Abstract
Background: Cardiac remodelling after acute myocardial infarction (AMI) is characterised by molecular and cellular mechanisms involving both left and right ventricles, and biventricular failure identifies patients with an extremely unfavourable prognosis.
Aims: To assess whether a link exists between increased myocardial apoptotic rates (AR) at sites of recent infarction and patterns of unfavourable cardiac remodelling, such as biventricular enlargement after left ventricular (LV) infarction.
Methods: Twelve patients with recent AMI involving the LV and not the right ventricle (RV) and with permanent infarct related artery occlusion were selected at necropsy. Gross pathological characteristics, such as LV and RV dilatation, and AR at site of infarction were assessed. Potential false positive results (DNA synthesis and RNA splicing) were excluded from the cell count.
Results: RV enlargement, defined as a tricuspidal ring greater than 120 mm, was found in five cases and was associated with LV dilatation. These patients showed significantly higher AR than the others. When the subjects were divided into three groups according to progressive cardiac remodelling (absence of cardiac dilatation, isolated LV dilatation, and biventricular enlargement), the last group had significantly higher ARs than the other two groups, showing that myocardiocyte apoptosis is increased in more unfavourable forms of cardiac remodelling.
Conclusion: Patients with severely unfavourable cardiac remodelling, such as biventricular enlargement, have extremely high myocardiocyte apoptosis at necropsy, even late after LV myocardial infarction, supporting the role of myocardiocyte loss in determining post-infarction adverse remodelling.
Keywords: apoptosis, heart failure, infarction, ischaemia, remodelling
Acute myocardial infarction (AMI) is associated with early and late compensatory mechanisms in the attempt to optimise ventricular filling and cardiac output. The greater the initial myocardial insult the greater the remodelling response and less favourable the longterm prognosis. In experimental models of AMI in animals, right ventricular (RV) remodelling is associated with left ventricular (LV) remodelling, even if the RV is spared from the initial ischaemic damage.1,2 Hirose et al have shown that RV dilatation and remodelling variably occur in subjects with transmural LV myocardial infarction.3 Biventricular dilatation is more pronounced in large AMI and is often associated with pulmonary congestion. The presence of signs and/or symptoms resulting from RV failure identifies a subgroup of patients with an extremely unfavourable prognosis, and survival is usually less than two years.4,5
“It is still unclear whether a link exists between increased apoptotic rates and extremely unfavourable forms of cardiac remodelling, such as that characterised by biventricular enlargement”
Several molecular and cellular mechanisms, occurring both at the site of infarction and in remote unaffected sites, lead to cardiac enlargement and dysfunction after AMI.6,7 Recently, myocardiocyte loss as a result of apoptosis in early and subacute phases of AMI has been consistently shown to be present in human observational studies and in experimental animal models, and increased myocardial apoptotic rates (AR) are associated with severe and progressive heart failure (HF).8–14 However, it is still unclear whether a link exists between increased AR and extremely unfavourable forms of cardiac remodelling, such as that characterised by biventricular enlargement. To investigate this issue, the extent of post-infarction myocardial apoptosis was assessed at postmortem examination in a cohort of subjects who died within two months of AMI, and who had a variable degree of cardiac remodelling.
METHODS
Twelve white subjects were selected at postmortem examination according to the following inclusion criteria: (1) death occurring 10 days to two months after an AMI involving only the LV and not the RV; (2) no clinical or pathological evidence of reinfarction; (3) persistent infarct related artery occlusion (IRA) at postmortem examination; and (4) absence of conditions likely to affect RV remodelling (such as primary lung disease, pulmonary embolism, pulmonary valvulopathy, and intracardiac shunts). Initial treatment and past and recent medical history were obtained from clinical records. Reinfarction was excluded on the basis of clinical, laboratory, and pathology data. Patients were considered to be suffering from symptomatic HF on the basis of signs, symptoms, and clinical characteristics (according to American College of Cardiology/American Heart Association guidelines for the evaluation and management of HF, stages C or D and New York Heart Association class IV).15 To stratify patients into high and low risk groups, the Norris coronary prognostic index was calculated for each subject,16 computing baseline and clinical data obtained at the time of initial hospitalisation.
