Abstract
Aims: To obtain further information regarding the frequency and degree of positivity for smooth muscle markers in a large number of malignant fibrous histiocytomas (MFHs), as an aid to accurate diagnosis.
Method: The immunohistochemical features of 100 MFHs were studied and the results were compared with those for 30 leiomyosarcomas. Eighteen cases of MFH with smooth muscle actin (SMA) positivity were examined ultrastructurally.
Results: Immunoreactivity for smooth muscle markers, such as desmin, SMA, muscle specific actin (MSA) and h-caldesmon (HCD), which is a specific marker for smooth muscle cells and their tumours, was found in 28, 30, 29, and 29 of 30 leiomyosarcomas. Immunoreactivity for desmin, SMA, MSA, and HCD was found in 17, 30, 14, and two of the MFHs. On electron microscopic examination, approximately half of the cases contained a varying proportion of myofibroblastic cells. The others had only fibroblastic or undifferentiated tumour cells. At least 30% of the cases were found to display features consistent with limited smooth muscle or myofibroblastic differentiation.
Conclusion: A large subset of so called MFH in fact shows poorly differentiated smooth muscle or myofibroblastic features, and perhaps such tumours should be regarded as pleomorphic leiomyosarcomas and/or pleomorphic myofibroblastic sarcomas.
Keywords: malignant fibrous histiocytoma, leiomyosarcoma, myofibroblastic differentiation, smooth muscle markers, electron microscopy
In a previous study,1 the reproducibility of determining the histological type in the diagnosis of adult soft tissue sarcomas was examined by comparing the independent diagnoses made by five different expert pathologists. The trend for some pathologists to diagnose malignant fibrous histiocytoma (MFH) less frequently than others may result from different diagnostic criteria for MFH among pathologists, reflecting the concept of MFH as a common morphological manifestation of a variety of poorly differentiated sarcomas, and the diagnosis of MFH through a process of exclusion.2,3 It was concluded that a re-evaluation of the diagnostic criteria was essential for so called MFH.
“Ultrastructural observation of neoplastic cells suggests that the expression of smooth muscle markers in so called malignant fibrous histiocytoma is a result of myofibroblastic differentiation”
Smooth muscle markers, such as smooth muscle actin (SMA) and occasionally even desmin, have been demonstrated immunohistochemically in a subset of “MFH”. In this situation, the distinction between leiomyosarcoma and “MFH” becomes more difficult. Ultrastructural observation of neoplastic cells suggests that the expression of smooth muscle markers in “MFH” is a result of myofibroblastic differentiation.4,5 There are limited data on the frequency and degree of positivity for smooth muscle markers in a large number of “MFHs”. Therefore, we studied the immunohistochemical features of 100 “MFHs”, along with their ultrastructural features, and compared the results with those for 30 leiomyosarcomas as an aid to accurate diagnosis.
MATERIALS AND METHODS
Subjects
One hundred patients with MFH, including pleomorphic (n = 47) and myxoid (n = 53) types, and 30 patients with leiomyosarcoma, all diagnosed and treated at the National Cancer Centre (NCC), Japan, were retrieved from the pathology files of the NCC. MFH occurred in the extremities (n = 67), trunk (n = 31), and retroperitoneum (n = 2), and leiomyosarcoma in the extremities (n = 5), trunk (n = 7), retroperitoneum (n = 7), inferior vena cava (n = 2), uterus (n = 5), kidney (n = 1), small intestine (n = 1), large intestine (n = 1), and rectum (n = 1). There were 60 male and 40 female patients with MFH, with a median age of 61 years (range, 26–88), and there were nine men and 21 women with leiomyosarcoma, with a median age of 52.5 years (range, 30–83).
