Abstract
Background: The hallmark of neurofibromatosis type 1 (NF1) is the development of multiple neurofibromas. Solitary neurofibroma may occur in an individual who does not have NF1, but multiple neurofibromas tend to develop only in those with NF1. It has been suggested that hormones may influence the neurofibromas of patients with NF1. The evidence that hormones may influence the growth of neurofibromas comes mainly from the observation that localised neurofibromas of patients with NF1 commonly grow during puberty and pregnancy. Because growth hormone (GH) concentrations increase during puberty, it is possible that GH influences the growth of these tumours.
Aims: To investigate the presence of GH receptors (GHRs) in neurofibromas.
Methods: By means of immunohistochemistry, the presence of GHRs was investigated in two groups of patients: 16 patients without NF1 with solitary neurofibromas (group A) and 10 patients with NF1 with localised neurofibromas (group B).
Results: Six of the 16 patients in group A had neurofibromas that were immunopositive for GHR, whereas nine of the 10 patients in group B were immunopositive.
Conclusions: Most patients with NF1 have localised neurofibromas that express GHR. This suggests that GH may play some role in the development of localised neurofibromas in patients with NF1.
Keywords: growth hormone, growth hormone receptor, neurofibroma, neurofibromatosis
The term neurofibromatosis (NF) is used for a group of genetic disorders that primarily affect the cell growth of neural tissues.1 There are at least two clinical forms of NF, namely: neurofibromatosis type 1 (NF1) and neurofibromatosis type 2 (NF2).2,3 These two forms of NF have few common features and they are caused by mutations on different genes.2 NF1, also known as von Recklinghausen’s disease, is the most common type of NF and accounts for about 90% of all cases.3 It is one of the most frequent human genetic diseases, with a prevalence of one case in 3000 births.3–7 NF1 is caused by a spectrum of mutations that affect the gene located on chromosome 17q11.2, known as the NF1 gene.8,9
The hallmark of NF1 is the development of multiple neurofibromas. Solitary neurofibroma may occur in an individual who does not have NF1, but multiple neurofibromas tend to develop only in those with NF1.2 Other common features of NF1 include Lisch nodules, café au lait spots, and freckling in the inguinal and axillary regions.2,4 Patients with NF1 are also at increased risk for the development of certain malignancies.2 Malignant peripheral nerve sheath tumour is the most common malignancy that occurs in patients with NF1.2,10
“Localised neurofibromas increase both in size and number during adolescence and also during pregnancy in patients with neurofibromatosis type 1”
Neurofibromas are complex benign tumours that arise from peripheral nerve sheaths from small or large nerves. There are two major types of neurofibromas of NF1, which may differ widely in their natural history, namely: “localised”, and “plexiform” neurofibromas.2
The number of localised neurofibromas can vary from one person to another.2 Although NF1 has been subjected to a century of clinical observation, much is still unknown about the pathogenesis of neurofibromas in these patients.
Localised neurofibromas are rarely, if ever, present at birth, and they are usually not evident in the first 5 years of life.2 Most often they begin to appear in the later part of childhood, especially in early puberty. Localised neurofibromas increase both in size and number during adolescence and also during pregnancy in patients with NF1.2,11
The appearance of neurofibromas in adolescence and in pregnancy suggests that hormones influence the development of these tumours. The basis for any possible hormonal influence is unknown. Some investigators have studied the presence of oestrogen receptors in neurofibromas. Martuza and colleagues12 only detected these receptors in one of six neurofibromas. Chaudhuri and colleagues13 could not detect steroid hormone receptors (receptors for oestrogen, progesterone, androgen, and glucocorticoid) in five neurofibromas. These studies suggest that the alterations seen in the neurofibromas of patients with NF1 during puberty and pregnancy are not related to steroids.
