Abstract
Background: Tangier disease (TD) is the phenotypic expression of rare familial syndromes with mutations in the ABCA1 transporter. TD results in extremely low high density lipoprotein (HDL) cholesterol and reduced low density lipoprotein cholesterol, with normal or mildly increased fasting triglyceride (TG) concentrations. Although there is a close relation between HDL cholesterol values and atherogenesis, the risk of coronary artery disease is variable in TD. Raised fasting or postprandial TG values frequently accompany low HDL cholesterol and can add to the risk of a vascular event.
Aims: To investigate the postprandial TG response in TD.
Patients and methods: Five patients (three homozygotes (HTD) and two heterozygotes (hTD)) from one family were studied. One was defined by DNA analysis as homozygous for a new mutation (C2033A) resulting in truncation of the ABCA1 protein. Their TG concentrations were measured before and four, six, and eight hours after a standardised fat load and compared with a control group.
Results: Two patients with HTD had high fasting TG concentrations. The third patient with HTD, the two with hTD, and the control group had TG concentrations within the reference range. The patients with HTD had increased postprandial peak TG values when compared with those with hTD and controls.
Conclusion: Patients with HTD, with or without fasting hypertriglyceridaemia, may have an increased TG response to a fatty meal. The small number of patients does not allow definitive conclusions to be made. However, postprandial hypertriglyceridaemia could be a reason why some patients with TD develop premature atherosclerosis.
Keywords: postprandial hypertriglyceridaemia, Tangier disease, triglycerides, ABCA1
Tangier disease (TD) is a rare genetic disorder that was first described 42 years ago.1 In 1999, three groups2–4 independently reported that mutations in the ATP binding cassette A1 transporter (ABCA1) are associated with TD. The metabolic hallmark of TD is a combination of extremely low high density lipoprotein (HDL) cholesterol1,5 and reduced low density lipoprotein (LDL) cholesterol concentrations.6 As yet, there is no definitive pattern described for serum triglyceride (TG) levels.
“A greater triglyceride increase postprandially has been reported in patients with coronary artery disease, diabetes mellitus, and arterial hypertension”
A substantial part of our life is spent in the postprandial state. Therefore, several authors7–9 have described fat load tests to evaluate postprandial lipid metabolism. A greater TG increase postprandially has been reported in patients with coronary artery disease (CAD),10 diabetes mellitus,11 and arterial hypertension.12 Despite this evidence, postprandial TG concentrations are often not considered.13
The limited surveys of the literature indicate that there is heterogeneity with regard to CAD risk in TD.14–16 One explanation could be that the extremely low levels of protective HDL cholesterol increase the risk of CAD in spite of the low serum LDL cholesterol concentrations.14–16 Variability in postprandial hypertriglyceridaemia in these patients could also be relevant in terms of CAD risk. Therefore, we assessed the postprandial TG response in three homozygous patients with TD and compared it with that in two heterozygous patients with TD and 25 normal individuals.
PATIENTS AND METHODS
Patients
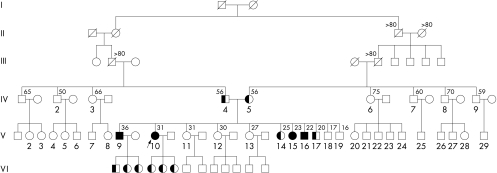
We studied five patients from one Greek family affected by TD, recently described by us.17 In this large six generation family (fig 1), we found no history of premature atherosclerosis, diabetes mellitus, arterial hypertension, or dyslipidaemia. Here, we analyse the proband (C2033A mutation) and four of the 10 siblings. Based on the fact that TD mutations are very rare and that we are dealing with one family, we assume that the C2033A mutation is probably present in each case. However, further studies are required to confirm this assumption.
Figure 1.
Pedigree of the family with Tangier disease. The arrow indicates the proband (individual V10). Individuals IV4 and IV5, the parents of the proband V10, are obligate heterozygotes. The numbers above symbols indicate age. Roman numerals indicate the number of the generation. Solid symbols indicate homozygotes and half filled symbols indicate obligate heterozygotes.