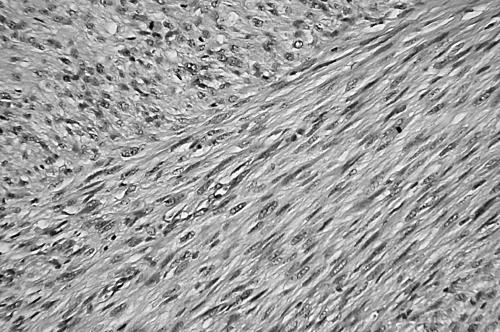
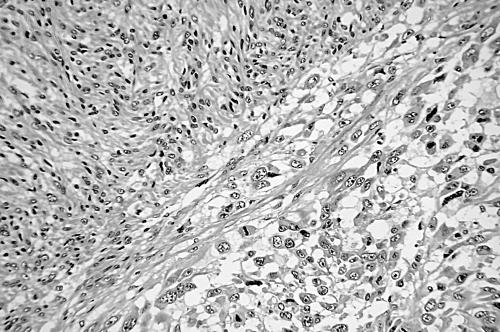
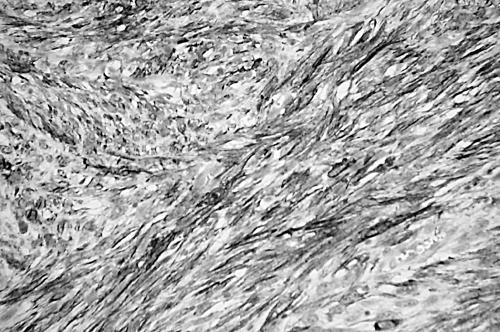
Leiomyosarcomas were classified, primarily on the basis of morphological appearance and strong immunoreactivity for SMA with or without desmin, into conventional, well to moderately differentiated type (n = 20), and pleomorphic, poorly differentiated type (n = 10). This last group comprised lesions of the external soft tissue (n = 6), retroperitoneum (n = 3), and rectum (n = 1). Conventional leiomyosarcomas were characterised by interlacing, tightly organised fascicles composed of elongated cells with easily seen eosinophilic cytoplasm and elongated nuclei with rounded ends (fig 1). Pleomorphic tumours displayed predominantly pleomorphic areas containing anaplastic spindle or oval cells, often admixed with bizarre giant cells mimicking pleomorphic MFH, in addition to at least an ordinary fascicular pattern of the elongated cells described above. In most tumours (n = 8), fascicular spindle cell areas were blended with pleomorphic areas (fig 2), and in retroperitoneal and rectal tumours (n = 2) the interface was abrupt (fig 3). Necrosis, haemorrhage, and mitotic figures were common in these tumours.
Figure 1.
Deeply eosinophilic intersecting fascicles of elongated spindle cells with blunt ended nuclei of a conventional leiomyosarcoma (haematoxylin and eosin stain; original magnification, ×200).
Figure 2.
Pleomorphic and spindle cells as seen in “malignant fibrous histiocytoma” of a pleomorphic leiomyosarcoma (haematoxylin and eosin stain; original magnification, ×200).
Figure 3.
The abrupt transition from fascicular area (upper left) to pleomorphic area (lower right) of a pleomorphic leiomyosarcoma (haematoxylin and eosin stain; original magnification, ×200).
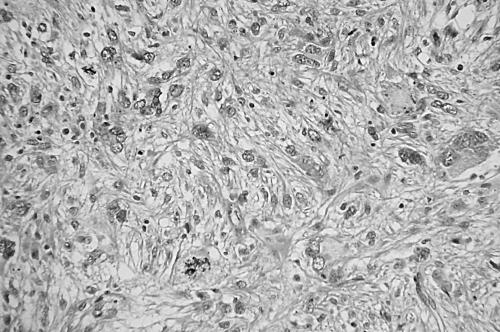
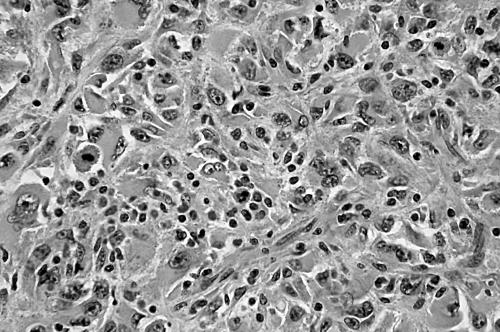
Pleomorphic MFH was originally defined as a tumour consisting of pleomorphic or spindle shaped cells arranged in a storiform or small fascicular pattern, with variable inflammatory infiltrate (fig 4). The neoplastic cells had pointed tapering nuclei, abundant pale eosinophilic or amphophilic cytoplasm, and indistinct cell borders. The stroma had a variable amount of collagen. Myxoid MFH contained a myxoid stroma occupying more than 50% of the tumour area; in these tumours, there were predominantly nodular myxoid regions with atypical hyperchromatic spindled cells, bizarre giant cells, and distinct curvilinear vessels, in addition to minor solid pleomorphic or spindle cell components (fig 5). Mitotic figures including abnormal mitoses were usually numerous, and most tumours displayed necrosis. In addition, a soft tissue sarcoma with morphological features identical to those of pleomorphic or myxoid MFH, along with variable immunoreactivity for SMA, was classified as MFH with myofibroblastic features.