Pathology
Gross examination of the hearts was performed to measure cardiac parameters and to define the infarcted area and the IRA. Infarct areas were classified as transmural or non-transmural infarcts and as small or large (involving more than one LV wall) infarcts. Permanent IRA occlusion at time of death was defined as a complete absence of residual lumen at pathological examination as a result of atheroma and/or thrombosis. Cardiac diameters were calculated at the atrioventricular section, and LV free wall thickness was measured at the median third of the unaffected free wall (usually the posterior wall). A transverse LV diameter greater than 90 mm and/or a cardiac diameter to free wall thickness ratio > 9 in the absence of RV enlargement were used to define LV dilatation. RV dilatation was defined as an enlargement of the right ventricle, characterised by a tricuspidal ring circumference greater than 120 mm.
Tissue specimens were obtained at sites of infarction and in remote unaffected LV free wall (usually posterior wall), and they were processed as described previously.13 Briefly, specimens were fixed in 10% paraformaldehyde, terminal deoxynucleotidyl transferase (TdT) mediated dUTP nick end labelling (TUNEL) was performed using the Apoptag kit (Oncor, Gaithersburg, Maryland, USA), according to the supplier’s instructions. For immunohistochemistry, the sections already treated for the TUNEL assay were heated and then incubated with antibodies against muscle actin (mouse monoclonal antihuman actin HHF35; dilution, 1/50; Dako, Carpenteria, California, USA) and activated caspase 3 (anti-cleaved caspase 3 (Asp 175) antibody; dilution, 1/50; Cell Signaling Technology, Piscataway, New Jersey, USA) and visualised by means of the streptavidin–biotin system (Dako), using either 3-amino-9-ethylcarbazide or diaminobenzidine as the final chromogen. Myocardiocytes were defined as apoptotic if colocalisation of markers of DNA fragmentation (TUNEL) and activated caspase 3 were evident, in accordance with the fact that high immunohistochemical expression of caspase 3 is present in myocardiocytes undergoing apoptosis and colocalises with TUNEL positive myocardiocytes. The AR was expressed as the ratio of the number of myocardiocytes showing both TUNEL and activated caspase 3 positivity out of the total number of nucleated cells in each field (magnification, ×250), calculated after counting 100 fields. Muscle actin negative cells, in addition to myocardiocytes showing both TUNEL positivity and specific staining for markers of DNA synthesis (proliferating cell nuclear antigen (PCNA); using mouse monoclonal antihuman PCNA PC10 antibody; Dako; dilution, 1/100) and/or markers of transcription activity (RNA splicing factor SC-35; using mouse monoclonal anti-SC-35; dilution, 1/200 Sigma, Milan, Italy) were not included in the cell count, because they were considered potential false positive results.9,13,17 Suitable negative and positive controls for TUNEL and caspase 3 were performed, as defined elsewhere.13 Briefly, controls for TUNEL were performed as indicated by the supplier (using a normal female rodent mammary gland three to five days after weaning of rat pups for the positive control and sham stainings leaving out active TdT, but including proteinase K digestion, to control for non-specific incorporation of nucleotides or for non-specific binding of the enzyme conjugate). A “stringent approach” (leaving proteinase K digestion out of the reaction) was used as a control to avoid false positive results potentially associated with pretreatment by proteinase K. A human lymph node was used as a control for activated caspase 3 (strong immunoreactivity was evident in the apoptosis prone germinal centre B cells but not in the mantle zone of the lymph node). Moreover, negative controls indicating the non-interference of TUNEL and secondary antibodies were performed by leaving out the primary antibodies (antibodies against actin, caspase 3, PCNA, and SC-35, respectively). Immunohistochemistry assays and AR counts were performed by two pathologists who were unaware of the clinical and macroscopical pathological data (FB and AB).
Statistical analysis
The software SPSS 10.0 for Windows (SPSS, Chicago, Illinois, USA) was used for statistical analysis. Quantitative results are expressed as median (interquartile range). The non-parametric Mann-Whitney U test and the Kruskal-Wallis test for non-paired data were used to compare AR values among different subjects, when comparing two or more than two groups, respectively. The χ2 test was used to compare discrete variables, and Fisher’s exact test was used when one or more cells contained a value lower than 5. Logarithmic transformation was used to perform the post hoc test for linear trend at ANOVA univariate analysis.