Case 1
This patient (fig 1, V10), the proband, has been described previously.17 She is a 34 year old woman who initially presented with anaemia. Clinical examination only revealed mild hepatosplenomegaly. No enlarged tonsils, pathological lymph nodes, peripheral neuropathy or atherosclerosis were present. Her lipid profile was typical of TD (table 1). DNA analysis revealed a new null mutation (C2033A) in both alleles, causing a premature stop codon at the amino acid residue 573, which resulted in truncation of the ABCA1 protein.
Table 1.
The fasting lipid profile of the patients and controls (mmol/l)
| TC | TG | HDL-C | Apo A1 | Apo B | |
| Proband | |||||
| Case 1 (V10) HTD | 2.0 | 1.6 | 0.05 | 0.5 | 2.2 |
| Siblings | |||||
| Case 2 (V9) HTD | 1.4 | 3.1 | 0.05 | <0.5 | 2.0 |
| Case 3 (V16) HTD | 1.3 | 3.1 | 0.05 | <0.5 | 1.7 |
| Case 4 (V17) hTD | 2.9 | 0.9 | 0.5 | 1.9 | 1.8 |
| Case 5 (V14) hTD | 3.4 | 0.7 | 0.7 | 2.6 | 1.7 |
| Controls | |||||
| Mean (SD) | 5.3 (0.9) | 1.2 (0.3) | 1.3 (0.3) | 3.8 (0.8) | 3.5 (1.3) |
Apo, apolipoprotein; HDL-C, high density lipoprotein cholesterol; HTD, homozygous Tangier disease; hTD, heterozygous Tangier disease; TC, total cholesterol; TG, triglycerides. The “V + a number” abbreviations refer to the family tree shown in fig 1.
Case 2
This patient (fig 1, V9) was a 36 year old, asymptomatic man. His history only revealed a β thalassaemia trait. Clinical examination showed mild hepatosplenomegaly. Enlarged tonsils or enlarged lymph nodes were not present. Neurological examination was normal. Blood tests were normal except for a decreased haematocrit (30%) and thrombocytopenia (87 × 109/litre). His plasma lipid values were typical for homozygous TD (HTD) (table 1).
Case 3
This patient (fig 1, V16) was a 22 year old, asymptomatic man with no relevant medical history. His tonsils appeared normal. His blood tests were normal except for the unusual lipid values (table 1). These findings led to the diagnosis of HTD.
Case 4
This patient (fig 1, V17) was a 20 year old man in good health with normal tonsils, liver, and spleen. His lipid profile was consistent with a diagnosis of heterozygous TD (hTD) (table 1).
Case 5
This patient (fig 1, V14) was a 25 year old woman with normal tonsils, liver, and spleen. No anaemia was found. Her lipid profile was consistent with a diagnosis of hTD (table 1).
All five patients showed no pathological findings on echocardiography and no ischaemic changes were seen in their resting electrocardiograms and exercise tests. They had normal arterial blood pressures and fasting glucose and were not obese. None used drugs and they were not heavy drinkers. Patient 2 was a smoker, case 1 was an ex-smoker; the others had never smoked.
Control group
The control group consisted of 25 healthy men, mean (SD) age 51 (9) years, with no family history of premature atherosclerosis, diabetes mellitus, arterial hypertension, or dyslipidaemia. Their fasting TG values were < 1.7 mmol/litre and their non-HDL cholesterol was <5.2 mmol/litre; all had never smoked.
All participants gave their informed consent and the ethics committee of the Onassis Cardiac Surgery Centre, Athens, Greece, approved the study protocol.
METHODS
Fat loading with a test meal was carried out and plasma TG concentrations were measured. The ingredients of the standardised fatty meal have been described previously.12 It contained 802.1 kcal/m2 of body surface and consisted of 2.5% protein, 14.0% carbohydrate, and 83.5% fat (from heavy whipped cream).