Because growth hormone (GH) concentrations increase during adolescence, it is possible that GH influences the growth of localised neurofibromas of NF1.2,14 GH exerts a wide variety of biological actions in many different tissues and cell types. The actions of GH at the cellular level can be divided into three categories: those affecting mitogenesis, differentiation, and metabolism.15 The ability of GH to promote its various effects is dependent upon interaction of the hormone with membrane receptors in target tissues. The activation of the GH receptor (GHR) by GH is responsible for initiating a variety of signalling pathways. These include pathways involving signal transducer and activator of transcription (STAT), Ras–mitogen activated protein (MAP) kinase, and insulin receptor substrate (IRS).16
The localisation of GHRs in the neurofibromas of patients with NF1 is an important step in evaluating the role of GH in the progression of neurofibromas. The objective of our study was to identify GHRs in the neurofibromas of patients with NF1.
METHODS
Our study was approved by the ethics committee of Fluminense Federal University, Brazil.
Patients
Lesions diagnosed as neurofibromas were retrieved from the files of the pathological anatomy service of Antônio Pedro University Hospital of Fluminense Federal University (tables 1 and 2). Thirty four neurofibromas from 26 patients were selected and separated into two groups:
Group A: 16 patients without NF1 with solitary neurofibroma (16 solitary neurofibromas).
Group B: 10 patients with NF1 who had localised neurofibromas (18 localised neurofibromas).
Table 1.
Clinical data from patients without neurofibromatosis type 1 with solitary neurofibroma (group A)
| Patient | Neurofibroma no. | Age (years) | Sex | Ethnicity | Localisation |
| 1 | 1 | NA | NA | NA | Skin |
| 2 | 2 | NA | M | NA | Forearm (skin) |
| 3 | 3 | 41 | M | Black | Frontotemporal region (skin) |
| 4 | 4 | 44 | M | White | Vertebral canal |
| 5 | 5 | NA | M | NA | Back (skin) |
| 6 | 6 | NA | F | White | Breast (skin) |
| 7 | 7 | NA | F | White | Skin |
| 8 | 8 | 55 | F | White | Back (skin) |
| 9 | 9 | NA | NA | NA | Forearm (skin) |
| 10 | 10 | 81 | F | White | Leg (skin) |
| 11 | 11 | NA | F | NA | Shoulder (skin) |
| 12 | 12 | NA | NA | NA | Scalp (skin) |
| 13 | 13 | NA | M | White | Scalp (skin) |
| 14 | 14 | 68 | F | White | Knee (skin) |
| 15 | 15 | 52 | M | White | Vertebral canal |
| 16 | 16 | 47 | F | NA | Mentonian region (skin) |
NA, not available.
Table 2.
Clinical data from patients with neurofibromatosis type 1 who have localised neurofibroma (group B)
| Patient | Neurofibroma no. | Age (years) | Sex | Ethnicity | Localisation |
| 1 | 1 | 26 | F | Black | Arm (skin) |
| 1 | 2 | 26 | F | Black | Forearm (skin) |
| 1 | 3 | 26 | F | Black | Forearm (skin) |
| 1 | 4 | 22 | F | Black | Skin |
| 2 | 5 | 33 | F | White | Skin |
| 2 | 6 | 33 | F | White | Skin |
| 3 | 7 | 8 | M | Black | Mandible (intraosseous lesion) |
| 4 | 8 | 49 | F | White | Leg (skin) |
| 5 | 9 | 31 | F | White | Shoulder (skin) |
| 6 | 10 | 34 | F | White | Back (skin) |
| 7 | 11 | 16 | F | White | Leg (skin) |
| 8 | 12 | 25 | F | White | Skin |
| 8 | 13 | 25 | F | White | Skin |
| 8 | 14 | 25 | F | White | Skin |
| 8 | 15 | 25 | F | White | Skin |
| 8 | 16 | 25 | F | White | Skin |
| 9 | 17 | 28 | F | Black | Arm (skin) |
| 10 | 18 | 19 | F | Black | Skin |
Group B had more neurofibromas than patients because some patients had been submitted to more than one biopsy. It is important to emphasise that there were no coincidental biopsies of the same lesion in our study.
All patients with NF1 included in our study had a diagnosis of NF1 according to the diagnostic criteria established at the 1987 National Institutes of Health consensus development conference on neurofibromatosis (table 3).
Table 3.
