Abstract
Background: Gene expression profiling of diffuse large B cell lymphoma (DLBCL) revealed three disease types: germinal centre B cell-like (GC), activated B cell-like (ABC), and a “third” type. Expression of CD44 variant isoforms (CD44v) is associated with an unfavourable clinical outcome in DLBCL, but previous studies did not consider the clinicopathological heterogeneity of this disease.
Aims: To analyse the expression and prognostic significance of CD44 in DLBCL types.
Methods: A tissue microarray (TMA) comprising 90 DLBCLs was constructed. CD10, CD20, bcl-2, bcl-6, CD44 standard isoform (CD44s), and CD44v4, CD44v6, and CD44v9 were analysed immunohistochemically and correlated with clinical follow up.
Results: TMA expression of CD10, CD20, bcl-2, and bcl-6 showed 100% concordance with results from conventional sections in 60 cases. Samples were segregated into 22 GC (bcl-6+/CD10+/bcl-2−), 25 ABC (bcl-6−/CD10−/bcl-2+), and 35 unclassifiable DLBCLs. Overall survival (OS) at 30 months was 89%, 44%, and 58% in GC, ABC, and unclassified types, respectively. CD44v6 was coexpressed with bcl-2, appeared predominantly on bcl-6 negative cases, and correlated with disease stage. Cases negative for CD44s could be separated into CD44v6 negative (OS, 82% at 70 months) and CD44v6 positive (OS, 58%).
Conclusions: TMA technology is useful for immunophenotyping and clinicopathological analysis of large lymphoma populations. The GC phenotype of DLBCL is of independent prognostic significance for OS. Expression of CD44v6 correlates with disease stage, and might contribute to lymphoma dissemination. CD44v6 is expressed predominantly in ABC DLBCL, and in CD44 negative cases is associated with worse OS.
Keywords: diffuse large B cell lymphoma, CD44, prognosis, tissue microarray
Diffuse large B cell lymphoma (DLBCL) is the most common lymphoid malignancy, comprising 35–40% of adult non-Hodgkin lymphomas (NHLs).1 Although listed as a specific entity in the World Health Organisation classification,1 it represents a heterogeneous disease—morphologically, phenotypically, genetically, and clinically. DLBCL either develops de novo, or occurs as a consequence of low malignant B cell diseases.1,2 Recent gene expression profiling of DLBCL using cDNA and oligonucleotide microarrays revealed that this single entity can be divided into three categories: DLBCL of germinal centre B cell-like type (GC), DLBCL of activated B cell-like type (ABC), or a “third” type.3–6 Among the differentially expressed genes within the ABC and GC subgroups, CD44 is of particular interest.6 CD44 standard isoform (CD44s) and its variants (CD44v) represent cell surface glycoproteins, which are generated by the alternative splicing of CD44 mRNA. These molecules play a key role in lymphocyte migration, homing, and activation and participate in the transmission of signals that regulate haemopoiesis and apoptosis. They are either constitutively expressed on the surface of peripheral lymphocytes (CD44s) or are induced upon antigen mediated activation (CD44v3 and CD44v6) (reviewed by Sneath and Mangham7). CD44v are necessary for tumour spread and metastasis, and their expression is generally associated with an unfavourable prognosis7–16 in DLBCL and in several other haematological malignancies, such as multiple myeloma, NHL, Hodgkin lymphoma, and acute myelogenous leukaemia.13–24
“CD44 isoforms play a key role in lymphocyte migration, homing, and activation and participate in the transmission of signals that regulate haemopoiesis and apoptosis”
With respect to CD44 expression in DLBCL, previous studies largely failed to take into account the clinicopathological heterogeneity of this disease and distinguished cases based on the CD44 expression profile alone. Therefore, we analysed the expression and prognostic significance of CD44 and its variant isoforms CD44v4, CD44v6, and CD44v9 on the background of the recently identified ABC and GC subgroups of DLBCL using a lymphoma tissue microarray (TMA) comprising 90 immunophenotypically profiled DLBCLs with complete clinical follow up. TMA technology allows simultaneous morphological and immunohistochemical analysis of up to 1000 different specimens on a single slide under identical processing conditions.25,26 Recently, we and others demonstrated that it is also a reliable and highly effective method for in situ lymphoma studies.2,27–31