RESULTS
Clinical data
Table 1 shows the clinical characteristics of the patients. The median time to death after AMI was 20 days. Seven patients had been given a diagnosis of HF at the time of initial hospitalisation for AMI or subsequently before death. All but one subject had had a transmural infarct, six of which involved the anterior wall and/or interventricular septum. Large infarcts were present in nine of 12 patients. Five patients had large transmural anterior and septal AMI. The median Norris coronary prognostic index calculated at the time of admission was 10 (interquartile range, 4–13), indicating a high risk clinical profile in this population.
Table 1.
Clinical and demographic characteristics of the patients
| RV dilatation | No RV dilatation | |
| Number of cases | 5 | 7 |
| Median age in years (interquartile range) | 78 (76–80) | 71* (63–74) |
| Sex (males) | 4 | 4 |
| Clinical features | ||
| Transmural AMI | 4 | 7 |
| Septal AMI | 2 | 5 |
| Anterior AMI | 2 | 4 |
| Large AMI | 3 | 6 |
| Multivessel coronary disease | 3 | 4 |
| Signs and symptoms of HF | 5 | 2* |
| Previous additional AMI | 3 | 4 |
| LV dilatation | 3 | 4 |
| Cardiac weight in grams (interquartile range) | 530 (460–625) | 490 (467–545) |
| Median Norris index (interquartile range) | 12 (10–13) | 6 (4–13) |
| Median time from AMI to death in days (interquartile range) | 25 (18–44) | 16 (14–23) |
*p<0.05.
AMI, acute myocardial infarction; HF, heart failure; LV, left ventricular; RV, right ventricular.
Cardiac remodelling
Table 1 summarises the gross anatomical characteristics of the hearts. Nine patients had LV dilatation, which was isolated in four patients but combined with RV dilatation in five (fig 1). No patients had isolated RV dilatation. Subjects with RV dilatation had similar clinical characteristics to the other patients (table 1), although they were significantly older (78 v 71 years old; p = 0.030) and more frequently had had symptoms or signs of HF (five of five v two of seven; p = 0.027). Interestingly, although all patients with biventricular remodelling had had symptoms and/or signs of HF, only half of the subjects with isolated LV dilatation and none of those with more favourable cardiac remodelling and undilated hearts had had a diagnosis of HF (p = 0.019). Moreover, the Norris index was significantly different between the three groups (4 v 9 v 12; p = 0.040).
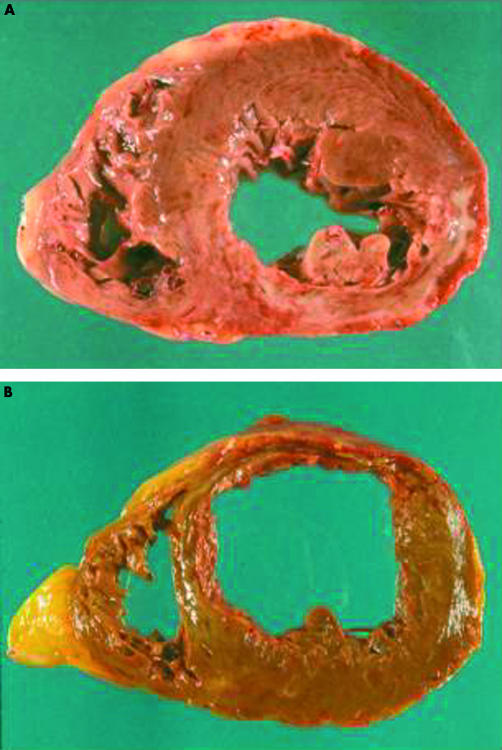
Figure 1.
Left ventricular and biventricular enlargement. (A) A case of recent posterior wall acute myocardial infarction (day 17) complicated by mild left ventricular dilatation. (B) A case of severe biventricular remodelling following anterior wall acute myocardial infarction (day 15).