Diagnostic criteria for neurofibromatosis type 1 (NF1) established by the National Institutes of Health (NIH) consensus development conference1
| Individual is affected with NF–1 if two or more of the following are met |
| • Six or more café au lait macules over 5 mm in greatest diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals |
| • Two or more neurofibromas of any type or one plexiform neurofibroma |
| • Freckling in the axillary or inguinal regions |
| • Optic glioma |
| • Two or more Lisch nodules (iris hamartomas) |
| • A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudoarthrosis |
| • A first degree relative with NF–1 by the above criteria (NIH 1988) |
Immunohistochemistry
Serial 5 μm sections were cut from paraffin wax blocks and collected on silane coated slides. After dewaxing, the presence of GHRs was demonstrated immunohistochemically by means of the EnVision kit™ (code K1392; Dako, Carpenteria, California, USA). Antigen retrieval was performed using microwave ovens and citrate buffer in a pressure cooker. Endogenous peroxidase activity was eliminated by incubation for 10, 15, and 20 minutes in 6% H2O2 in distilled water at room temperature. Non–specific protein binding was blocked by incubation with a 1/100 dilution of normal goat serum in antibody diluent with background reducing component (code S3022; Dako), for 30 minutes at 37°C. Sections were incubated: (1) overnight at 4°C with a 1/100 dilution of the primary monoclonal antibody against GHR (263; code MCA 1555; Serotec, Raleigh, North Carolina, USA); (2) for one hour at room temperature with EnVision™. Visualisation was performed by incubation for five minutes in diaminobenzidine. Between each step, sections were washed three times for 10 minutes in Tris buffered saline. All incubations were carried out in humidified chambers to prevent evaporation. Sections were counterstained in Mayer’s haematoxylin and they were coverslipped with Entellan (Merck Frankfurt Brandenburg, Germany; code 107961). Negative controls were performed by omission of the primary monoclonal antibody and normal fetal bone marrow was used as the positive control.
RESULTS
Normal epidermis and dermal appendages, when present in sections, showed immunoreactivity for GHR and served as positive internal controls. All layers of the epidermis showed immunopositivity for GHR, except for the keratin layer. Hair follicles and sebaceous glands were also immunopositive for the GHR. The excretory ducts of sweat glands showed immunoreactivity only in the basal cells and no immunoreaction was seen in the cells of the secretory portion of sweat glands (fig 1A ). Vascular endothelial cells and skeletal muscle cells also possessed GHR immunoreactivity.
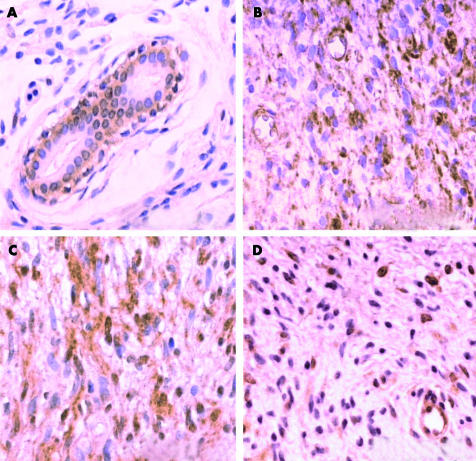
Figure 1.
Typical immunohistochemistry results using the monoclonal antibody (263) specific for the growth hormone receptor (GHR). (A) Immunoreactivity for the GHR seen in the cells of the basal layer of the excretory ducts of sweat glands. There is a lack of staining in the cells of the superficial ductal (group B; neurofibroma number 4). (B) Neurofibroma staining positively for the GHR. Note the immunopositive endothelial cells and immunonegative red blood cells (group B; neurofibroma number 6). (C) Neurofibroma staining positively for the GHR (group B; neurofibroma number 8). (D) Neurofibroma staining positively for the GHR. Note the immunopositive endothelial cells (group B; neurofibroma number 15).
The control sections were negative for GHR.
Immunohistochemical analyses in neurofibromas
Of the 34 neurofibromas studied, 22 were immunopositive for the GHR. The GHR displayed immunoreactivity in the cytoplasm, cellular membrane, and nucleus of tumorous cells. In one neurofibroma, staining was seen only over the nucleus, whereas in the remainder, it was seen both in the cytoplasm and the nucleus. Of these neurofibromas, six lesions also presented scarce cells with immunopositivity in the cellular membrane. In all neurofibromas, staining was granular. In 13 of the 22 immunopositive neurofibromas the pattern of staining was heterogeneous, whereas in the remaining nine the staining had a homogeneous distribution.