Figure 4.
Pleomorphic or spindle cells arranged in a storiform pattern with variable inflammatory infiltrate of a so called pleomorphic malignant fibrous histiocytoma (haematoxylin and eosin stain; original magnification, ×200).
Figure 5.
Atypical spindle and pleomorphic cells with an abundant myxoid stroma of a myxoid malignant fibrous histiocytoma/myxofibrosarcoma (haematoxylin and eosin stain; original magnification, ×200).
Immunohistochemistry
Tumour slides of all 130 cases, stained with haematoxylin and eosin, were reviewed, and representative sections from paraffin wax blocks were examined by the labelled streptavidin–biotin method, with the appropriate use of positive and negative controls. The sections were dewaxed, rehydrated, and moistened with phosphate buffered saline (pH 7.4). They were pretreated in an autoclave at 121°C for 10 minutes in 10 mmol/litre citrate buffer (pH 6.0), before being incubated with antibodies to the following molecules on an automated immunostaining system (i6000™; BioGenex, San Ramon, California, USA) for 30 minutes: vimentin (clone V9; 1/200 dilution; Dako, Glostrup, Denmark), desmin (clone D33; 1/200 dilution; Dako), α smooth muscle actin (clone 1A4; 1/100 dilution; Dako), muscle specific actin (clone HHF35; 1/100 dilution; Enzo, New York, USA), h-caldesmon (clone h-CD; 1/100 dilution; Dako), CD34 (clone My10; 1/100 dilution; Becton Dickinson, San Jose, California, USA), S-100 protein (polyclonal; 1/2000 dilution; Dako), epithelial membrane antigen (clone E29; 1/100 dilution; Dako), and cytokeratin (clone AE1/3; 1/100 dilution; Dako). Heat induced epitope retrieval was not used for sections stained with antibodies to S-100 protein and epithelial membrane antigen (EMA).
Immunostaining for CD117/c-kit protein (polyclonal antibody; 1/50 dilution; Dako) with autoclave based epitope retrieval was performed to eliminate the possibility of gastrointestinal stromal tumour in three cases of leiomyosarcoma of the gastrointestinal tract. Sporadic faint CD117 positivity was observed in a large intestinal tumour, but the other two tumours were negative, and thus the diagnosis was confirmed. All cases were graded according to our grading system1 on the basis of three criteria (tumour differentiation, necrosis, and MIB-1 score) because this system, modified from the French system, proved to be more valid and reproducible than the original system, and was a strong prognostic factor in adult patients with the main histological types of soft tissue sarcoma.6
Electron microscopy
Eighteen cases of MFH with myofibroblastic features, 14 of the pleomorphic type and four of the myxoid type, were available for ultrastructural analysis. Small fresh fragments of tumour tissue were fixed in 2.5% glutaraldehyde, postfixed in 1% osmium tetroxide, and embedded in epoxy resin. After contrasting with uranyl acetate and lead citrate, ultrathin sections were examined with a transmission electron microscope (H-7500; Hitachi High-Technologies, Tokyo, Japan).
