Abstract
Aims: Spurious platelet counts can be found in acute leukaemias, as a result of the fragmentation of blood cells. Microscopic examination of a blood smear should be performed to detect the presence of these so called pseudoplatelets. When present, the platelet count should be corrected because of the important clinical consequences that a lower platelet count may have in these patients.
Methods: K3EDTA anticoagulated blood was measured on an automated blood cell counter, and a blood smear was made and stained according the May Grünwald–Giemsa method for microscopic observation. A 500 cell/particle differentiation was performed and the automated platelet count was corrected.
Results: The incidence of pseudoplatelets in 169 patients with acute leukaemia was studied. Pseudoplatelets were detected in 43 patients (25.4%), and seven patients (4.1%) were re-classified as having a major bleeding risk (platelet count, < 15 × 109/litre).
Conclusions: Platelets should be determined morphologically in patients with acute leukaemia and a routine screening method for the detection of pseudoplatelets should be developed.
Keywords: pseudoplatelets, platelet counts, acute leukaemia, bleeding risk
Haemorrhagic diathesis is a common manifestation of acute leukaemia and is usually caused by thrombocytopenia.1 Haemostatic disturbances seen in patients with acute leukaemia can be related to changes in vascular function, failure of the haematopoietic system, liver dysfunction, increased fibrinolysis, or disseminated intravascular coagulation (DIC).2 In such patients, a spurious platelet count as a result of red blood cell fragmentation3 or fragmentation of white blood cells4–11 has been reported previously. Stass et al reported a spurious increase in automated platelet counts in a patient with hairy cell leukaemia, with a significantly lower number of platelets being seen when counted microscopically.5 Armitage et al described a patient with acute leukaemia who had artificially raised platelet counts because of circulating fragments of leukaemic cells.6 Malcolm et al reported a case of spurious thrombocytosis in a patient with acute myelocytic leukaemia, who had thrombopenia when the blood smear was viewed under the microscope7; the automated platelet count in this patient was 10 times higher than the microscopic count. Stass et al reported a case of spurious platelet counts caused by cell fragmentation in a patient with a poorly differentiated lymphoma; after chemotherapy the number of platelet-like fragments increased.8 Hammerstrom9 reported a patient with a newly diagnosed acute myeloid leukaemia (AML: FAB M5) with DIC, for which the patient was treated with low dose heparin. Although the platelet count was 129 × 109/litre, the patient developed intracerebral bleeding and died. Staining the blood smears with platelet specific antigen resulted in only 4% positive cell particles, whereas one third of the particles showed the same staining characteristics as the leukaemia cells, indicating their leukaemic origin. Sugimoto et al reported a case of AML (FAB M2) with cell fragments resembling giant platelets positive for myeloperoxidase.10 Finally, Li et al reported a patient with secondary acute monocytic leukaemia with tumour lysis syndrome; numerous fragments of leukaemic cells resulted in a falsely raised platelet count.11 Moreover, apoptotic cells with pycnotic nuclei were seen, suggesting a relation between apoptosis and pseudoplatelets.
In our present study, we investigated 169 patients with leukaemia, both de novo diagnosed and relapsed (table 1). Furthermore we looked at the bleeding tendency of patients with pseudoplatelets who are classified into the risk group for bleeding disorders after correction of the automated platelet count.
Table 1.
Distribution of leukaemia type, age, onset/relapse, and sex in the group of 169 patients
| Mean age (min–max) | Onset/relapse | Male/female | |
| Total | 35 (2–83) | 131/38 | 93/76 |
| ALL | 15 (2–64) | 53/7 | 40/20 |
| AML-M0 | 48 (32–79) | 1/3 | 1/3 |
| AML-M1 | 53 (9–79) | 11/4 | 9/6 |
| AML-M2 | 50 (9–82) | 17/1 | 10/8 |
| AML-M3 | 40 (19–59) | 10/0 | 3/7 |
| AML-M4 | 51 (14–83) | 17/3 | 13/7 |
| AML-M5 | 44 (7–70) | 7/3 | 3/7 |
| AML-M6 | 53 | 1/0 | 1/0 |
| AML-M7 | 26 (2–51) | 2/0 | 0/2 |
| Not specified | 45 (11–75) | 12/16 | 13/15 |
ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia.
“Haemorrhagic diathesis is a common manifestation of acute leukaemia and is usually caused by thrombocytopenia”
To exclude the possibility that pseudoplatelets are an intrinsic result of the slide preparation method, both the correlation of pseudoplatelets with smudge cells and pseudoplatelets with leucocytosis was studied.