MATERIALS AND METHODS
Patients
Ninety formalin fixed, paraffin wax embedded DLBCL tissue samples from the archives of the institute of pathology at the University of Bologna (Italy) were included in our study. They consisted of 82 previously untreated, newly diagnosed de novo DLBCLs, six cases of transformed follicular lymphoma (FL), and two cases of transformed small lymphocytic lymphoma (SLL). Follow up data of 71 patients (36 women and 35 men) aged between 29 and 90 years (mean, 62.1) were available. According to the Ann-Arbor classification, 12 cases were at clinical stage I, 15 at stage II, 15 at stage III, and 21 at stage IV. Twenty one patients had an international prognostic index (IPI) of 0, 14 had an IPI of 1, 13 had an IPI of 2, nine had an IPI of 3, three had an IPI of 4, and one had an IPI of 5. Disease remission was defined as absence of disease for at least one month after cessation of the last treatment regimen, as assessed by laboratory and imaging studies and physical examination. Disease relapses were defined as disease recurring at least one month after disease remission. Freedom from treatment failure was defined as lack of relapses and treatment resistance. Twelve relapses occurred. Twenty three patients died as a result of treatment failure and two because of cardiovascular disease. Within the follow up period of one to 130 months (mean, 27), cumulative overall survival (OS) was 49.5%.
Tissue microarray construction
For TMA construction, a haematoxylin and eosin (H&E) stained slide was made from each block and reviewed by at least two expert haematopathologists. Representative tumour regions were morphologically identified and marked on the H&E stained slides. Tissue cylinders with a diameter of 0.6 mm were punched from the marked areas of each block and incorporated into a recipient paraffin block using a precision instrument (Beecher Instruments, Silver Spring, Maryland, USA), as described previously.25,26 To overcome the problem of tissue microheterogeneity and to increase the number of evaluable cases, each donor tissue block was punched three times for the construction of the recipient block, so that each block contained 270 tissue cores. Sections (4 μm thick) of these TMA blocks were transferred to an adhesive coated glass slide system (Instrumedics Inc, Hackensack, New Jersey, USA).
Immunohistochemistry
Table 1 lists the primary antibodies against CD10, CD20, bcl-2, bcl-6, CD44s, CD44v4, CD44v6, and CD44v9, their dilutions, and the pretreatment conditions. Bound secondary antibodies were visualised by standard avidin–biotin–peroxidase techniques using diaminobenzidine as chromogen. Because weak staining for CD44s was consistently present in up to 20% of the small reactive background lymphocytes, similar staining patterns in lymphomas were not considered to be specific tumour associated CD44s expression; membranous staining in 20–70% of the malignant cells was considered positive, and in over 70% as strongly positive. CD44v staining in 10–20% of the malignant cells was considered weakly positive, in 20–70% as positive, and in over 70% as strongly positive.16 Positive cases were analysed together by the Spearman rank test and the Kaplan-Meier method. Normal tonsils were used as positive controls, and for negative controls the primary antibodies were omitted.
Table 1.
Antibodies and antigen retrieval techniques used
| Antibody/antigen | Dilution | Retrieval | Source |
| CD10 | 1/10 | Pressure cooker, 121°C, 5 minutes | Dako, Glostrup, Denmark |
| CD20 | 1/700 | Microwave oven, 800 W, 10 minutes | Dako |
| bcl-2 | 1/50 | Pressure cooker, 121°C, 5 minutes | Dako |
| bcl-6 | 1/40 | Pressure cooker, 121°C, 5 minutes | Dako |
| CD44s | 1/10 | Microwave oven, 800 W, 60 minutes | * |
| CD44v4 | 1/10 | Microwave oven, 800 W, 60 minutes | * |
| CD44v6 | 1/10 | Microwave oven, 800 W, 60 minutes | * |
| CD44v9 | 1/10 | Microwave oven, 800 W, 60 minutes | * |
*Monoclonal antibodies produced and kindly given by Dr U Günthert.
CD44s, CD44 standard isoform; CD44v, CD44 variant isoform.