RESULTS
Immunohistochemical features
Tables 1 and 2 summarise the immunohistochemical results of the 30 leiomyosarcomas and the 100 MFHs, classified according to tumour location for the leiomyosarcomas and subtype for the MFHs. All of the leiomyosarcomas and the MFH tumours were positive for vimentin; most cases showed vimentin reactivity in more than 50% of the tumour cells.
Table 1.
Frequency and degree of immunoreactivity for each antibody in 30 leiomyosarcomas
| Vimentin (%) | Desmin (%) | SMA (%) | MSA (%) | HCD (%) | CD34 (%) | S-100 (%) | EMA (%) | CK (%) | |
| Leiomyosarcoma | 30 (100) | 28 (93) | 30 (100) | 29 (97) | 29 (97) | 0 | 0 | 14 (47) | 0 |
| External soft tissue (n=12) | 12 (100) | 10 (83) | 12 (100) | 11 (92) | 11 (92) | 0 | 0 | 4 (33) | 0 |
| 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 8 | 0 |
| 1+ | 0 | 3 | 0 | 6 | 8 | 0 | 0 | 4 | 0 |
| 2+ | 2 | 3 | 1 | 5 | 0 | 0 | 0 | 0 | 0 |
| 3+ | 10 | 4 | 11 | 0 | 3 | 0 | 0 | 0 | 0 |
| Retroperitoneum and abdomen (n=18) | 18 (100) | 18 (100) | 18 (100) | 18 (100) | 18 (100) | 0 | 0 | 10 (56) | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 |
| 1+ | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 5 | 0 |
| 2+ | 2 | 3 | 0 | 9 | 0 | 0 | 0 | 5 | 0 |
| 3+ | 14 | 14 | 18 | 8 | 17 | 0 | 0 | 0 | 0 |
CK, cytokeratin; EMA, epithelial membrane antigen; HCD, heavy-caldesmon; MSA, muscle specific actin; SMA, α smooth muscle actin.
Degree of immunoreactivity: 0, negative; 1+, less than 10% positivity; 2+, 10–50% positivity; 3+, more than 50% positivity.
Table 2.
Frequency and degree of immunoreactivity for each antibody in 100 malignant fibrous histiocytomas (MFHs)
| Vimentin (%) | Desmin (%) | SMA (%) | MSA (%) | HCD (%) | CD34 (%) | S-100 (%) | EMA (%) | CK (%) | |
| MFH | 100 (100) | 17 (17) | 30 (30) | 14 (14) | 2 (2) | 22 (22) | 0 | 5 (5) | 13 (13) |
| Pleomorphic (n=47) | 47 (100) | 15 (32) | 22 (47) | 11 (24) | 2 (4) | 2 (4) | 0 | 5 (11) | 7 (15) |
| 0 | 0 | 32 | 25 | 36 | 45 | 45 | 0 | 42 | 40 |
| 1+ | 0 | 11 | 8 | 4 | 2 | 1 | 0 | 4 | 5 |
| 2+ | 12 | 3 | 4 | 7 | 0 | 1 | 0 | 1 | 2 |
| 3+ | 35 | 1 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Myxoid (n=53) | 53 (100) | 2 (4) | 8 (15) | 3 (6) | 0 | 20 (38) | 0 | 0 | 6 (11) |
| 0 | 0 | 51 | 45 | 50 | 0 | 33 | 0 | 0 | 47 |
| 1+ | 0 | 1 | 3 | 2 | 0 | 8 | 0 | 0 | 4 |
| 2+ | 22 | 1 | 3 | 1 | 0 | 5 | 0 | 0 | 2 |
| 3+ | 31 | 0 | 2 | 0 | 0 | 7 | 0 | 0 | 0 |
CK, cytokeratin; EMA, epithelial membrane antigen; HCD, heavy-caldesmon; MSA, muscle specific actin; SMA, α smooth muscle actin.
Degree of immunoreactivity: 0, negative; 1+, less than 10% positivity; 2+, 10–50% positivity; 3+, more than 50% positivity.