Six of the 16 group A patients (patients without NF1 who had solitary neurofibroma) had neurofibromas that stained positively for GHR. Nine of the 10 group B patients (patients with NF1 who had localised neurofibromas) had at least one neurofibroma that was immunopositive for GHR. Thus, group B patients had a higher proportion of patients with neurofibromas that were immunopositive for GHR than did the patients in group A.
Tables 4 and 5 summarise the results of the immunohistochemical analysis of neurofibromas. Figure 1B–D shows typical immunohistochemistry results for GHR.
Table 4.
Results of immunohistochemical analyses for the growth hormone receptor (GHR) in solitary neurofibromas (group A)
| Patient | Neurofibroma no. | GHR | Localisation of immunoreaction | Pattern of immunoreaction | Distribution of immunoreaction |
| 1 | 1 | + | C, N, CM | Granular | Homogeneous |
| 2 | 2 | – | |||
| 3 | 3 | – | |||
| 4 | 4 | – | |||
| 5 | 5 | – | |||
| 6 | 6 | – | |||
| 7 | 7 | – | |||
| 8 | 8 | – | |||
| 9 | 9 | + | C, N, CM | Granular | Homogeneous |
| 10 | 10 | – | |||
| 11 | 11 | + | C, N | Granular | Homogeneous |
| 12 | 12 | + | C, N | Granular | Homogeneous |
| 13 | 13 | – | |||
| 14 | 14 | + | C, N | Granular | Heterogeneous |
| 15 | 15 | – | |||
| 16 | 16 | + | C, N | Granular | Heterogeneous |
C, cytoplasm; CM, cytoplasmic membrane; N, nucleus.
Table 5.
Results of immunohistochemical analyses for the growth hormone receptor (GHR) in localised neurofibromas (group B)
| Patient | Neurofibroma no. | GHR | Localisation of immunoreaction | Pattern of immunoreaction | Distribution of immunoreaction |
| 1 | 1 | + | C, N | Granular | Heterogeneous |
| 1 | 2 | + | C, N | Granular | Homogeneous |
| 1 | 3 | + | C, N | Granular | Homogeneous |
| 1 | 4 | + | C, N | Granular | Homogeneous |
| 2 | 5 | – | |||
| 2 | 6 | + | C, N, CM | Granular | Heterogeneous |
| 3 | 7 | + | C, N | Granular | Heterogeneous |
| 4 | 8 | + | C, N, CM | Granular | Heterogeneous |
| 5 | 9 | – | |||
| 6 | 10 | + | N | Granular | Heterogeneous |
| 7 | 11 | + | C, N, CM | Granular | Homogeneous |
| 8 | 12 | + | C, N, CM | Granular | Heterogeneous |
| 8 | 13 | + | C, N, CM | Granular | Heterogeneous |
| 8 | 14 | + | C, N | Granular | Heterogeneous |
| 8 | 15 | + | C, N | Granular | Heterogeneous |
| 8 | 16 | + | C, N | Granular | Heterogeneous |
| 9 | 17 | + | C, N | Granular | Homogeneous |
| 10 | 18 | + | C, N | Granular | Heterogeneous |
C, cytoplasm; CM, cytoplasmic membrane; N, nucleus.