All patients were studied in the outpatient clinic of the Onassis Cardiac Surgery Centre between 8.00–9.00 am after a 12 hour overnight fast. The fatty meal was consumed within 20 minutes and plasma TG concentrations were measured before and four, six, and eight hours after the fat load. During this eight hour period, the participants did not eat; they could only drink water and they did not smoke.
Areas under the curve (AUC) for individual patients and for the control group were calculated using the trapezoid rule. For the control group, values are presented as means (1 SD).
RESULTS
All participants ate the individually calculated fatty meal and tolerated it well. Table 2 and fig 2 show the TG and AUC values of the patients with TD and the controls before and during the postprandial state.
Table 2.
Fasting and postprandial triglyceride concentrations (mmol/l)
| TG 0 | TG 4 | TG 6 | TG 8 | AUC | |
| TD patients | |||||
| Case 1 (V10) | 1.6 | 2.9 | 2.2 | 1.5 | 17.8 |
| Case 2 (V9) | 3.1 | 3.8 | 4.6 | 3.0 | 29.8 |
| Case 3 (V16) | 3.1 | 4.1 | 4.9 | 3.6 | 31.9 |
| Case 4 (V17) | 0.9 | 2.4 | 2.2 | 1.4 | 14.8 |
| Case 5 (V14) | 0.7 | 2.4 | 1.9 | 1.1 | 13.5 |
| Control group | |||||
| Mean (SD) | 1.2 (0.3) | 1.7 (0.4) | 1.8 (0.5) | 1.3 (0.4) | 12.4 |
TG 0, baseline fasting plasma triglyceride (TG) concentrations; TG 4/6/8, plasma TG concentrations four/six/eight hours after the test meal; AUC, area under the curve (mmol/litre/hour). The “V + a number” abbreviations refer to the family tree shown in fig 1.
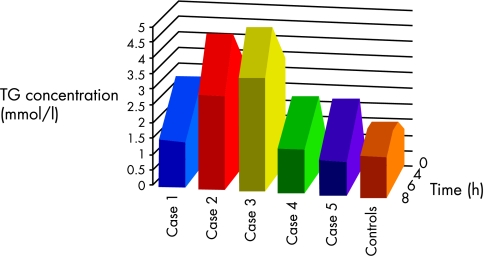
Figure 2.
Schematic representation of triglyceride (TG) concentrations for cases and controls in relation to time.
Fasting TG concentrations (table 1)
Of the three patients with HTD, two (patients 2 and 3) had mild hypertriglyceridaemia, and one (patient 1) had normal values. The patients with hTD and the controls had normal fasting TG concentrations.
Postprandial TG concentration (table 2)
TG values four hours after the fat load
Patients 1, 2, and 3 had greatly increased postprandial TG concentrations compared with the controls. Because of the small numbers of patients we could not carry out a statistical analysis. However, we can express the results of the patients as deviations from the mean value for the controls. Thus, in the controls four hours postprandially, the calculated TG values +1 SD would be 2.1 mmol/litre; the corresponding values +2 SD would be 2.6 mmol/litre, +3 SD 3.0 mmol/litre, +4 SD 3.4 mmol/litre, and +5 SD 3.8 mmol/litre. Therefore, some of the patients had four hour TG values that were up to 5 SD higher than seen in the controls. The TG concentrations of patients 4 and 5 remained within the “normal” range. The highest TG values were seen in patients 2 and 3.
TG values six hours after the fat load
Patients 2 and 3 reached their highest postprandial TG concentrations (more than 6 SD above that seen in the controls) at this time. In the other three patients, there was a decrease in postprandial TG concentrations. The controls had approximately the same TG concentrations as was seen four hours after the fat load.
TG value eight hours after the fat load
The patients and controls reached TG concentrations close to their fasting values.
DISCUSSION
The TD homozygotes, with or without fasting hypertriglyceridaemia, had postprandial TG values well above those seen in the controls. This postprandial response is similar to that reported in patients with CAD.18 The patients with hTD demonstrated a normal postprandial TG response. To our knowledge, this is the first report involving a fat load test in patients with TD.