Immunoreactivity for the smooth muscle markers desmin, SMA, muscle specific actin (MSA), and h-caldesmon (HCD) was found in 28, 30, 29, and 29 of the 30 leiomyosarcomas. All 18 leiomyosarcomas of the retroperitoneum and abdomen displayed coexpression of all smooth muscle markers (fig 6). Except for MSA, this positive reactivity was diffuse and found in more than 50% of cells in most tumours. In the 12 cases of external soft tissue leiomyosarcoma, reactivity for desmin, SMA, MSA, and HCD was found in 10, 12, 11, and 11 tumours, respectively. Eight of the 12 tumours of external soft tissue coexpressed all smooth muscle markers. Their reactivity for MSA and HCD was sporadic (1+) to focal (2+) in most cases. There was no difference in the frequency and degree of immunoreactivity for the smooth muscle markers between conventional and pleomorphic leiomyosarcomas, and among tumours of different grades. Fourteen of the 30 leiomyosarcomas were positive for EMA; its reactivity was sporadic (1+) to focal (2+) in 10 of the 18 tumours of the retroperitoneum and abdomen, and sporadic (1+) in four of the 12 tumours of the external soft tissue.
Figure 6.
A conventional leiomyosarcoma of external soft tissue shows diffuse and intense immunoreactivity for heavy-caldesmon (immunoperoxidase; original magnification, ×400).
Immunoreactivity for CD34, S-100 protein, and cytokeratin was negative in all leiomyosarcomas.
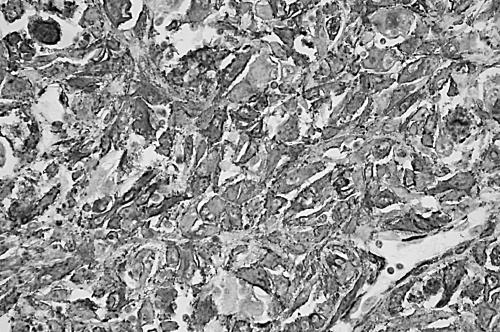
Immunoreactivity for desmin, SMA, MSA, and HCD was found in 17, 30, 14, and two of the MFHs. These smooth muscle markers were expressed in 15, 22, 11, and two of the 47 pleomorphic type tumours , and two, eight, three, and none of the 53 myxoid type tumours. Therefore, 30 of the 100 tumours were classified as MFH with myofibroblastic features, and these comprised 22 pleomorphic and eight myxoid types. Among the 22 pleomorphic MFHs with SMA positivity (fig 7), eight tumours were positive for both desmin and MSA, and two were also positive for HCD. Their positive reaction for the smooth muscle markers other than SMA was sporadic (1+) to focal (2+) in the otherwise typical pleomorphic areas. Of eight SMA positive tumours of the myxoid type, three showed a sporadic (1+) to focal (2+) reactivity for MSA. There was no difference in morphological appearances between tumours with and without myofibroblastic features.
Figure 7.
A so called pleomorphic malignant fibrous histiocytoma shows diffuse and strong immunoreactivity for α smooth muscle actin (immunoperoxidase; original magnification, ×400).
A positive reaction for CD34 was evident in 22 of the 100 MFHs, 20 of which were myxoid MFH, with a varying degree of reactivity. Five of the 47 pleomorphic MFHs expressed sporadic (1+) to focal (2+) EMA reactivity. Similarly, sporadic to focal reactivity for cytokeratin was seen in seven of the 47 pleomorphic MFHs and six of the 53 myxoid MFHs. None of the histological types of MFH showed immunoreactivity for S-100 protein.
By our histological grading system, 19 of the 30 leiomyosarcomas were classified as grade 3, 10 as grade 2, and one as grade 1. All 10 pleomorphic leiomyosarcomas were grade 3 tumours. Of the 47 pleomorphic and 53 myxoid MFH tumours, 36 and 11 were assessed as grade 3, nine and 35 as grade 2, and two and 7 as grade 1, respectively. There was no difference in histological grade between tumours with and without myofibroblastic features.