MATERIALS AND METHODS
Blood was obtained by venapuncture or by finger prick and anticoagulated with K3EDTA. Within four hours, the blood was measured for complete blood cell count (CBC) and a blood smear was made by the wedge method. The slides were stained according the May Grünwald–Giemsa method. Stained slides were embedded and stored in a dark place at room temperature.
CBC was performed by an automated analyser: H*3 from Bayer (Tarrytown, New York, USA) or Sysmex NE-8000 from Toa (Kobe, Japan). The principle of platelet counting is based on flow cytometry (H*3) or impedance (NE-8000). Both platelets and erythrocytes are counted in the same channel: discrimination between platelets and erythrocytes is made on particle size.
The patients included in our study were diagnosed with acute leukaemia at onset or at relapse and had blast cells in the peripheral blood. Table 1 lists the various types of leukaemia, onset/relapse, age and sex of the patients. The acute leukaemias were further specified according the FAB classification12 with the aid of immunophenotyping and cytochemistry.
A 500 particle differentiation was made of each sample (platelets, pseudoplatelets, leucocytes, and smear cells) and the percentage of pseudoplatelets was calculated. In cases of high white blood cell count, the platelets and pseudoplatelets were counted separately. Arbitrarily, if more than 5% pseudoplatelets were present the smear was considered positive for the presence of pseudoplatelets. These positive samples were also reviewed by an independent expert. Both manual counts were averaged and used to correct the platelet count. We used 15 × 109/litre (the mean of 10 × 109/litre and 20 × 109/litre) as the cut off value for serious bleeding risk, as published previously.13,14
RESULTS
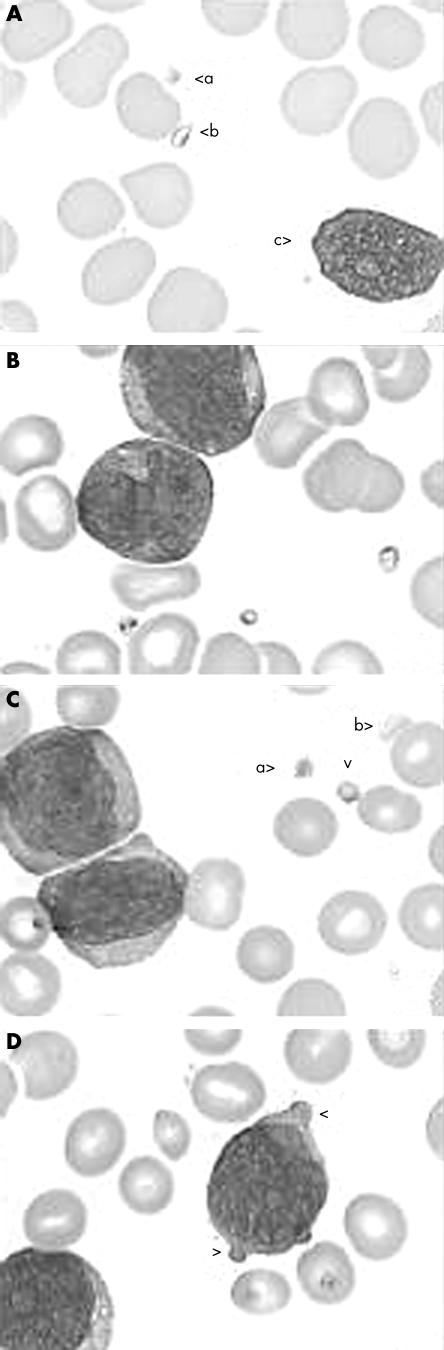
Pseudoplatelets were found in the blood smears of 43 (25%) of the 169 patients: 32 (30%) smears from 107 patients with AML and 11 (18%) smears from 62 patients with ALL (table 2). The morphology of the pseudoplatelets was comparable to agranular platelets, but with a deeply stained cytoplasm in accordance with the cytoplasm of malignant cells (fig 1). Note that in these patients red cell fragments were not found. Of this group of 43 patients, 11 patients were retrospectively thought to be at risk of major bleeding events (table 3). The platelet count obtained with a haematology analyser varied from 10 × 109/litre to 75 × 109/litre in these patients, whereas the corrected platelet counts ranged from 2 × 109/litre to 15 × 109/litre, with a mean difference of 20 × 109/litre or 40%. Two patients with a corrected platelet count of 10 × 109/litre and 3 × 109/litre were not included because of technically poor blood smears.