DISCUSSION
In the past few years, GH has been identified as a potent inducer of cell growth in many tumours.18–22 High plasma concentrations of GH have been seen in individuals with malignant conditions.18,23 Therefore, some authors believe that alterations of serum concentrations of GH might be important in the pathogenesis of several tumours. In addition, studies have detected an increase in the incidence of benign and malignant tumours in individuals with acromegaly.24–27 There have also been reports of an increased incidence of leukaemia in individuals treated with recombinant GH.28,29 Using immunohistochemistry, Lincoln et al reported that the ratio of immunopositive cells for GHR was higher in tumours than in normal tissues.22 A role for GH in neoplastic dissemination has also been suggested because it was reported that patients with prostate cancer who developed metastases had higher serum concentrations of GH than patients without metastases.30
Short stature has long been known to be a feature of NF1.31 In some instances, children with NF1 and short stature of unknown cause have been treated with recombinant GH. Because children with NF1 have an increased risk of developing benign and malignant tumours, there has been some concern about the safety of recombinant GH treatment in this situation.31
Our study investigated the presence of the GHR in neurofibromas. Our results, using an immunohistochemical technique, demonstrated that GHR was expressed on some neurofibromas. Nine of the 10 patients with NF1 had neurofibromas that were immunopositive for the GHR. Only six of the 16 patients without NF1 had immunopositive neurofibromas. This suggests that GH may have a greater influence on NF1 neurofibromas than on non–NF1 neurofibromas.
Solitary neurofibromas and localised neurofibromas are histologically identical lesions, yet the expression of the GHR was higher in neurofibromas from patients with NF1 than in neurofibromas from patients without NF1. The question of why the expression of GHR is more common in neurofibromas of patients with NF1 than in neurofibromas of patients without NF1 remains to be solved.
The mechanisms leading to the formation of neurofibromas remain unclear. Most mutations of the NF1 gene prevent expression of the intact NF1 product, called neurofibromin. Neurofibromin contains a central domain homologous to a family of proteins known as GTPase activation proteins (GAPs), which function as negative regulators of the Ras proteins. GAPs attenuate signalling from Ras, thus blocking the transmission of signals leading to increased growth or differentiation.32
Activation of GHR by GH is thought to be responsible for the initiation of a variety of signalling pathways.1 These include pathways involving STATs, Ras, MAP kinases (designated extracellular signal regulated kinases 1 and 2), and IRSs.16
It is known that the neurofibroma cells of patients with NF1 have high concentrations of the activated form of the Ras protein (Ras–GTP). This happens because neurofibromin, which is an important regulator of the Ras pathway, is modified as a consequence of mutation of the NF1 gene.33 It is possible that neurofibroma cells have increased Ras–GTP concentrations because of the action of GH, which leads to an increase in the cell number. This could explain why during adolescence there is an increase in the size of localised neurofibromas. The increase of GH secretion seen in this period could result in the super stimulation of the Ras protein signalling pathway in the neurofibromas cells.
“Because growth hormone has proliferative activity in several other benign and malignant tumours, its role in the development of the neurofibromas of neurofibromatosis type 1 should not be ignored”
Although GHR is localised to the cytoplasmic membrane, the expression of GHR in this location was rarely seen in our study. Staining of most of the immunopositive tumorous cells was localised to the cytoplasm and the nucleus. These locations are identical to those reported in a study performed by Lincoln et al with several tumour types (for example, lymphomas, melanomas, and melanocytic naevi) and normal tissues from humans, rats, and rabbits.22 The authors used the same monoclonal antibody (263) that was used in our study. Lobie and Breipohl,34 in a study on the normal gastrointestinal tract of rats, found cytoplasmic immunostaining for GHR, and immunoreactivity for GHR was also seen in the nucleus in some cells. Ginarte et al,20 in a study performed with normal skin and proliferative benign cutaneous diseases, found no staining at the cell membrane but did observe immunoreactivity in the cytoplasm and the nucleus.
In our study, the identification of GHR in the nucleus is compatible with the results of Lobie et al,35 who showed that GH is internalised into the cell by a receptor mediated process. There are several intracellular destinations for GH after internalisation, including lysosomal bodies, the Golgi apparatus, mitochondria, and the nucleus.35
Based on our findings, it can be presumed that treatment with recombinant GH in patients with NF1 can increase the size and number of localised neurofibromas. However, the concept that GH stimulates the growth of neurofibromas of patients with NF1 is still only theoretical, although our results suggest that GHR expression in these tumours is capable of transmitting mitogenic signs to the neoplastic cells. Although the presence of GHRs does not prove that this hormone has a role in the development of the neurofibromas of NF1, its presence suggests that these lesions can respond to GH. Because GH has proliferative activity in several other benign and malignant tumours, its role in the development of the neurofibromas of NF1 should not be ignored. Unfortunately, some children with NF1 and low height without apparent cause are indiscriminately treated with recombinant GH.