Ultrastructural features
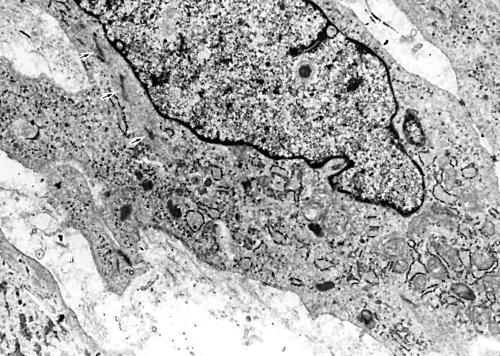
Among the 18 MFHs with myofibroblastic features that were examined, eight of 14 pleomorphic type and two of four myxoid type tumours contained a varying proportion of myofibroblastic cells, in addition to fibroblastic cells with a prominent Golgi apparatus and well developed rough endoplasmic reticulum, and fewer histiocytic cells containing numerous cytoplasmic lysosomes and multiple Golgi complexes. The myofibroblastic tumour cells were recognised by the presence of an often well developed branching rough endoplasmic reticulum and primarily peripheral bundles of actin microfilaments with interspersed fusiform densities (fig 8). Subplasmalemmal attachment plaques, focal basal lamina-like material, and micropinocytotic vesicles were present to variable degrees in these cells. The other tumours consisted of fibroblastic and undifferentiated mesenchymal cells with few organelles. There was no difference in expression of smooth muscle markers between tumours with and without myofibroblastic differentiation.
Figure 8.
A myofibroblastic tumour cell containing a well developed rough endoplasmic reticulum, prominent Golgi areas, and a few bundles of myofilaments with focal densities as indicated by arrows (uranyl acetate and lead citrate stain; original magnification, ×6000).
DISCUSSION
In accordance with previous findings,7 leiomyosarcomas in our series were more common in women than in men. Retroperitoneal and abdominal tumours outnumbered those in external soft tissue. Leiomyosarcomas were morphologically classified into conventional and pleomorphic types. Conventional leiomyosarcoma was characterised by interlacing, tightly organised fascicles composed of elongated cells with easily seen eosinophilic cytoplasm and elongated nuclei with rounded ends. Pleomorphic type tumours displayed predominantly pleomorphic areas containing anaplastic spindle or oval cells, often admixed with bizarre giant cells mimicking pleomorphic MFH, in addition to at least an ordinary fascicular pattern of elongated smooth muscle-like cells. Leiomyosarcomas showed a distinctive smooth muscle phenotype, as shown by a high frequency and degree of immunoreactivity for desmin, SMA, MSA, and HCD.
In contrast, so called MFH occurred predominantly in the extremities and trunk of elderly men. “MFH” was characterised by pleomorphic or spindle cells arranged in a storiform or small fascicular pattern, with a varying amount of inflammatory infiltrate, and myxoid and collagenous stroma. The neoplastic cells had pointed tapering nuclei, abundant pale eosinophilic or amphophilic cytoplasm, and indistinct cell borders. In our study, 30% of tumours were classified as “MFH” with myofibroblastic features, which had a morphological appearance identical to that of “MFH” and immunoreactivity for SMA.
In our immunohistochemical survey of leiomyosarcoma, the frequency and degree of positivity for smooth muscle markers, particularly HCD, in tumours of the retroperitoneum exceeded that in tumours of the external soft tissue. Recently, heavy caldesmon (HCD), an actin binding cytoskeleton associated protein, has been shown to be a specific immunohistochemical marker for smooth muscle cells and their tumours.8,9 Such variable expression of HCD according to location has been reported,10 but the histological subtype (conventional or pleomorphic) or different grade of leiomyosarcoma did not affect the results. We also found that EMA immunoreactivity was present in approximately half of all leiomyosarcomas, as described in a previous study.11
“As reflected in the latest World Health Organisation classification of soft tissue tumours, so called malignant fibrous histiocytoma can no longer be regarded as a definable entity, and is now viewed as a synonym for undifferentiated pleomorphic sarcoma”
In our present study, S-100 was always negative in so called MFH, and a small proportion of cases showed sporadic to focal immunoreactivity for cytokeratin and EMA, in keeping with previous results.12 CD34 corresponds to the cluster designation of a transmembrane glycoprotein that is expressed in human haemopoietic progenitor cells and vascular endothelial cells. In addition to its expression in primitive leukaemias and vascular tumours, CD34 has been found to be positive in Kaposi’s sarcoma, dermatofibrosarcoma protuberans, giant cell fibroblastoma, solitary fibrous tumour, gastrointestinal stromal tumour, epithelioid sarcoma, and inflammatory fibroid polyps of the stomach.13 The presence of CD34 has been reported in sporadic cases of “MFH”,14,15 but we found that CD34 positivity was a feature of myxoid MFH, being seen in 38% of cases. On the basis of the observation of CD34 expression in dendritic fibroblastic cells and a family of tumours derived from such cells,16 a subset of myxoid MFH, recently termed as myxofibrosarcoma, might share a common histogenesis, distinct from the pleomorphic type.
