLS/FUS–CHOP gene expression and the heterogeneity of its junction in human myxoid and round cell liposarcoma. Am J Pathol 1995;147:1221–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Hisaoka M, Tsuji S, Morimitsu Y, et al. Detection of TLS/FUS–CHOP fusion transcripts in myxoid and round cell liposarcomas by nested reverse transcription-polymerase chain reaction using archival paraffin-embedded tissues. Diagn Mol Pathol 1998;7:96–101. [DOI] [PubMed] [Google Scholar]
- 6.Enzinger FM, Winslow DJ. Liposarcoma: a study of 103 cases. Virchows Arch Pathol Anat 1962;335:367–88. [PubMed] [Google Scholar]
- 7.Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol 1979;3:507–23. [DOI] [PubMed] [Google Scholar]
- 8.Evans HL. Liposarcomas and atypical lipomatous tumors: a study of 66 cases followed for a minimum of 10 years. Surg Pathol 1988;1:41–54. [Google Scholar]
- 9.Hashimoto H, Enjoji M. Liposarcoma: a clinicopathologic subtyping of 52 cases. Acta Pathol Jpn 1982;32:933–48. [PubMed] [Google Scholar]
- 10.Hasegawa T, Yokoyama R, Lee YH, et al. Prognostic relevance of a histological grading system using MIB-1 for adult soft-tissue sarcoma. Oncology 2000;58:66–74. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa T, Yamamoto S, Yokoyama R, et al. Prognostic significance of grading and staging systems using MIB-1 score in adult patients with soft tissue sarcoma of the extremities and trunk. Cancer 2002;95:843–51. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa T, Yamamoto S, Nojima T, et al. Validity and reproducibility of histologic diagnosis and grading for adult soft-tissue sarcomas. Hum Pathol 2002;33:111–15. [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick SE, Doyon J, Choong PFM, et al. The clinicopathologic spectrum of myxoid and round cell liposarcoma: a study of 95 cases. Cancer 1996;77:1450–8. [DOI] [PubMed] [Google Scholar]
- 14.Smith TA, Easley KA, Goldblum JR. Myxoid/round cell liposarcoma of the extremities: a clinicopathologic study of 29 cases with particular attention to extent of round cell liposarcoma. Am J Surg Pathol 1996;20:171–80. [DOI] [PubMed] [Google Scholar]
- 15.Antonescu CR, Tschernyavsky SJ, Decuseara R, et al. Prognostic impact of p53 status, TLS–CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: molecular and clinicopathologic study of 82 cases. Clin Cancer Res 2001;7:3977–87. [PubMed] [Google Scholar]
- 16.Lack EE, Steinberg SM, White DM, et al. Extremity soft tissue sarcomas: analysis of prognostic variables in 300 cases and evaluation of tumor necrosis as a factor in stratifying high-grade sarcomas. J Surg Oncol 1989;41:263–73. [DOI] [PubMed] [Google Scholar]
- 17.Mandard AM, Petiot JF, Marnay J, et al. Prognostic factors in soft tissue sarcomas. A multivariate analysis of 109 cases. Cancer 1989;63:1437–51. [DOI] [PubMed] [Google Scholar]
- 18.Choong PFM, Gustafson P, Willen H, et al. Prognosis following locally recurrent soft tissue sarcoma. A staging system based on primary and recurrent tumor characteristics. Int J Cancer 1995;6:33–7. [DOI] [PubMed] [Google Scholar]
- 19.Dei Tos AP, Piccinin S, Doglioni C, et al. Molecular aberrations of the G1–S checkpoint in myxoid and round cell liposarcoma. Am J Pathol 1997;151:1531–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Pilotti S, Lavarino C, Mezzelani A, et al. Limited role of Tp53 and Tp53-related genes in myxoid liposarcoma. Tumori 1998;84:571–7. [DOI] [PubMed] [Google Scholar]
- 21.Orson GG, Sim FH, Reiman HM, et al. Liposarcoma of the musculoskeletal system. Cancer 1987;60:362–70. [DOI] [PubMed] [Google Scholar]
- 22.Reitan JB, Kaazhus O, Brenhoud IO, et al. Prognostic factors in liposarcoma. Cancer 1985;55:2482–90. [DOI] [PubMed] [Google Scholar]