In conclusion, our results indicate that most patients with NF1 have localised neurofibromas that express the GHR. Our results also suggest that GH may play a role in the development of localised neurofibromas of patients with NF1. Therefore, caution must be excercised in the treatment of children with NF1 and low height with recombinant GH.
Take home messages.
Most of our patients with neurofibromatosis type 1 (NF1) had localised neurofibromas that expressed the growth hormone (GH) receptor
GH probably plays a role in the development of localised neurofibromas in patients with NF1
These results bring into question the safety of treating patients with NF1 and short stature with recombinant GH
Acknowledgments
We thank Dr A Pires, who helped in material analyses and figures. We also thank Dr EP Dias, Dr AS Cardoso, and Dr M Geller for comments and suggestions.
Abbreviations
GAP, GTPase activation protein
GH, growth hormone
GHR, growth hormone receptor
IRS, insulin receptor substrate
MAP, mitogen activated protein
NF1, neurofibromatosis type 1
STAT, signal transducer and activator of transcription
REFERENCES
- 1.Neurofibromatosis. NIH Consens Statement 1987;6:1–19. [Google Scholar]
- 2.Friedman JM, Gutmann DH, Maccollin M, et al. Neurofibromatosis. Phenotype, natural history and pathogenesis, 3rd ed. Baltimore: The Johns Hopkins University Press, 1999.
- 3.Gorlin RJ, Cohen MM, Levin LS, et al. Syndromes of the head and neck, 3rd ed. Oxford: Oxford University Press, 1990.
- 4.Cotran R, Kumar V, Robbins S. Robbins patologia estrutural e funcional, 5th ed. Rio de Janeiro: Guanabara Koogan, 1996.
- 5.Heim RA, Kam–Morgan LN, Binnie CG, et al. Distribution of 13 truncating mutations in the neurofibromatosis 1 gene. Hum Mol Genet 1995;4:975–81. [DOI] [PubMed] [Google Scholar]
- 6.Kitano Y, Okamoto E, Saito K, et al. Effects of several growth factors on cultured neurofibroma cells. J Dermatol Sci 1992;3:137–44. [DOI] [PubMed] [Google Scholar]
- 7.Vogel KS, Klesse LJ, Velasco–Miguel S, et al. Mouse tumor model for neurofibromatosis type 1. Science 1999;286:2176–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace MR, Marchuk DA, Andersen LB, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three nf1 patients. Science 1990;249:181–6. [DOI] [PubMed] [Google Scholar]
- 9.Cawthon RM, Weiss R, Xu G, et al. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell 1990;62:193–201. [DOI] [PubMed] [Google Scholar]
- 10.Shearer P, Parham D, Kovnar E. Neurofibromatosis type I and malignancy: review of 32 pediatric cases treated at a single institution. Med Pediatr Oncol 1994;22:78–83. [DOI] [PubMed] [Google Scholar]
- 11.Geller M, Bonalumi Filho A. Neurofibromatose. In: Carakushansky G, ed. Doenças genéticas em pediatria. Rio de janeiro: Guanabara–Koogan, 2001:377–90.