Statistical analysis
Statistical analysis was performed using the statistical package of social sciences (SPSS). Failure free survival (FFS) and OS were analysed by the Kaplan-Meier method and compared by the log rank test. The Spearman rank test and Fisher’s exact test were applied to demonstrate correlations. Multivariate analysis for the prognostic effect of the expression of CD44 and its variant isoforms (CD44v4, CD44v6, and CD44v9), age, sex, Ann-Arbor stage, IPI, bcl-2, bcl-6, and CD10 was performed using a general linear model. p Values below 0.05 were considered significant.
RESULTS
Histopathology and immunohistochemistry
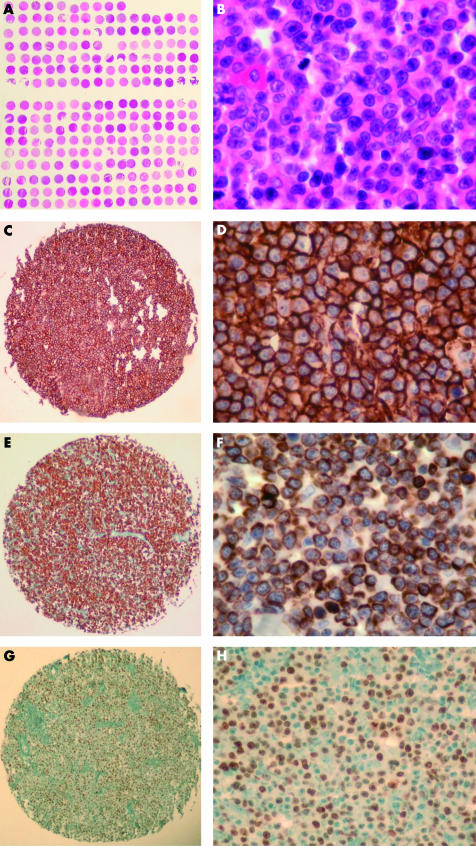
All 90 cases tested were representative by H&E morphology. There was no tissue at 21 positions of the TMA, and at two positions no tumour was seen. However, each sample was punched three times; therefore, at least one evaluable core was present in almost all cases. All examined samples showed strong expression of CD20; 26 of the 90 cases were also positive for CD10, 52 of 89 for bcl-2, and 38 of 90 for bcl-6 (table 2; fig 1).
Table 2.
Tissue microarray immunoprofiling of de novo and secondary diffuse large B cell lymphomas (DLBCLs)
| Lymphoma subtype | CD10 | CD20 | bcl-2 | bcl-6 |
| De novo DLBCL | 22/82 | 78/78 | 45/81 | 35/82 |
| Transformed FL | 4/6 | 6/6 | 5/6 | 3/6 |
| Transformed SLL | 0/2 | 2/2 | 2/2 | 0/2 |
FL, follicular lymphoma; SLL, small lymphocytic lymphoma.
Figure 1.
Diffuse large B cell lymphoma (DLBCL) tissue microarray (TMA). (A) Overview of the lymphoma TMA. Haematoxylin and eosin (H&E) stain; original magnification, ×2. (B) DLBCL TMA, high magnification. H&E stain; original magnification, ×400. (C) CD20 expression in a tissue core of DLBCL. Immunoperoxidase stain; original magnification, ×20. (D) CD20 expression in a tissue core of DLBCL. Immunoperoxidase stain; original magnification, ×400. (E) Bcl-2 expression in a tissue core of DLBCL. Immunoperoxidase stain; original magnification, ×20. (F) Bcl-2 expression in a tissue core of DLBCL. Immunoperoxidase stain; original magnification, ×400. (G) Bcl-6 expression in a tissue core of DLBCL. Immunoperoxidase stain; original magnification, ×20. (H) Bcl-2 expression in a tissue core of DLBCL. Immunoperoxidase stain; original magnification, ×200. Note the excellent representativity of all TMA cores shown