Table 2.
Incidence of the presence of pseudoplatelets in relation to the different types of leukaemia
| Type | Subtype | Total number of patients | Number of patients with pseudoplatelets | Per cent |
| ALL | 62 | 11 | 18 | |
| AML | 107 | 32 | 30 | |
| AML-M0 | 4 | 1 | 25 | |
| AML-M1 | 15 | 8 | 53 | |
| AML-M2 | 18 | 6 | 33 | |
| AML-M3 | 10 | 3 | 30 | |
| AML-M4 | 20 | 6 | 30 | |
| AML-M5 | 10 | 4 | 40 | |
| AML-M6 | 1 | 0 | 0 | |
| AML-M7 | 2 | 0 | 0 | |
| Not further specified | 27 | 4 | 15 | |
| Total | 169 | 43 | 25 |
ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia.
Figure 1.
Morphological aspects of pseudoplatelets: (A) acute myeloid leukaemia (AML)-M1 showing a true platelet (a) pseudoplatelet (b), and smear cell (c); (B) AML-M5 showing three pseudoplatelets and one blast cell; (C) AML-M2 showing a true platelet (a), two pseudoplatelets (b) and two (pro)blast cells (arrowhead); (D) acute lymphoblastic leukaemia showing pseudoplatelet budding (arrowheads) of a lymphoblast.
Table 3.
Overview of the patients with a corrected platelet count of 15×109/litre or less
| No | Type | Age/sex | Automated platelet count (×109/litre) | Corrected platelet count (×109/litre) (range) |
| 1 | ALL | 43/M | 10 | 2 (1–2) |
| 2 | AML-M1 | 44/M | 24 | 12 (10–13) |
| 3 | AML-M1 | 55/M | 57 | 14 (12–16) |
| 4 | AML-M1 | 9/M | 10 | 6 (5–6) |
| 5 | AML-M1 | 63/M | 28 | 14 (10–18) |
| 6 | AML-M2 | 74/v | 75 | 15 (10–20) |
| 7 | AML-M2 | 46/M | 13 | 8 (7–9) |
| 8 | AML-M2 | 81/v | 48 | 8 (5–10) |
| 9 | AML-M3 | 22/v | 23 | 14 (12–16) |
| 10 | AML-M5 | 56/v | 14 | 8 (7–9) |
| 11 | AML-M5 | 24/M | 21 | 9 (9) |
ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia.
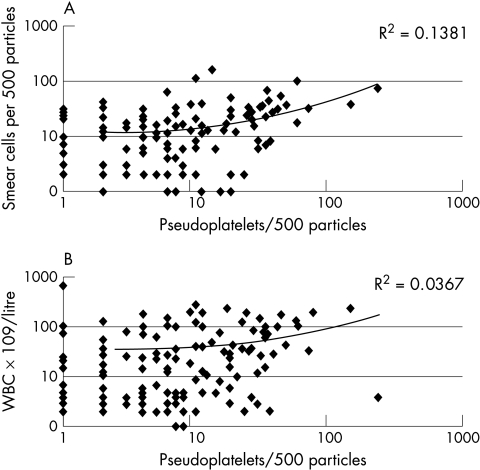
There was no correlation between pseudoplatelets and smudge cells (R2, 0.14) or between pseudoplatelets and leucocytosis (R2, 0.04) (fig 2).
Figure 2.
Correlation between number of (A) pseudoplatelets and smear cells, and (B) pseudoplatelets and total white blood cells (WBC). No correlations were found.
DISCUSSION
Modern haematology analysers are able to produce platelet counts with great precision and accuracy. Even in the very low range (below 15 × 109/litre), the counts are usually reliable. However, in certain cases these analysers produce erroneous platelet results. Pseudothrombopenia is a well known phenomenon and via various procedures (for example, recounting platelets in citrated blood) the laboratory is able to produce a correct result. We report an opposite phenomenon with important clinical implications: pseudothrombocytosis or at least obvious overestimation of the real number of platelets in patients with acute leukaemia. Because of their shape and size, haematology analysers add several undefined particles to the platelet cluster. In some cases, this may even lead to the masking of a (possible life threatening) thrombopenia, and consequently the withholding of proper medication or other crucial supportive measures.
Take home messages.
