Abstract
Aims: To clarify the clinicopathological profile of osteosarcomas showing an intensely positive immunoreaction for cytokeratin.
Methods: Clinicopathological and immunohistochemical features were analysed in 131 patients with non-metastatic, conventional osteosarcoma, treated in Akita University and National Cancer Centre in Tokyo between 1972 and 1999.
Results: Six patients (4.5%; mean age, 32 years; four men, two women) had osteosarcomas showing intense cytokeratin expression. Tumours were located on the long bones of the extremities in five patients and the ilium in one. Osteoid formations were found in biopsied specimens in all cases. Three tumours were classified as osteoblastic osteosarcoma, two as fibroblastic, and one as chondroblastic. In three tumours classified as the osteoblastic subtype, epithelioid features were prominent, and four tumours showed pronounced cellular pleomorphism. In contrast to the expression of cytokeratin, epithelial membrane antigen was negative in all cases. Surgery with a wide excisional margin was performed in six patients. Preoperative and postoperative chemotherapy was given to five of the six patients, but the effects of these agents were negligible. Three of the six patients developed lung metastases, whereas the other three patients have remained well with no evidence of local recurrence or distant metastasis.
Conclusions: Osteosarcoma with intense immunoreaction for cytokeratin was rare. The clinicopathological features were similar to those of patients with conventional osteosarcoma, except for a higher age, chemotherapy resistance, histological epithelioid features, and pleomorphism. This study indicates that osteoid formation and negative expression of epithelial membrane antigen are key features in the differentiation from metastatic carcinoma.
Keywords: osteosarcoma, cytokeratin, diagnosis, epithelial membrane antigen
There have been several reports of osteosarcomas with epithelioid features. In 1975, Scranton et al first reported an osteosarcoma showing epithelioid features.1 After the first report, several authors described epithelioid features in osteosarcomas that were histologically indistinguishable from metastatic carcinoma.2–6 Some osteosarcomas show an intense and diffuse reaction for cytokeratin on histochemical analysis, although this finding is rare.7 Therefore, in cases of epithelioid osteosarcoma with intense and diffuse expression of cytokeratin, the differential diagnosis between osteosarcoma and metastatic carcinoma is a difficult diagnostic problem.
We use immunohistochemistry to examine 131 cases of osteosarcomas treated at Akita University and National Cancer Centre, Japan between 1972 and 1999. Six cases showed an intense and diffuse immunohistochemical reaction for cytokeratin. We describe here a clinicopathological profile of osteosarcomas with intense and diffuse expression of cytokeratin, with special attention to the differential diagnosis between these osteosarcomas and metastatic carcinoma.
“In cases of epithelioid osteosarcoma with intense and diffuse expression of cytokeratin, the differential diagnosis between osteosarcoma and metastatic carcinoma is a difficult diagnostic problem”
MATERIALS AND METHODS
We reviewed more than 200 cases of osteosarcoma involving the extremities or axial bones filed at the Akita University Hospital in Akita and the National Cancer Centre in Tokyo, Japan between 1972 and 1999. Microscopic haematoxylin and eosin slides of the biopsied specimen and slides of the surgical specimen were reviewed. Patients with osteosarcoma in the jawbones or cranium, and patients who underwent surgery only for metastatic foci were excluded from our study. Similarly, the following conditions were excluded from our study: low grade and high grade surface variants of osteosarcoma, dedifferentiated chondrosarcoma with foci of osteosarcoma, patients with primary metastasis at presentation, and secondary osteosarcomas after radiotherapy. Consequently, 131 patients with non-metastatic, high grade osteosarcoma were investigated in our current study. In all those patients who died, death was the result of tumour progression. All protocols were accepted by the institutional review boards of our institutions. Informed consent was obtained from all patients or their legal guardians, depending on the patient’s age.
In all 131 cases, we obtained formalin fixed, paraffin wax embedded tissues before treatment, including chemotherapy. Representative blocks were cut to a thickness of 4 μm, and were examined by the labelled streptavidin–biotin method with the appropriate use of negative controls throughout, after pretreatment with heat induced epitope unmasking in a 10mM citrate buffer, pH 6.0, in an autoclave at 121°C for 10 minutes. The primary antibodies were applied as follows: cytokeratin (clone AE1/3; 1/100 dilution; Dako, Glostrup, Denmark) and epithelial membrane antigen (EMA; clone E29; 1/100 dilution; Dako). When evaluating the immunostaining for AE1/3 and EMA, only homogeneous staining, along with staining of the cell membranes or cytoplasm, that was of the same intensity and pattern as that in the adjacent normal epithelium was assessed as 2+ positive, and heterogeneous or focal staining was assessed as 1+.