In our study, so called MFH displayed less frequent, and a lower degree of, immunoreactivity for the smooth muscle markers than did leiomyosarcoma. Among the histological subtypes of “MFH”, frequent positivity for SMA (22 of 47) and desmin (15 of 47) was seen in the pleomorphic type. A previous study also found frequent expression of smooth muscle markers in “MFH” of bone.17 Our present electron microscopic examination of “MFH” tumours with SMA positivity identified a myofibroblastic ultrastructure in approximately half of these tumours. The others contained only fibroblastic or undifferentiated tumour cells. Although various stages of ultrastructurally detectable myofibroblastic differentiation seem to be present in “MFH”,18 it is not meaningful to base the distinction on the ultrastructural examination of just 18 cases, particularly when electron microscopy is inevitably associated with sampling error, and the non-specificity of myofilaments means that electron microscopy should be used as an adjunctive study combined with other modalities.
From a morphological viewpoint, pleomorphic leiomyosarcoma may have areas that closely simulate so called pleomorphic MFH.19 As reflected in the latest World Health Organisation classification of soft tissue tumours,20 “MFH” can no longer be regarded as a definable entity, and is now viewed as a synonym for undifferentiated pleomorphic sarcoma. It has become a diagnosis of exclusion to be reserved for tumours showing no other line of differentiation—yet at least 30% of our cases showed another line of differentiation— that is, features consistent with limited smooth muscle or myofibroblastic differentiation. It has been reported that most “MFH” tumours and leiomyosarcomas have very similar comparative genomic hybridisation imbalances,21 and therefore an important subset of “MFH” could be leiomyosarcomas.
In conclusion, a large subset of so called MFH in fact shows poorly differentiated smooth muscle or myofibroblastic features and perhaps such tumours should be regarded as pleomorphic leiomyosarcomas and/or pleomorphic myofibroblastic sarcomas.
Take home messages.
A large subset of so called malignant fibrous histiocytoma shows poorly differentiated smooth muscle or myofibroblastic features
Perhaps tumours within this subset should be regarded as pleomorphic leiomyosarcomas and/or pleomorphic myofibroblastic sarcomas
Acknowledgments
This work was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour, and Welfare, Japan.
Abbreviations
EMA, epithelial membrane antigen
HCD, heavy-caldesmon
MFH, malignant fibrous histiocytoma
MSA, muscle specific actin
NCC, National Cancer Centre
SMA, smooth muscle actin
REFERENCES
- 1.Hasegawa T, Yamamoto S, Nojima T, et al. Validity and reproducibility of histologic diagnosis and grading for adult soft-tissue sarcomas. Hum Pathol 2002;33:111–15. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CDM. Commentary: malignant fibrous histioctyoma? Histopathology 1987;11:433–7. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher CDM. Pleomorphic malignant fibrous histiocytoma: fact or fiction? A critical reappraisal based on 159 tumours diagnosed as pleomorphic sarcoma. Am J Surg Pathol 1992;16:213–28. [PubMed] [Google Scholar]
- 4.Hirose T, Kudo E, Hasegawa T, et al. Expression of intermediate filaments in malignant fibrous histiocytomas. Hum Pathol 1989;20:871–7. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery E, Fisher C. Myofibroblastic differentiation in malignant fibrous histiocytoma (pleomorphic myofibrosarcoma): a clinicopathological study. Histopathology 2001;38:499–509. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa T, Yamamoto S, Yokoyama R, et al. Prognostic significance of grading and staging systems using MIB-1 score in adult patients with soft tissue sarcoma of the extremities and trunk. Cancer 2002;95:843–51. [DOI] [PubMed] [Google Scholar]
- 7.Weiss SW, Goldblum JR. Leiomyosarcoma. In: Enzinger and Weiss’s soft tissue tumours, 4th ed. St Louis: Mosby, 2001:727–48.