To validate the TMA approach for our study, we compared the expression of CD10, CD20, bcl-2, and bcl-6 with the results obtained on conventional full tissue sections of 60 cases and found a concordance of 100% for each marker. According to the immunohistochemically detectable expression of bcl-2, bcl-6, and CD10, the analysed DLBCLs were tentatively subclassified into GC type DLBCL (bcl-6+/CD10+/bcl-2−; 22 cases) and ABC type DLBCL (bcl-6−/CD10−/bcl-2+; 25 cases), in addition to unclassifiable cases (all other expression profiles; 35 cases).3–6,32
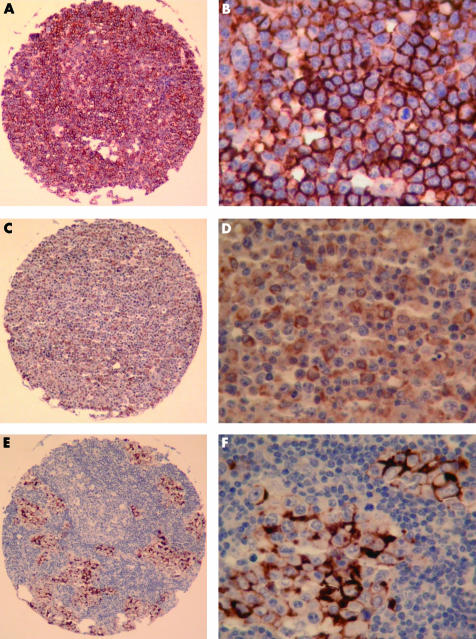
Because of factors related to the TMA technology,27 nine (CD44s), seven (CD44v4, CD44v6), and three (CD44v9) cases of de novo DLBCL contained no tissue on the slides and, therefore, could not be evaluated. Table 3 summarises the expression profile of CD44s and CD44v in our study group. The staining pattern was intense and confined to the cell membrane, with a characteristic submembranous rim (fig 2). Reactive background cells expressed none of the examined CD44v isoforms. Only two de novo DLBCLs expressed CD44v4.
Table 3.
Tissue microarray analysis of the expression of CD44 standard isoform (CD44s) and its variant isoforms CD44v4, CD44v6, and CD44v9 in diffuse large B cell lymphoma (DLBCL)
| CD44s | CD44v6 | CD44v9 | |||||
| Lymphoma subtype | 10–20% | 20–70% | >70% | 10–20% | 20–70% | >70% | >70% |
| All de novo DLBCLs | 12/73 | 19/73 | 28/73 | 14/75 | 5/75 | 4/75 | 15/79 |
| DLBCL ABC type | 8/23 | 5/23 | 10/23 | 5/22 | 2/22 | 2/22 | 4/22 |
| DLBCL GC type | 4/22 | 8/22 | 7/22 | 2/22 | 2/22 | 0/22 | 7/22 |
| Transformed FL | 0/6 | 1/6 | 3/6 | 0/6 | 0/6 | 0/6 | 0/6 |
| Transformed SLL | 1/2 | 1/2 | 0/2 | 0/2 | 1/2 | 0/2 | 1/2 |
Positive cases out of evaluable cases are shown.
ABC, activated B cell-like; FL, follicular lymphoma; GC, germinal centre B cell-like; SLL, small lymphocytic lymphoma.
Figure 2.
Tissue microarray (TMA) analysis of CD44 expression in diffuse large B cell lymphoma (DLBCL). (A) CD44 standard isoform (CD44s) expression in DLBCL. Immunoperoxidase stain; original magnification, ×20. (B) CD44s expression in DLBCL. Immunoperoxidase stain; original magnification, ×400. (C) CD44 variant isoform 6 (CD44v6) expression in DLBCL. Immunoperoxidase stain; original magnification, ×20. (D) CD44v6 expression in DLBCL. Note the weaker staining intensity compared with CD44s; the background lymphocytes remain unstained. Immunoperoxidase stain; original magnification, ×400. (E) CD44v9 expression in a focal infiltrate of a DLBCL. Even small lymphoma foci can be identified on a TMA core. Immunoperoxidase stain; original magnification, ×20. (F) CD44v9 expression in a focal infiltrate of a DLBCL. Immunoperoxidase stain; original magnification, ×400.