- 12.Martuza RL, Maclaughlin DT, Ojemann RG. Specific estradiol binding in schawnnomas, meningiomas and neurofibromas. Neurosurgery 1981;9:665–71. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri PK, Walker MJ, Das Gupta TK, et al. Steroid receptors in tumors of nerve sheath origin. J Surg Oncol 1982;20:205–6. [DOI] [PubMed] [Google Scholar]
- 14.Reed ML, Jacoby RA. Cutaneous neuroanatomy and neuropathology. Normal nerves, neural–crest derivates, and benign neural neoplasms in the skin. Am J Dermatopathol 1983;5:335–62. [PubMed] [Google Scholar]
- 15.Billestrup N, Hansen JA, Hansen LH, et al. Molecular mechanism of growth hormone signaling. Endocr J 1998;45:s41–5. [DOI] [PubMed] [Google Scholar]
- 16.Carter–Su C, Rui L, Stofega MR. SH2–B and SIRP: JAK2 binding proteins that modulate the actions of growth hormone. Recent Prog Horm Res 2000;55:293–311. [PubMed] [Google Scholar]
- 17.Withdrawn
- 18.Emerman JT, Leahy M, Gout PW. Elevated growth hormone levels in sera from breast cancer patients. Horm Metab Res 1985;17:421–4. [DOI] [PubMed] [Google Scholar]
- 19.Garcia–Caballero T, Mertani HM, Lambert A, et al. Increased expression of growth hormone and prolactin receptors in hepatocellular carcinomas. Endocrine 2000;12:265–71. [DOI] [PubMed] [Google Scholar]
- 20.Ginarte M, Garcia–Callabero T, Fernandez–Redondo V, et al. Expression of growth hormone receptor in benign and malignant cutaneous proliferative entities. J Cutan Pathol 2000;27:276–82. [DOI] [PubMed] [Google Scholar]
- 21.Kaulsay KK, Zhu T, Bennett W, et al. The effects of autocrine human growth hormone (hgh) on human mammary carcinoma cell behavior are mediated via the hgh receptor. Endocrinology 2001;142:767–77. [DOI] [PubMed] [Google Scholar]
- 22.Lincoln DT, Sinowatz F, Temmim–Baker L, et al. Growth hormone receptor expression in the nucleus and cytoplasm of normal and neoplastic cells. Histochem Cell Biol 1998;109:141–59. [DOI] [PubMed] [Google Scholar]
- 23.Andrews GS. Growth hormone and malignancy. J Clin Pathol 1983;36:935–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander L, Appleton D, Hall R, et al. Epidemiology of acromegaly in the Newcastle region. Clin Endocrinol 1980;12:71–9. [DOI] [PubMed] [Google Scholar]
- 25.Barzilay J, Heatley GJ, Cushing GW. Benign and malignant tumors in patients with acromegaly. Arch Intern Med 1991;151:1629–32. [PubMed] [Google Scholar]
- 26.Bengtsson BA, Edén S, Ernest I, et al. Epidemiology and long–term survival in acromegaly: a study of 166 cases diagnosed between 1955 and 1984. Acta Med Scand 1988;223:327–35. [DOI] [PubMed] [Google Scholar]
- 27.Ratner RE, Hare JW. Association of acromegaly and chondrosarcoma. South Med J 1983;76:1181–2. [DOI] [PubMed] [Google Scholar]
- 28.Aktan M, Tanakol R, Nalcaci M, et al. Leukemia in a patient treated with growth hormone. Endocr J 2000;47:471–3. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe S, Mizuno S, Oshima LH, et al. Leukemia and other malignancies among GH users. J Pediatr Endocrinol 1993;6:99–108. [DOI] [PubMed] [Google Scholar]
- 30.British Prostate Study Group. Evaluation of plasma hormone concentrations in relation to clinical staging in patients with prostate cancer. Br J Urol 1979;51:382–9. [DOI] [PubMed] [Google Scholar]
- 31.Saenger P. Growth hormone in von Recklinghausen’s disease: reckless or recommended? J Pediatr 1998;133:172–4. [DOI] [PubMed] [Google Scholar]
- 32.Declue JE, Heffelfinger S, Benvenuto G, et al. Epidermal growth factor receptor expression in neurofibromatosis type 1–related tumors and NF1 animal models. J Clin Invest 2000;105:1233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherman LS, Tit R, Rosenbaum T, et al. Single cell Ras–GTP analysis reveals altered Ras activity in a subpopulation of neurofibroma schwann cells but not fibroblasts. J Biol Chem 2000;275:30740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lobie PE, Breipohl W. Growth hormone receptor expression in the rat gastrointestinal tract. Endocrinology 1990;126:299–306. [DOI] [PubMed] [Google Scholar]
- 35.Lobie PE, Mertani H, Morel G, et al. Receptor–mediated nuclear translocation of growth hormone. J Biol Chem 1994;269:21330–9. [PubMed] [Google Scholar]