Although TGs are not found in atheromatous plaques,19,20 postprandial hypertriglyceridaemia is considered to be a vascular risk. TG rich lipoproteins are involved in atherogenesis in several ways, namely: (1) they are carriers of cholesteryl esters to the vessel wall,20 (2) they induce endothelial dysfunction,21 and (3) hypertriglyceridaemia is accompanied by small dense LDL (which is more susceptible to oxidation)22 and low concentrations of HDL.23 Hypertriglyceridaemia may also be involved in the activation of haemostasis,24 and probably provokes the activation of nuclear factor κB, which has been proposed as a mediator of atherogenesis.25
Postprandial hypertriglyceridaemia is probably a consequence of competition between chylomicrons and very low density lipoprotein (VLDL) cholesterol for lipoprotein lipase. Classically, chylomicron clearance occurs in two sequential steps: (1) TG hydrolysis by lipoprotein lipase and (2) uptake of the remnants by the liver. Delay in the second step leads to the accumulation of remnants and is generally thought to be responsible for the increased atherogenic risk associated with postprandial hypertriglyceridaemia. A chylomicron particle must gain access to an unoccupied lipoprotein lipase molecule on the capillary surface of adipose tissue or cardiac and skeletal muscle. Therefore, the TG clearance rate is the result of many variables,26 such as the size of the respective capillary beds, the amount of active lipoprotein lipase, and the number of VLDL particles competing with chylomicrons.
When describing patients with TD, Fredrickson and colleagues1 reported that they have “too high” plasma TG concentrations compared with normal subjects. Later it was confirmed that fasting hypertriglyceridaemia is usual in TD patients.5 Interestingly, the chylomicrons from patients with TD were found to have an unusually low cholesterol content and to leave plasma more slowly than chylomicron remnants from normal subjects.5 However, fasting hypertriglyceridaemia is not present in all patients with TD,14 and there is a lack of data concerning postprandial TG abnormalities in these patients. The metabolic abnormalities associated with TD led us to hypothesise that postprandial hypertriglyceridaemia is probably present in patients with HTD even if fasting hypertriglyceridaemia is absent. The lack of adequate maturation of HDL in TD may prevent the cholesterol ester transport protein dependent transfer of tissue deposited cholesterol from HDL to TG rich lipoproteins and LDL.27 Patients with TD also have a disproportional TG distribution in the LDL fraction, resulting in TG rich LDL.28–30 In a patient with normal TG values (1.4–1.7 mmol/litre), most of the TG (60%) was located in the LDL rather than the VLDL fraction.31 It has been suggested32 that in TD the unusually high concentration of Apo A-II in the VLDL and LDL fractions make those lipoproteins poor substrates for lipoprotein lipase. This may explain the hypertriglyceridaemia seen in many of these patients.32 However, in some patients with TD, despite this abnormality and the accompanying low lipoprotein lipase activity, hypertriglyceridaemia is absent.31
Schaefer and colleagues16 reported that 45% of patients with TD between the age of 35 and 65 years had developed cardiovascular disease. Other workers15 observed cardiovascular disease in 20% of patients with TD and in 44% of those aged 35–65 years (compared with 6.5% in male and 3.2% in female age matched controls). Premature CAD has mostly been described in patients with TD over 40 years old,14,16 and only in a few younger subjects with TD.33,34 Of seven patients with TD who died after the age of 40, three deaths were caused by CAD and two by stroke.15 This observation is of particular interest because there is evidence that both HDL and TG values predict the risk of strokes, in addition to CAD related events.35,36
Take home messages.