Statistics
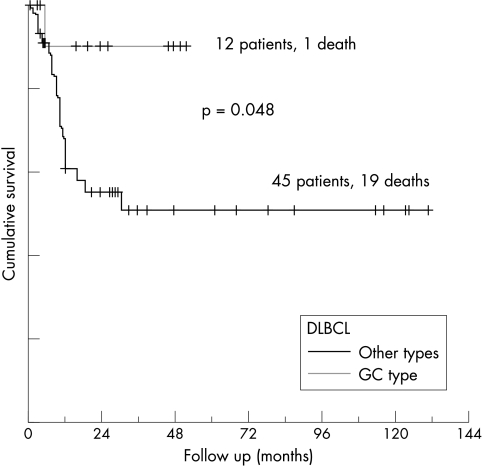
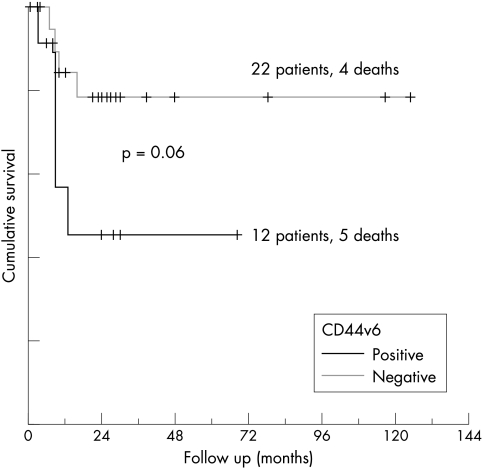
In primary DLBCL, univariate analysis showed that CD10 and bcl-6 were coexpressed (22 of 35 bcl-6 positive cases also expressed CD10; p = 0.005), whereas there was an inverse correlation between the distribution of bcl-2 and bcl-6 (13 of 45 bcl-2 positive cases expressed bcl-6; p < 0.05). CD44v6 was colocalised with bcl-2 (p < 0.05), and appeared predominantly on bcl-6 negative cases (p = 0.05). Applying Fisher’s exact test, OS correlated positively with the expression of CD10, bcl-6, and CD44s (p < 0.05), and inversely with IPI, disease stage, and relapse rate (p < 0.001). FFS correlated positively with the expression of CD44s and inversely with IPI, disease stage, and relapse rate. The GC type of DLBCL presented with a mean IPI score of 0.6, tumours of the ABC type with 1, and the unclassified group with 1.88 (p = 0.001). OS in the CD10 positive group at 60 months after diagnosis was 80%, compared with 48% in the CD10 negative group, 48% in bcl-2 expressing cases, 70% in bcl-2 negative cases, 72% in bcl-6 positive cases, and 46% in bcl-6 negative cases, although these differences were not significant in the log rank test. The OS of patients with GC type DLBCL at 30 months after diagnosis was 89%, compared with 58% in the unclassified group, and 44% in the ABC type (fig 3). Multivariate analysis revealed disease stage to be an independent prognostic factor for OS and FFS, as was CD10 and bcl-6 coexpression for OS (p < 0.05). OS and FFS correlated with CD44s expression (14 treatment failures in 19 CD44s negative cases compared with 12 in 34 CD44s positive cases; p < 0.005). Disease stage and IPI correlated inversely with CD44s expression, whereas disease stage showed a linear correlation with CD44v6 expression (p < 0.05). CD44v6 expression separated cases negative for CD44s into two prognostically different groups: the CD44v6 negative group had an OS of 82% versus 58% in the CD44v6 positive group at 70 months after diagnosis (p = 0.06) (fig 4). CD44v9 expression had no prognostic value in this study group.
Figure 3.
Overall survival in diffuse large B cell lymphoma (DLBCL) correlates with the coexpression of bcl-6 and CD10 (germinal centre (GC) B cell phenotype).
Figure 4.
Overall survival in CD44 standard isoform negative diffuse large B cell lymphomas with respect to expression of CD44 variant isoform 6 (CD44v6).