In the cases showing 2+ reactivity for cytokeratin, clinical details and follow up information were obtained by reviewing all medical charts, and a histological subtype was determined in each case by biopsy review. Then, the intensity of pleomorphism and the epithelioid features of the tumour cells were evaluated. In surgical specimens after preoperative chemotherapy, the effect of chemotherapy was evaluated by the degree of tumour necrosis as follows: good, ≥ 95%; moderate, 90–95%; and poor, < 90%.
RESULTS
Immunohistochemical findings
Six of the 131 tumours (4.5%) showed 2+ immunoreactivity for cytokeratin, and 12 tumours (9%) showed 1+ immunoreactivity. Epithelioid features were extensive in three of the six tumours with 2+ immunoreactivity for cytokeratin. Immunoreactivity for cytokeratin was intensely positive in the epithelioid areas. These three tumours were classified as the osteoblastic subtype according to their predominant matrix production. In two other fibroblastic subtypes and the remaining one chondroblastic subtype, cytokeratin was intensely positive in pleomorphic spindle cells and chondrocytes. EMA was negative in these six cases.
Clinicopathological findings
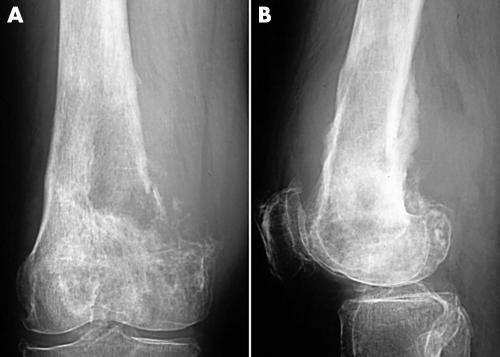
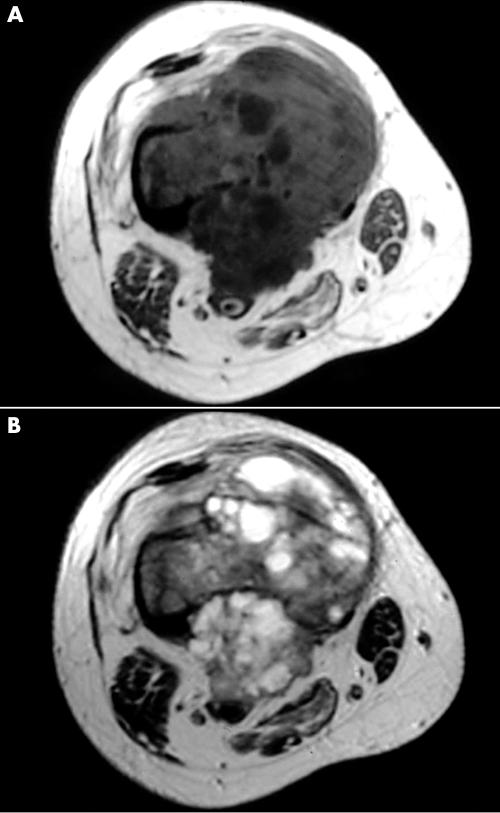
The six patients ranged in age from 13–66 years (mean, 32). Four patients were male and two were female. Tumours were located on the long tubular bones of the extremities in five patients (upper extremities, two; lower extremities, three), and the ilium in one. The tumours ranged from 4 to 11 cm at the maximal diameter (mean, 6.5). The presenting symptom was pain in all six patients, and the duration before they visited our institutes ranged from one to 11 months (mean, seven). The serum alkaline phosphatase concentration was high in two of the six patients, and within normal limits in the other four patients. Radiological findings were not specific. In four patients whose radiographs were available for review, all four tumours showed osteolytic and destructive lesions in the metaphyses, with various amounts of mineralisation (figs 1 and 2).
Figure 1.
Case 4. (A) Anteroposterior radiograph and (B) lateral radiograph showing a destructive lesion in the metaphysis of the femur with a small amount of fluffy mineralisation and thick periosteal reactions.
Figure 2.
Case 4. Magnetic resonance images of the femoral lesion showing isosignal intensity on (A) T1 weighted images (TR, 519; TE, 10.1) and isosignal to high signal intensity on (B) T2 weighted images (TR, 4000; TE, 10.1).