Patients with homozygous Tangier disease (TD), with or without fasting hypertriglyceridaemia, often have an increased triglyceride response to a fatty meal
Postprandial hypertriglyceridaemia could be a reason why some patients with TD develop premature atherosclerosis
This abnormality may increase cardiovascular risk in these patients, so that further studies are warranted
“Investigation of low high density lipoprotein syndromes, such as Tangier disease, could contribute to our understanding of reverse cholesterol transport and may open the way for new beneficial treatments”
Although ABCA1 is mainly implicated in cellular lipid efflux and HDL metabolism, additional roles have been described. Analyses of knockout mice and overexpression studies have pointed out the importance of ABCA1 in cellular trafficking of cholesterol and cholinephospholipids, and in total body lipid homeostasis (intestinal cholesterol and fat soluble vitamin absorption and modulation of steroidogenesis). Furthermore, in the absence of ABCA1, compensatory mechanisms involving other members of the family such as MDR1, MDR3, and MRP family members, could become active.37 In addition, when leucocyte ABCA1 is absent, mice develop more advanced and larger atherosclerotic lesions.38 This effect operated independently of HDL concentrations, indicating a potential direct atherogenic action related to an ABCA1 defect.38 These properties may contribute to the observed heterogeneity in the relation between TD and CAD.15
Although a causal relation between low HDL and atherosclerosis has been confirmed,13,39–41 the exact mechanisms involved are not completely understood. Investigation of low HDL syndromes, such as TD, could contribute to our understanding of reverse cholesterol transport and may open the way for new beneficial treatments.
Our study has limitations. We did not perform statistical analysis because of the small number of patients. Furthermore, the mean age of the control group was at least 20 years higher than the patients with TD, but there are many studies suggesting that aging is accompanied by increased postprandial TG values.42 Another limitation is that all the patients with TD were related. Therefore, our observation that patients with homozygous TD had an increased TG response to a fat meal, whereas heterozygotes had normal maximal postprandial TG values, may not be valid for other patients with TD. Whether patients with TD and abnormal postprandial TG responses will benefit from treatment (for example, with a fibrate or fish oils) should be investigated.
In conclusion, some homozygous patients with TD may have postprandial hypertriglyceridaemia. Whether this abnormality increases cardiovascular risk in these patients remains to be established.
Abbreviations
ABCA1, ATP binding cassette A1 transporter
AUC, area under the curve
HDL, high density lipoprotein
hTD, heterozygous Tangier disease
HTD, homozygous Tangier disease
LDL, low density lipoprotein
TD, Tangier disease
TG, triglyceride
VLDL, very low density lipoprotein
REFERENCES
- 1.Fredrickson DS, Altrocehi PH, Avioli LV, et al. Tangier disease. Combined clinical staff conference at the National Institutes of Health. Ann Intern Med 1961;55:1016–31. [Google Scholar]
- 2.Brooks-Wilson A, Marcil M, Clee SM, et al. Mutation in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 1999;22:336–45. [DOI] [PubMed] [Google Scholar]
- 3.Rust S, Rosier M, Funke H, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet 1999;22:352–5. [DOI] [PubMed] [Google Scholar]
- 4.Bodzioch M, Orsó E, Klucken J, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet 1999;22:347–51. [DOI] [PubMed] [Google Scholar]
- 5.Ferrans VJ, Fredrickson DS. The pathology of Tangier disease. A light and electron microscopic study. Am J Pathol 1975;78:101–58. [PMC free article] [PubMed] [Google Scholar]
- 6.Heinen RJ, Herbert PN, Fredrickssos DS, et al. Properties of the plasma very low and low density lipoproteins in Tangier disease. J Clin Invest 1978;61:120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parks EJ. Recent findings in the study of postprandial lipemia. Curr Atheroscler Rep 2001;3:462–70. [DOI] [PubMed] [Google Scholar]