DISCUSSION
We report the construction and validation of a DLBCL TMA containing 270 cores of 90 different samples. This lymphoma array was used to analyse the expression and prognostic significance of CD44. Application of the TMA technology enabled us to explore CD44 protein expression in a higher number of tumour specimens than was possible in previous studies.13,21 To assess the accuracy of the TMA technology for immunohistochemical analysis, we directly compared the expression of CD10, CD20, bcl-2, and bcl-6 with the results obtained on the corresponding conventional full sections of 60 cases, and found a concordance of 100% for each marker if three cores/case were used. The absence of a single discordant case, similar to a recent TMA study on Hodgkin lymphoma,27,31 demonstrates that the TMA methodology is very useful for clinicopathological investigations of large lymphoma series. A similarly high degree of correlation (86–100%) for nine commonly used lymphoma markers was recently reported in a lymphoma TMA validation study using two cores/case.30
The immunophenotypical profile of the tumours showed a clear segregation of the de novo DLBCLs into a bcl-2−/bcl-6+/CD10+ (GC type; 22 cases) and a bcl-2+/bcl-6−/CD10− group (ABC type; 25 cases), in addition to a third group showing coexpression or lack of expression of these three markers (35 cases). Coexpression of CD10 and bcl-6 was, along with disease stage, of independent prognostic significance for OS. The OS of patients with GC type DLBCL at 30 months after diagnosis was 89%, compared with 58% in the unclassified cases and 44% in ABC type DLBCL. Our results confirm and extend recent observations using cDNA and oligonucleotide microarrays,2–6 indicating that de novo DLBCLs consist of at least three histogenetically, phenotypically, and prognostically different entities. Thus, bcl-2, bcl-6, and CD10 might be useful as surrogate markers for the complex gene expression pattern of the molecularly defined DLBCL subtypes.32
“Disease stage correlated inversely with CD44 standard isoform expression and showed a linear correlation with CD44 variant isoform 6 expression”
We observed a complex expression pattern of CD44s and its variant isoforms CD44v4, CD44v6, and CD44v9 in DLBCL. Whereas de novo DLBCLs expressed CD44s and all variants, transformed FLs, similar to their normal counterparts,33 expressed only CD44s and no variant isoforms. Of particular interest was CD44v expression in the tentative GC and ABC subgroups of de novo DLBCL. Univariate analysis showed that CD44v6 was predominantly expressed in bcl-2 positive cases (major marker of activated B cells), but not in bcl-6 positive tumours (major marker of germinal centre B cells). This distribution of CD44v6 corresponds to the gene expression profile of ABC type DLBCL.3,6 Importantly, CD44v6 expression is considered to be a signature of activated B cells because it is upregulated in peripheral blood B cells upon activation.6,7,10,33
Disease stage correlated inversely with CD44s expression and showed a linear correlation with CD44v6 expression. These results support the concept that the expression of CD44v6 might contribute to lymphoma dissemination.8,12 In our study group, CD44v6 expression per se was not prognostically significant, but in CD44s positive cases, it played an important albeit minor role (p = 0.06), as suggested in many previous studies.13–15,18,21 In CD44s negative cases, CD44v6 positive lymphomas had a worse OS. Furthermore, CD44v6 was expressed predominantly in advanced stage disease and in DLBCLs of the ABC type. The expression of CD44v6 in CD44s positive and CD44s weakly positive cases had no prognostic impact, perhaps because of the accumulation of unclassifiable DLBCL cases in this group. This could also explain the better clinical outcome observed in the CD44s positive group. Whether these prognostic differences result from direct CD44v6 mediated effects, such as lymphoma spread and increased resistance to chemotherapy induced apoptosis, or CD44v6 expression is only a bystander signature of the different genetically defined DLBCLs, remains to be determined. Nonetheless, searching for additional immunohistochemical markers, such as CD44v6, which can be used in conjunction with bcl-2, bcl-6, and CD10 to help subclassify DLBCL cases into ABC and GC types might be of practical importance for lymphoma diagnosis and individualised treatment.
Take home messages.