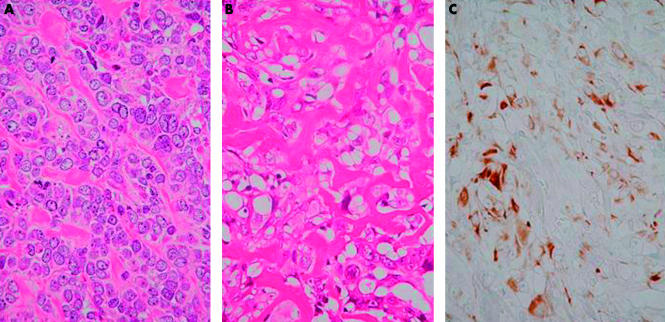
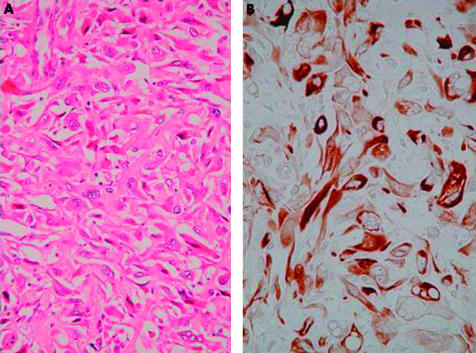
Histologically, all six tumours were conventional, high grade osteosarcoma. Definite osteoid formation was identified in the biopsied specimen in all cases. Three of the six tumours were classified as osteoblastic osteosarcoma, two as fibroblastic osteosarcoma, and one as chondroblastic osteosarcoma, depending on the predominant matrix production. In the three tumours classified as the osteoblastic subtype, epithelioid cytological features of the tumour cells—eccentrically located vesicular nuclei with prominent nucleoli and abundant palely eosinophilic cytoplasm—were prominent (fig 3). Four of the six tumours (two fibroblastic and two osteoblastic subtypes) showed pronounced pleomorphism (fig 4), and in the other two tumours, cellular pleomorphism was evaluated as moderate. Malignant appearing cartilage was prominent in one case that was diagnosed as chondroblastic osteosarcoma, but neoplastic cartilage was not found in the other cases. Table 1 (table 1) summarises the clinicopathological features of the six cases.
Figure 3.
Case 2. (A) Photomicrograph showing the epithelioid cytological features of the tumour cells—eccentrically located vesicular nuclei with prominent nucleoli and abundant palely eosinophilic cytoplasm (haematoxylin and eosin stained; original magnification, ×400). (B) Photomicrograph showing an area of lace-like osteoid formation (haematoxylin and eosin stained; original magnification, ×400). (C) Immunohistochemical stain for cytokeratin showing diffuse and intense positivity of tumour cells (original magnification, ×400).
Figure 4.
Case 4. (A) Photomicrograph showing proliferation of spindle cells with pronounced pleomorphism and lace-like osteoid formation (haematoxylin and eosin stained; original magnification, ×200). (B) Immunohistochemical stain for cytokeratin showing diffuse and intensely positive pleomorphic spindle shaped tumour cells (original magnification, ×400).
Table 1.
Clinicopathological profile of the patients with osteosarcoma with cytokeratin expression
| No. | Age/Sex | Site | Symptoms | Size | Histology | Ep | CK | EMA | Pleo | Ch Ef | Surgery | Follow up |
| 1 | 27 M | Humerus | Pain | 8 | Os | + | 2+ | – | 2+ | Poor | Wide | 113 m, NED |
| 2 | 43 M | Ilium | Pain | 12 | Os | + | 2+ | – | + | Poor | Wide | 24 m, DOD |
| 3 | 28 M | Humerus | Pain | 4 | Fi | – | 2+ | – | 2+ | Poor | Wide | 38 m, AWD |
| 4 | 66 F | Femur | Pain | 11 | Os | + | 2+ | – | 2+ | Wide | 30 m, NED | |
| 5 | 18 M | Tibia | Pain | 6 | Fi | – | 2+ | – | 2+ | Poor | Wide | 319 m, NED |
| 6 | 13 F | Tibia | Pain | 8 | Chon | – | 2+ | – | + | Poor | Wide | 53 m, AWD |
Size, size (cm); Ep, epithelioid feature; CK, cytokeratin; EMA, epithelial membrane antigen; Pleo, pleomorphism; Ch Ef, chemotherapy effect; OS, osteoblastic; Fi, fibroblastic; Chon, chondroblastic; NED, no evidence of disease; DOD, dead of disease; AWD, alive with disease.