Myocardial apoptosis in RV dilatation
Gross pathological and standard microscopic examination showed that scar tissue consistent with previous necrotic cell death was evident in all patients, in the absence of signs of very recent or acute ongoing necrosis. Indeed, the presence of ongoing necrosis was an exclusion criterion because it may be a confounding factor when assessing the role of apoptosis. TUNEL and immunostaining for activated caspase 3 colocalised in more than 90% of cells. Only double positive myocardiocytes were considered apoptotic. A median of 21% of myocardiocytes were apoptotic at sites of recent infarction and 0.7% in remote regions of the LV. Intense immunostaining for activated caspase 3 was evident at sites of infarction (including some TUNEL negative cells) but not in remote regions, where few myocardiocytes were considered positive. A comparison of immunostaining for both non-activated (rabbit polyclonal antihuman caspase 3; dilution, 1/10011,12; Upstate Biotechnology, Lake Placid, New York, USA) and activated caspase 3 showed almost identical results, supporting the hypothesis that caspase 3 is both overexpressed and subsequently activated at sites of infarction (fig 2).
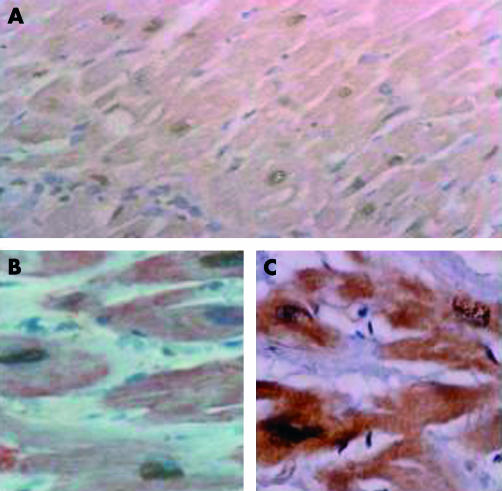
Figure 2.
Demonstration of apoptosis. (A) Labelling of nuclear DNA fragmentation using terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL). Colocalisation of (B) TUNEL and actin and of (C) TUNEL and activated caspase 3.
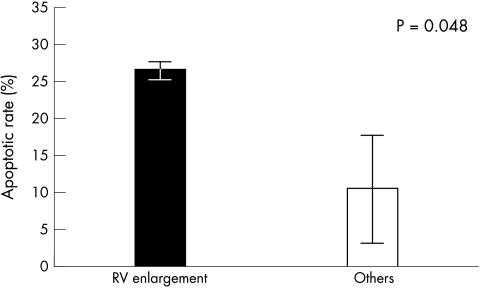
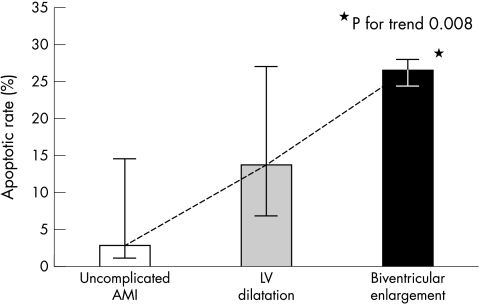
RV dilatation (five cases) was associated with significantly higher AR at the site of infarction (median, 26% (interquartile range, 24–27%) v 10% (3–18%); p = 0.048; fig 3). When subjects were divided into three groups (non-dilated hearts, isolated LV dilatation, and combined LV and RV dilatation), there was a significant difference in AR among the three groups (3% v 14% v 26%, respectively; p = 0.050; figs 4, 5). Logarithmic transformation was also performed in case of potential deviations from normality and linearity. This confirmed the association between the three different groups and increasing AR in ANOVA univariate analysis (p = 0.023), showing a p value for trend of 0.008 in the post hoc test for linear trend (fig 5). No significant differences were found when comparing AR values at remote LV sites between the three different groups.
Figure 3.
Myocardial apoptosis and right ventricular (RV) dilatation. Subjects with RV remodelling after left ventricular acute myocardial infarction have a significantly higher apoptotic rate at the site of recent infarction compared with the other groups. The columns represent the median value and the vertical bars show the interquartile range.