Tissue microarray technology is useful for immunophenotyping and the clinicopathological analysis of large lymphoma populations and correlated 100% with the results from conventional analysis
Overall survival (OS) was significantly better in those patients with the germinal centre B cell-like phenotype (bcl-2−/bcl-6+/CD10+) of diffuse large B cell lymphoma (DLBCL)
Expression of CD44 variant isoform 6 (CD44v6) correlates with disease stage, and might contribute to lymphoma dissemination
CD44v6 was expressed predominantly in activated B cell-like (ABC) DLBCL (bcl-2+/bcl-6−/CD10−), and in CD44 negative cases it was associated with worse OS
CD44v6 might be useful in conjunction with bcl-2, bcl-6, and CD10 to help subclassify DLBCL cases into ABC and GC types, and this might be of practical importance for lymphoma diagnosis and individualised treatment
Abbreviations
ABC, activated B cell-like
CD44s, CD44 standard isoform
CD44v, CD44 variant isoform
DLBCL, diffuse large B cell lymphoma
FFS, failure free survival
FL, follicular lymphoma
GC, germinal centre B cell-like
H&E, haematoxylin and eosin
IPI, international prognostic index
NHL, non-Hodgkin lymphoma
OS, overall cumulative survival
SLL, small lymphocytic lymphoma
TMA, tissue microarray
REFERENCES
- 1.Gatter KC, Warnke RA. Diffuse large B-cell lymphoma. In: Jaffe ES, Harris NL, Stein H, et al, eds. Pathology and genetics of tumours of the haematopoietic and lymphoid system. 2001, Lyon: IARC Press:171–4.
- 2.Pileri SA, Dirnhofer S, Ascani S, et al. Diffuse large B-cell lymphoma: one or more entities? Present controversies and possible tools for its subclassification. Histopathology 2002;41:482–509. [DOI] [PubMed] [Google Scholar]
- 3.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503–21. [DOI] [PubMed] [Google Scholar]
- 4.Staud LM. Molecular diagnosis and pathogenesis of lymphoma using gene expression profiling [abstract]. J Clin Pathol 2002;55(suppl 1):A24. [Google Scholar]
- 5.Shipp MA, Ross KN, Tamayo P, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med 2002;8:68–74. [DOI] [PubMed] [Google Scholar]
- 6.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937–47. [DOI] [PubMed] [Google Scholar]
- 7.Sneath RJS, Mangham DC. The normal structure and function of CD44 and its role in neoplasia. J Clin Pathol 1998;51:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartolazzi A, Jackson D, Bennett K, et al. Regulation of growth and dissemination of a human lymphoma by CD44 splice variants. J Cell Sci 1995;108:1723–33. [DOI] [PubMed] [Google Scholar]
- 9.Gunthert U, Stauder R, Mayer B, et al. Are CD44 variant isoforms involved in human tumour progression? Cancer Surv 1995;24:19–42. [PubMed] [Google Scholar]
- 10.Gunthert U, Schwarzler C, Wittig B, et al. Functional involvement of CD44, a family of cell adhesion molecules, in immune responses, tumour progression and haematopoiesis. Adv Exp Med Biol 1998;451:43–49. [DOI] [PubMed] [Google Scholar]
- 11.Naot D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res 1997;71:241–319. [DOI] [PubMed] [Google Scholar]
- 12.Pals ST, Drillenburg P, Radaszkiewicz T, et al. Adhesion molecules in the dissemination of non-Hodgkin’s lymphomas. Acta Haematol 1997;97:73–80. [DOI] [PubMed] [Google Scholar]
- 13.Ristamaki R, Joensuu H, Soderstrom KO, et al. CD44v6 expression in non-Hodgkin’s lymphoma: an association with low histological grade and poor prognosis. J Pathol 1995;176:259–67. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki K, Niitsu N. Elevated serum levels of soluble CD44 variant 6 are correlated with shorter survival in aggressive non-Hodgkin’s lymphoma. Eur J Haematol 2000;65:195–202. [DOI] [PubMed] [Google Scholar]