The excisional margins of the initial surgical treatment were wide in all six patients. Chemotherapy was given preoperatively and postoperatively in five of the six patients. In these five patients, three were treated with combination chemotherapy, mainly high dose methotrexate (8–12 g/m2 for each course with leucovorin rescue), doxorubicin (60–90 mg/m2 for each course), and cisplatinum (80–120 mg/m2 for each course), with/without ifosfamide (6–10 g/m2 for each course). Two other patients were treated with combination chemotherapy of vincristin, doxorubicin, and mitomycin C in varying combinations. Systemic chemotherapy was not administrated to one patient who was 66 years old. The effects of chemotherapy in the five patients who underwent systemic preoperative chemotherapy were poor. Tumour necrosis rates in the excisional surgical specimen were less than 90% in the five patients. Follow up information on all six patients was available and covered periods ranging from 24 to 319 months (mean, 96). None of the patients developed local recurrence. Three (cases 2, 3, and 6) of the six patients developed metastases in the lungs; one died of the expansion of the metastatic foci (case 2) 24 months after surgery, and the other two have remained alive with disease for 38 months, and 53 months, respectively. Three (cases 1, 4, and 5) patients have remained well with no evidence of local recurrence or distant metastasis for 113 months, 30 months, and 319 months, respectively.
DISCUSSION
On immunohistochemical examination of 131 cases of osteosarcoma treated at Akita University Hospital and National Cancer Centre in Japan between 1972 and 1999, six patients showed a positive immunohistochemical reaction for cytokeratin. The age distribution of these six patients (13–66 years; mean, 32 years) was slightly higher than that of patients with conventional osteosarcomas. Other clinical profiles including sex distribution, site of the lesion, size, and symptoms are similar to those of conventional osteosarcomas.6 Similarly, the radiological findings were not specific in most patients, showing an osteolytic and destructive lesion in the metaphyses with varied degrees of mineralisation.
Histologically, three were the osteoblastic subtype, and epithelioid features were also extensive in these three cases. Immunoreactivity for cytokeratin was intensely positive in the epithelioid area. Similarly, cytokeratin expression was seen in osteosarcoma cases with extensive epithelioid cell proliferation in the literature.2–5 However, in the other two fibroblastic subtypes and the remaining one chondroblastic subtype an epithelioid element was not seen, and cytokeratin was intensely positive in pleomorphic spindle shaped cells and chondrocytes. Conventional osteosarcoma can show a positive immunoreaction for cytokeratin, even in the fibroblastic or chondroblastic subtypes without an epithelioid appearance.
Intense and diffuse positivity for cytokeratin, mainly seen in epithelial tumours, is rarely seen in osteosarcoma,7 although it has also been described in chordomas,8 and other soft tissue sarcomas, including synovial sarcomas,9 epithelioid sarcomas,10 malignant rhabdoid tumours,11 and extraskeletal Ewing’s sarcomas.12 In these tumours, chordoma principally occurs in the spine, and is never seen in bones of the extremities. Therefore, it is probably easy to exclude chordoma when a bony lesion of the extremities shows cytokeratin expression. In other soft tissue tumours with immunoreactivity for cytokeratin, location of the lesion is the most important issue in the differential diagnosis.
“Although a poor chemotherapy effect may indicate that an alternative method of treating this type of osteosarcoma is needed, two of the five patients who received chemotherapy and one patient who did not receive chemotherapy remained continuously disease free”
Thus, the differential diagnosis between metastatic carcinoma to the bones and cytokeratin positive osteosarcoma with extensive epithelioid cell proliferation is the most difficult. History of cancer and the following systemic survey for any lesion is mandatory in the differential, although these do not provide specific information. Careful attention should be paid because osteoid or bone formation in cases of melanoma13 and metastatic carcinoma6 have been reported in the literature. However, the most important histological findings to make a diagnosis of osteosarcoma are still osteoid formation and malignant appearing cartilage.6 In our current series, all six cases showed osteoid formation, but only one case contained malignant appearing cartilage. Furthermore, none of the patients had a history of cancer treatment and there was no lesion found on systemic survey. Thus, the diagnosis of cytokeratin positive osteosarcoma was made.
In 2001, we reported several osteoblastic osteosarcomas showed rosette-like features.14 These osteosarcomas with rosette-like features also frequently had epithelioid histological features, and were frequently positive for EMA, but negative for cytokeratin. In contrast, the current six cases had intense and diffuse immunohistochemical positivity for cytokeratin only. Interestingly, osteosarcoma with rosette-like features had a worse prognosis than that of classic osteoblastic osteosarcoma, suggesting a correlation between EMA expression and poor prognosis. However, cytokeratin expression in osteosarcomas was not correlated with poor prognosis. Among the groups of epithelial markers, EMA and cytokeratin expression in osteosarcoma may show a different correlation with clinical behaviour. In our current study, none of the patients showed coexpression of EMA and cytokeratin. However, we have previously reported two cases of osteosarcoma in the thoracic and second lumbar vertebrae with immunoreactivity for both cytokeratin and EMA.3,4 Thus, it should be borne in mind that negative results for EMA in cytokeratin positive osteosarcomas are not always consistent findings.