Pseudoplatelets were found in the blood smears of 43 (25%) of the 169 patients with leukaemia and in almost 5% of the patients this resulted in a re-classification of the bleeding risk
The finding of pseudoplatelets has important consequences for the clinical management of patients with acute leukaemia
Platelets should be determined morphologically in patients with acute leukaemia
There is a need for a routine screening method for the detection of pseudoplatelets
“To establish a proper platelet count, we suggest that the blood smears at presentation and at unexpected bleeding disorders in patients with acute leukaemia (de novo or relapsed) should be examined for pseudoplatelets”
The undefined particles (or pseudoplatelets) are not formed as a result of mechanical destruction during the preparation of the smear. No correlation was found between the number and presence of smudge cells and the number and presence of pseudoplatelets, indicating a different origin.
To establish a proper platelet count, we suggest that the blood smears made at presentation and at unexpected bleeding disorders in patients with acute leukaemia (de novo or relapsed) should be examined for pseudoplatelets. In the presence of pseudoplatelets, the automated platelet count should be corrected. In our present study, the platelet counts had to be corrected in more than 25% of the patients investigated. Because in almost 5% of the patients this resulted in a re-classification of the bleeding risk, the finding of pseudoplatelets has important consequences for the clinical management of patients with acute leukaemia.
Abbreviations
ALL, acute lymphoblastic leukaemia
AML, acute myeloid leukaemia
CBC, complete blood cell count
DIC, disseminated intravascular coagulation
REFERENCES
- 1.Wilde JT, Davies JM. Haemostatic problems in acute leukaemia. Blood Rev 1990;4:245–51. [DOI] [PubMed] [Google Scholar]
- 2.Törnebohm E, Blombäck M, Lockner D, et al. Bleeding complications and coagulopathy in acute leukaemia. Leuk Res 1992;16:1041–8. [DOI] [PubMed] [Google Scholar]
- 3.Savage RA, Lucas FV, Hoffman GC. Spurious thrombocytosis caused by red blood cell fragmentation. Am J Clin Pathol 1983;1:144. [DOI] [PubMed] [Google Scholar]
- 4.Hanker JS, Giamarra BL. Neutrophil pseudoplatelets: their discrimination by myeloperoxidase demonstration. Science 1983;220:415–17. [DOI] [PubMed] [Google Scholar]
- 5.Stass SA, Holloway ML, Slease RB, et al. Spurious platelet counts in hairy cell leukemia. Am J Clin Pathol 1977;68:530–1. [DOI] [PubMed] [Google Scholar]
- 6.Armitage JO, Goeken JA, Feagler JR. Spurious elevation of the platelet count in acute leukemia. J Am Med Assoc 1978;239:433–4. [PubMed] [Google Scholar]
- 7.Malcolm ID, Monks P, Katz M. Spurious thrombocytosis in acute myelocytic leukemia. N Engl J Med 1978;298:1260–1. [DOI] [PubMed] [Google Scholar]
- 8.Stass SA, Holloway ML, Peterson V, et al. Cytoplasmatic fragments causing spurious platelet counts in the leukemic phase of poorly differentiated lymphocytic lymphoma. Am J Clin Pathol 1979;71:125. [PubMed] [Google Scholar]
- 9.Hammerstrom J. Spurious platelet counts in acute leukaemia with DIC due to cell fragmentation. Clin Lab Haematol 1992;14:239–43. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto T, Saigo K, Ryo R. Giant platelet-like cell fragments produced from abnormal promyelocytes in acute myelogenous leukemia. Rinsho Byori 1998;46:182–5. [PubMed] [Google Scholar]
- 11.Li S, Salhany KE. Spurious elevation of automated platelet counts in secondary acute monocytic leukemia associated with tumor lysis syndrome. Arch Pathol Lab Med 1999;11:1111–14. [DOI] [PubMed] [Google Scholar]
- 12.Bennett JM, Catowsky D, Daniel MT, et al. Proposal for the classification of acute leukaemias. Br J Haematol 1976;33:451–8. [DOI] [PubMed] [Google Scholar]
- 13.Beutler E. Platelet transfusions: the 20,000/μL trigger. Blood 1993;81:1411–13. [PubMed] [Google Scholar]
- 14.Wandt H, Frank M, Ehninger G, et al. Safety and cost effectiveness of a 10 × 109/l trigger: a prospective comparative trial in 105 patients with acute myeloid leukemia. Blood 1998;91:3601–6. [PubMed] [Google Scholar]