- 8.Watanabe K, Kusakabe T, Hoshi N, et al. h-Caldesmon in leiomyosarcoma and tumours with smooth muscle cell-like differentiation: its specific expression in the smooth muscle cell tumor. Hum Pathol 1999;30:392–6. [DOI] [PubMed] [Google Scholar]
- 9.Ceballos KM, Nielsen GP, Selig MK, et al. Is anti-h-caldesmon useful for distinguishing smooth muscle and myofibroblastic tumours? Am J Clin Pathol 2000;114:746–53. [DOI] [PubMed] [Google Scholar]
- 10.Hisaoka M, Wei-Qi S, Jian W, et al. Specific but variable expression of h-caldesmon in leiomyosarcomas: an immunohistochemical reassessment of a novel myogenic marker. Appl Immunohistochem Mol Morphol 2001;9:302–8. [DOI] [PubMed] [Google Scholar]
- 11.Iwata J, Fletcher CDM. Immunohistochemical detection of cytokeratin and epithelial membrane antigen in leiomyosarcoma: a systematic study of 100 cases. Pathol Int 2000;50:7–14. [DOI] [PubMed] [Google Scholar]
- 12.Brooks JSJ. Immunohistochemistry in the differential diagnosis of soft tissue tumours. In: Weiss SW, Brooks JSJ, eds. Soft tissue tumours. Baltimore: Williams & Wilkins, 1996:65–128. [PubMed]
- 13.Suster S. Recent advances in the application of immunohistochemical markers for the diagnosis of soft tissue tumours. Semin Diagn Pathol 2000;17:225–35. [PubMed] [Google Scholar]
- 14.Sirgi KE, Wick MR, Swanson PE. B72.3 and CD34 immunoreactivity in malignant epithelioid soft tissue tumours: adjuncts in the recognition of endothelial neoplasms. Am J Surg Pathol 1993;17:179–85. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens—evaluation of their specificity and sensitivity in the diagnosis of vascular tumours and comparison with von Willebrand factor. Mod Pathol 1994;7:82–90. [PubMed] [Google Scholar]
- 16.Van de Rijn M, Rouse RV. CD34. A review. Appl Immunohistochem Mol Morphol 1994;2:71–80. [Google Scholar]
- 17.Ueda T, Araki N, Mano M, et al. Frequent expression of smooth muscle markers in malignant fibrous histiocytoma of bone. J Clin Pathol 2002;55:853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erlandson RA, Woodruff JM. Role of electron microscopy in the evaluation of soft tissue neoplasms, with emphasis on spindle cell and pleomorphic tumours. Hum Pathol 1998;29:1372–81. [DOI] [PubMed] [Google Scholar]
- 19.Oda Y, Miyajima K, Kawaguchi K, et al. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol 2001;25:1030–8. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher CDM, Van den Berg E, Molenaar WM. Pleomorphic malignant fibrous histiocytoma/undifferentiated high grade pleomorphic sarcoma. In: Fletcher CDM, Unni KK, Mertens F, eds. World Health Organisation classification of tumours. Pathology and genetics of tumours of soft tissue and bone. Lyon, France: IARC Press, 2002:120–2.
- 21.Derre J, Lagace R, Nicolas A, et al. Leiomyosarcoma and most malignant fibrous histiocytomas share very similar comparative genomic hybridization imbalances: an analysis of a series of 27 leiomyosarcomas. Lab Invest 2001;81:211–15. [DOI] [PubMed] [Google Scholar]