Take home messages.
Osteosarcoma with intense immunoreaction for cytokeratin was rare and was seen in only 4.5% of patients with osteosarcoma
The clinicopathological features were similar to those of patients with conventional osteosarcoma, except for a higher age, chemotherapy resistance, histological epithelioid features, and pleomorphism
In the differential diagnosis between cytokeratin positive osteosarcoma and metastatic carcinoma, clinical history of cancer, existence of other cancers in the visceral region, osteoid formation on histological slides, and negativity for epithelial membrane antigen provide useful information
Three of the six cases showed good clinical results, but the other three developed lung metastases. Characteristically, the effect of chemotherapy on the five patients who received preoperative chemotherapy was negligible. Although a poor chemotherapy effect may indicate that an alternative method of treating this type of osteosarcoma is needed, two of the five patients who received chemotherapy and one patient who did not receive chemotherapy remained continuously disease free. Because of their rarity, further studies should be undertaken to evaluate the relation between the effect of chemotherapy and clinical outcome of this subtype of osteosarcoma.
In conclusion, in the differential diagnosis between cytokeratin positive osteosarcoma and metastatic carcinoma, clinical history of cancer, existence of other cancers in the visceral region, osteoid formation on histological slides, and negativity for EMA provide useful information.
REFERENCES
- 1.Scranton PE, Jr, DeCicco FA, Totten RS, et al. Prognostic factors in osteosarcoma: a review of 20 years’ experience at the University of Pittsburgh Health Center Hospitals. Cancer 1975;36:2179–91. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida H, Yumoto T, Adachi H, et al. Osteosarcoma with prominent epithelioid feature. Acta Pathol Jpn 1989;39:439–45. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa T, Hirose T, Kudo E, et al. Immunophenotypic heterogeneity in osteosarcomas. Hum Pathol 1991;22:583–590. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa T, Shibata T, Hirose T, et al. Osteosarcoma with epithelioid features. An immunohistochemical study. Arch Pathol Lab Med 1993;117:295–8. [PubMed] [Google Scholar]
- 5.Kramer K, Hicks DG, Palis J, et al. Epithelioid osteosarcoma of bone: immunocytochemical evidence suggesting divergent epithelial and mesenchymal differentiation in a primary osseous neoplasm. Cancer 1993;71:2977–82. [DOI] [PubMed] [Google Scholar]
- 6.Unni KK. Dahlin’s bone tumors: general aspects and data on 11,087 cases, 5th ed. Philadelphia: Lippincott-Raven, 1996:143–83.
- 7.Dorfman HA, Czerniak B. Bone tumors. St Louis: Mosby, 1997:128–252.
- 8.Salisbury JR, Isaacson PG. Demonstration of cytokeratins and an epithelial membrane antigen in chordomas and human fetal notochord. Am J Surg Pathol 1985;9:791–7. [DOI] [PubMed] [Google Scholar]
- 9.Fisher C. Synovial sarcoma: ultrastructural and immunohistochemical features of epithelial differentiation in monophasic and biphasic tumors. Hum Pathol 1986;17:996–1008. [DOI] [PubMed] [Google Scholar]
- 10.Daimaru Y, Hashimoto H, Tsuneyoshi M, et al. Epithelial profile of epithelioid sarcoma: an immunohistochemical analysis of eight cases. Cancer 1987;59:134–41. [DOI] [PubMed] [Google Scholar]
- 11.Tsuneyoshi M, Daimaru Y, Hashimoto H, et al. Malignant soft tissue neoplasms with the histologic features of renal rhabdoid tumors: an ultrastructural and immunohistochemical study. Hum Pathol 1985;16:1235–42. [DOI] [PubMed] [Google Scholar]
- 12.Fujii Y, Hongo T, Nakagawa Y, et al. Cell culture of small round cell tumor originating in the thoracopulmonary region: evidence for derivation from a primitive pluripotent cell. Cancer 1989;64:43–51. [DOI] [PubMed] [Google Scholar]
- 13.Lucas DR, Tazelaar HD, Unni KK, et al. Osteogenic melanoma. A rare variant of malignant melanoma. Am J Surg Pathol 1993;17:400–9. [PubMed] [Google Scholar]
- 14.Okada K, Hasegawa T, Yokoyama R. Rosette-forming epithelioid osteosarcoma. A histologic subtype with highly aggressive clinical behavior. Hum Pathol 2001;32:726–33. [DOI] [PubMed] [Google Scholar]