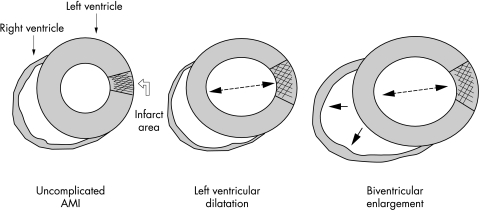
Figure 4.
Progressive post-infarction cardiac remodelling. Cardiac remodelling after acute myocardial infarction (AMI) involving the left ventricle may determine progressive left ventricular dilatation and subsequent right ventricular enlargement. Biventricular remodelling identifies a group of patients with an extremely unfavourable prognosis.
Figure 5.
Increase in the apoptotic rate in progressive post-infarction cardiac remodelling. When patients are divided into three groups according to increasing severity of cardiac remodelling (uncomplicated acute myocardial infarction (AMI) with undilated hearts, isolated left ventricular (LV) dilatation, and combined left ventricular and right ventricular enlargement), a significant difference in apoptotic rates between the three groups is evident (p for trend, 0.008). The box represents the median value and the vertical bar shows the interquartile range.
DISCUSSION
Post-infarction cardiac remodelling leading to HF represents a “dangerous intersection” in AMI patients,18 and biventricular failure is considered to be the terminal stage of cardiac remodelling4,5 Our study shows for the first time that this high risk condition is associated with extremely high peri-infarctual AR at postmortem examination, confirming and greatly expanding previous findings of a correlation between myocardiocyte loss caused by apoptosis and unfavourable LV remodelling.8–12 Although speculative, it is possible that the extremely unfavourable prognosis associated with biventricular remodelling (supported in our data by the raised Norris index and the presence of signs of HF in all patients) might be the result not only of greater infarct sizes but also of increased myocardial apoptosis. Interestingly, an association between high risk clinical features with a relevant prognostic value, as computed in the Norris index, and increased AR has been reported recently.19
Right sided HF is mainly characterised by a low cardiac output state and systemic congestion. Neurohormonal derangements (such as adrenergic hyperactivation and activation of the renin–angiotensin system) are particularly evident in this clinical feature, and may not only represent an epiphenomenon but may also have a direct pathophysiological role. Sabbah et al have shown that multiple myocardial infarctions in dogs lead to biventricular remodelling and increased mortality rates.20 The same authors have subsequently reported significantly increased AR values in the hearts of these animals (mainly in peri-infarct regions), and the potential modulation by angiotensin converting enzyme inhibitors and by β blockers.21–23
“Our results support the role of progressive myocardiocyte loss in determining unfavourable remodelling”
However, it is still unclear exactly how AMI of the LV affects remodelling of the RV. Pulmonary hypertension and secondary increases of the RV afterload are thought to be the main pathophysiological mechanisms, although other hypotheses should also be considered. Abnormal interventricular septum contractility, concomitant RV ischaemia, and afterload increase resulting from ischaemic mitral insufficiency may be responsible for additional RV strain. Interestingly, in our analysis, which was limited by the small sample size, no significant association was found between RV dilatation and the site of AMI or multivessel coronary artery disease. Taken together, our results identify the major determinant for the development of biventricular failure as the extent of myocardiocyte loss at the site of infarction. Among established and potential modulators of myocardial apoptosis, age, sex, ischaemia, infarct related artery occlusion, HF, and cardiac dilatation have all been shown to be predictors of increased apoptosis.12,19,24–26 Both increased wall stress (as a result of LV failure) and the enhanced production of angiotensin II and of catecholamines (as a result of HF) may further stimulate apoptosis in the ischaemic peri-infarctual myocardium, and may also cause increased apoptosis in areas of the myocardium remote from the infarction site, both in the LV and RV.8,9,12,27,28
In conclusion, patients showing severely unfavourable forms of cardiac remodelling, such as biventricular enlargement, have extremely high myocardiocyte apoptosis even at a late time after LV myocardial infarction. Our results support the role of progressive myocardiocyte loss in determining unfavourable remodelling. However, as with all observational studies, our study may be fraught with potential limitations, such as unrevealed selection biases.29 Moreover, the incomplete definition of the temporal and spatial burden of increased apoptosis is a limitation when interpreting these results. The extremely high AR (up to 25%) at the site of infarction may seem difficult to interpret. However, the following should be kept in mind: (1) this high rate was confined to the surviving myocardium at the infarct zone; (2) selection criteria included only cases with extremely unfavourable prognosis, enrolled at postmortem examination; (3) this persistent myocardiocyte loss may be limited in time following AMI; (4) all cases had persistent infarct related artery occlusion, which is known to be associated with increased AR25; and (5) the data presented agree with previous published studies enrolling individuals with similar clinical characteristics.12,13,30–32 It is possible that jeopardised myocardium (where the apoptotic cascade is already partially activated,33 but kept within the boundary of reversibility) may be detrimentally affected by perimortem events, leading to significantly higher rates of cell death at postmortem examination.[AQ:5]
Take home messages.