- 15.Stauder R, Eisterer W, Thaler J, et al. CD44 variant isoforms in non-Hodgkin’s lymphoma: a new independent prognostic factor. Blood 1995;85:2885–99. [PubMed] [Google Scholar]
- 16.Terpe HJ, Koopmann R, Imhof BA, et al. Expression of integrins and CD44 isoforms in non-Hodgkin’s lymphomas: CD44 variant isoforms are preferentially expressed in high-grade malignant lymphomas. J Pathol 1994;174:89–100. [DOI] [PubMed] [Google Scholar]
- 17.Angelopoulou MK, Kontopidou FN, Pangalis GA. Adhesion molecules in B-chronic lymphoproliferative disorders. Semin Hematol 1999;36:178–97. [PubMed] [Google Scholar]
- 18.Drillenburg P, Wielenga VJ, Kramer MH, et al. CD44 expression predicts disease outcome in localized large B cell lymphoma. Leukemia 1999;13:1448–55. [DOI] [PubMed] [Google Scholar]
- 19.Horst E, Meijer CJ, Radaskiewicz T, et al. Expression of a human homing receptor (CD44) in lymphoid malignancies and related stages of lymphoid development. Leukemia 1990;4:383–9. [PubMed] [Google Scholar]
- 20.Horst E, Meijer CJ, Radaszkiewicz T, et al. Adhesion molecules in the prognosis of diffuse large-cell lymphoma: expression of a lymphocyte homing receptor (CD44), LFA-1 (CD11a/18), and ICAM-1 (CD54). Leukemia 1990;4:595–9. [PubMed] [Google Scholar]
- 21.Inagaki H, Banno S, Wakita A, et al. Prognostic significance of CD44v6 in diffuse large B-cell lymphoma. Mod Pathol 1999;12:546–52. [PubMed] [Google Scholar]
- 22.Irving JA, Cain G, Howard M, et al. The role of alternative splicing of the adhesion molecule, CD44, in lymphoid malignancy. J Clin Pathol 1998;51:776–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucio PJ, Faria MT, Pinto AM, et al. Expression of adhesion molecules in chronic B-cell lymphoproliferative disorders. Haematologica 1998;83:104–11. [PubMed] [Google Scholar]
- 24.Salles G, Zain M, Jiang WM, et al. Alternatively spliced CD44 transcripts in diffuse large-cell lymphomas: characterization and comparison with normal activated B cells and epithelial malignancies. Blood 1993;82:3539–47. [PubMed] [Google Scholar]
- 25.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of hundreds of specimens. Nat Med 1998;4:844–7. [DOI] [PubMed] [Google Scholar]
- 26.Schraml P, Kononen J, Bubendorf L, et al. Tissue microarrays for gene amplification surveys in many different tumour types. Clin Cancer Res 1999;5:1966–75. [PubMed] [Google Scholar]
- 27.Tzankov A, Zimpfer A, Lugli A, et al. High-throughput tissue microarray analysis of the expression of cyclin D1, D3 and E1 and cyclin E1 gene amplification in 330 cases of classical Hodgkin lymphoma. J Pathol 2003;199:201–7. [DOI] [PubMed] [Google Scholar]
- 28.Zimpfer A, Bernasconi B, Glatz K, et al. Trisomy 3 and gain of an X-chromosome are frequent cytogenetic abnormalities in gastric B-cell lymphomas. Analysis by FISH on tissue microarrays of 226 surgically resected cases [abstract]. J Clin Pathol 2002;55(suppl 1):A20. [Google Scholar]
- 29.Tzankov A, Lugli A, Zimpfer A, et al. Tissue-microarray analysis of CD 44 expression in B-cell non-Hodgkin lymphomas [abstract]. J Clin Pathol 2002;55(suppl 1):A29. [Google Scholar]
- 30.Hedvat C, Hegde A, Chaganti R, et al. Application of tissue microarray technology to the study of non-Hodgkin’s and Hodgkin’s lymphoma. Hum Pathol 2002;33:968–74. [DOI] [PubMed] [Google Scholar]
- 31.Simon R, Sauter G. Tissue microarrays for miniaturized high-throughput molecular profiling of tumors. Exp Hematol 2002;30:1365–72. [DOI] [PubMed] [Google Scholar]
- 32.Dogan A, Bagdi E, Munson Ph, et al. CD10 and BCL-6 expression in paraffin sections of normal lymphoid tissue and B-cell lymphomas. Am J Surg Pathol 2000;24:846–52. [DOI] [PubMed] [Google Scholar]
- 33.Kremmidiotis G, Ridings J, Hicks M, et al. Heterogeneity of CD44 expression among human B-cell subpopulations. Tissue Antigens 1998;51:232–41. [DOI] [PubMed] [Google Scholar]