Patients with severely unfavourable cardiac remodelling, such as enlargement of both the right and left ventricles, have extremely high myocardiocyte apoptosis at necropsy, even late after left ventricular myocardial infarction
These results support the role of myocardiocyte loss in determining post-infarction adverse remodelling
Acknowledgments
Thanks to Dr V Di Trocchio (RomaTre University of Rome, Italy) for her writing, editorial, and graphical support. This study was supported by a MIUR Second University of Naples grant and by FUTURA Inc (Drs F Baldi and A Baldi).
Abbreviations
AMI, acute myocardial infarction
AR, apoptotic rates
HF, heart failure
IRA, infarct related artery occlusion
LV, left ventricular
PCNA, proliferating cell nuclear antigen
RV, right ventricular
TdT, terminal deoxynucleotidyl transferase
TUNEL, terminal deoxynucleotidyl transferase mediated dUTP nick end labelling
Footnotes
The first two authors contributed equally to this work.
REFERENCES
- 1.Patten RD, Aronovitz MJ, Deras-Meja L, et al. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol 1998;274:H1812–20. [DOI] [PubMed] [Google Scholar]
- 2.Brower GL, Janicki JS. Contribution of ventricular remodeling to pathogenesis of heart failure in rats. Am J Physiol 2001;280:H674–83. [DOI] [PubMed] [Google Scholar]
- 3.Hirose K, Shu NH, Reed JE, et al. Right ventricular dilatation and remodeling the first year after an initial transmural wall left ventricular myocardial infarction. Am J Cardiol 1993;72:1126–30. [DOI] [PubMed] [Google Scholar]
- 4.Oakley C. Importance of right ventricular function in congestive heart failure. Am J Cardiol 1988;62:14A–19A. [DOI] [PubMed] [Google Scholar]
- 5.Di Salvo TG, Mathier M, Semigran MJ, et al. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol 1995;25:1143–53. [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction—experimental observations and clinical implications. Circulation 1990;81:1161–72. [DOI] [PubMed] [Google Scholar]
- 7.Anversa P, Olivetti G, Capasso JM. Cellular basis of ventricular remodeling after myocardial infarction. Am J Cardiol 1991;68:7D–16D. [DOI] [PubMed] [Google Scholar]
- 8.Krijnen PAJ, Nijmeijer R, Meijer CJLM, et al. Apoptosis in myocardial ischaemia and infarction. J Clin Pathol 2002;55:801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbate A, Biondi-Zoccai GGL, Baldi A. Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol 2002;193:145–53. [DOI] [PubMed] [Google Scholar]
- 10.Saraste A, Pulkki K, Kallajoki M, et al. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest 1999;29:380–6. [DOI] [PubMed] [Google Scholar]
- 11.Metzger M, Higuchi LM, Moreira LF, et al. Relevance of apoptosis and cell proliferation for survival of patients with dilated cardiomyopathy undergoing partial left ventriculectomy. Eur J Clin Invest 2002;32:394–9. [DOI] [PubMed] [Google Scholar]
- 12.Abbate A, Biondi-Zoccai GGL, Bussani R, et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol 2003;41:753–60. [DOI] [PubMed] [Google Scholar]
- 13.Baldi A, Abbate A, Bussani R, et al. Apoptosis and post-infarction left ventricular remodeling. J Mol Cell Cardiol 2002;34:165–74. [DOI] [PubMed] [Google Scholar]
- 14.Rayment NB, Haven AJ, Madden B, et al. Myocyte loss in chronic heart failure. J Pathol 1999;188:213–19. [DOI] [PubMed] [Google Scholar]
- 15.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2001;38:2101–13. [DOI] [PubMed] [Google Scholar]
- 16.Norris RM, Caughey DE, Deeming LW, et al. Coronary prognostic index for predicting survival after recovery from acute myocardial infarction. Lancet 1970;2:485–7. [DOI] [PubMed] [Google Scholar]
- 17.Alison MR. Identifying and quantifying apoptosis; a growth industry in the face of death. J Pathol 1999;188:117–18. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer MA. Myocardial infarction and heart failure—a dangerous intersection. Am J Med 2002;113:341–3. [DOI] [PubMed] [Google Scholar]
- 19.Abbate A, Biondi-Zoccai GGL, Bussani R, et al. High-risk clinical features predict enhanced post-infarction myocardial apoptosis and the need to achieve infarct-related artery patency [abstract]. J Am Coll Cardiol 2003;41:544A. [DOI] [PubMed] [Google Scholar]
- 20.Sabbah HN, Stein PD, Kono T, et al. A canine model of chronic heart failure produced by multiple sequential coronary microembolization. Am J Physiol 1991;260:H1379–84. [DOI] [PubMed] [Google Scholar]
- 21.Sharov VG, Sabbah HN, Shimoyama H, et al. Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. Am J Pathol 1996;148:141–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Goussev A, Sharov VG, Shimoyama H, et al. Effects of ACE inhibition on cardiomyocyte apoptosis in dogs with heart failure. Am J Physiol 1998;275:H626–31. [DOI] [PubMed] [Google Scholar]
- 23.Sabbah HN, Sharov VG, Gupta RC, et al. Chronic therapy with metoprolol attenuates cardiomyocyte apoptosis in dogs with heart failure. J Am Coll Cardiol 2000;36:1698–705. [DOI] [PubMed] [Google Scholar]
- 24.Biondi-Zoccai GGL, Abbate A, Bussani R, et al. Reduced post-infarction myocardial apoptosis in women: a clue to their different clinical course [abstract]? J Am Coll Cardiol 2003;41:381A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbate A, Bussani R, Biondi-Zoccai GL, et al. Persistent infarct-related artery occlusion is associated with an increased myocardial apoptosis at postmortem examination in humans late after an acute myocardial infarction. Circulation 2002;106:1051–4. [DOI] [PubMed] [Google Scholar]
- 26.Bialik S, Geenen DL, Sasson IE, et al. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J Clin Invest 1997;100:1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ecarnot-Laubriet A, Assem M, Poirson-Bichat F, et al. Stage-dependent activation of cell cycle and apoptosis mechanisms in the right ventricle by pressure overload. Biochim Biophys Acta 2002;1586:233–42. [DOI] [PubMed] [Google Scholar]
- 28.Sam F, Sawyer DB, Chang DLF, et al. Progressive left ventricular remodeling and apoptosis late after myocardial infarction in mouse heart. Am J Physiol 2000;279:H422–8. [DOI] [PubMed] [Google Scholar]
- 29.Abbate A, Biondi-Zoccai GGL, Baldi A. Myocardiocyte loss due to apoptosis. Eur Heart J 2002;23:1889–90. [DOI] [PubMed] [Google Scholar]
- 30.Olivetti G, Quaini F, Sala R, et al. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol 1996;28:2005–16. [DOI] [PubMed] [Google Scholar]
- 31.Matturri L, Milei J, Grana DR, et al. Characterization of myocardial hypertrophy by DNA content, PCNA expression and apoptotic index. Int J Cardiol 2002;82:33–9. [DOI] [PubMed] [Google Scholar]
- 32.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430–6. [DOI] [PubMed] [Google Scholar]
- 33.Scheubel RJ, Bartling B, Simm A, et al. Apoptotic pathway activation from mitochondria and death receptors without caspase-3 cleavage in failing human myocardium: fragile balance of myocyte survival? J Am Coll Cardiol 2002;39:481–8. [DOI] [PubMed] [Google Scholar]