- 8.Funada H, Sekiya M, Hamada M, et al. Postprandial elevation of remnant lipoprotein leads to endothelial dysfunction. Circ J 2002;66:127–32. [DOI] [PubMed] [Google Scholar]
- 9.Bae JH, Bassenge E, Lee HJ, et al. Impact of postprandial hypertriglyceridemia on vascular responses in patients with coronary artery disease. Atherosclerosis 2001;158:165–71. [DOI] [PubMed] [Google Scholar]
- 10.Pirro M, Mauriege P, Tchernof A, et al. Plasma free fatty acid levels and the risk of ischemic heart disease in men: prospective results from the Quebec cardiovascular study. Atherosclerosis 2002;160:377–84. [DOI] [PubMed] [Google Scholar]
- 11.Ginsberg HN, Illingworth DR. Postprandial dyslipidema: an atherogenic disorder common in patients with diabetes mellitus. Am J Cardiol 2001;20; 88:9H–15H. [DOI] [PubMed] [Google Scholar]
- 12.Kolovou GD, Daskalova DCh, Iraklianou SA, et al. Postprandial lipemia and hypertension. J Am Coll Nutr 2003;22:80–7. [DOI] [PubMed] [Google Scholar]
- 13.Executive Summary of the Third Report of the National Cholesterol Educational Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- 14.Burnett JR, Law AJJ, Yeong ML, et al. Severe aortic stenosis and atherosclerosis in a young man with Tangier disease. Am J Cardiol 1994;73:923–5. [DOI] [PubMed] [Google Scholar]
- 15.Serfaty-Lacrosniere C, Civeira F, Lanzberg A, et al. Homozygous Tangier disease and cardiovascular disease. Atherosclerosis 1994;107:85–98. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer EJ, Zech LA, Schwartz DE, et al. Coronary heart disease prevalence and other clinical features in familial high-density lipoprotein deficiency (Tangier disease). Ann Intern Med 1980;93:261–6. [DOI] [PubMed] [Google Scholar]
- 17.Kolovou GD, Wade DP, Sengupta R, et al. Tangier disease with unusual clinical manifestations. Clin Genet 2003;63:323–4. [DOI] [PubMed] [Google Scholar]
- 18.Zhao SP, Liu L, Gao M, et al. Impairment of endothelial function after a high-fat meal in patients with coronary artery disease. Coron Artery Dis 2001;12:561–5. [DOI] [PubMed] [Google Scholar]
- 19.Oliver MF, Davies MJ. The atheromatous lipid core. Eur Heart J 1998;19:16–18. [DOI] [PubMed] [Google Scholar]
- 20.Zilveresmit D. Atherogenesis: a postprandial phenomenon. Circulation 1979;60:473–84. [DOI] [PubMed] [Google Scholar]
- 21.Jagla A, Schezenmeir J. Postprandial triglycerides and endothelial function. Exp Clin Endocrinol Diabetes 2001;109:S533–47. [DOI] [PubMed] [Google Scholar]
- 22.Lamarche B, Tchernof A, Moorjani S, et al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results form the Quebec cardiovascular study. Circulation 1997;95:69–75. [DOI] [PubMed] [Google Scholar]
- 23.Malloy MJ, Kane JP. A risk factor for atherosclerosis: triglyceride-rich lipoproteins. Adv Intern Med 2001;47:111–36. [PubMed] [Google Scholar]
- 24.Jastrzebska M, Przybycien K, Chelstowski K, et al. Increased levels of factor VII, fibrinogen and activity of plasminogen activator inhibitor during postprandial triglyceridemia in patients with ischemic disease confirmed by angiography. Nutr Metab Cardiovasc Dis 1999;9:33–40. [PubMed] [Google Scholar]
- 25.Collins T, Cybulsky MI. NF-κB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest 2001;107:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharrett AR, Heiss G, Chambless LE, et al. Metabolic and lifestyle determinants of postprandial lipemia differ from those of fasting triglycerides: the atherosclerosis risk in communities (ARIC) study. Arterioscler Thromb Vasc Biol 2001;21:275–81. [DOI] [PubMed] [Google Scholar]
- 27.von Eckardstein A, Huang Y, Wu S, et al. Reverse cholesterol transport in plasma of patients with different forms of familial high density lipoprotein deficiency. Arterioscler Thromb Vasc Biol 1995;15:691–703. [DOI] [PubMed] [Google Scholar]
- 28.Greten H, Hannemann T, Gusek W, et al. Lipoproteins and lipolytic plasma enzymes in case of Tangier disease. N Engl J Med 1974;291:548–52. [DOI] [PubMed] [Google Scholar]
- 29.Cheung MC, Mendez AJ, Wolf AC, et al. Characterization of apolipoprotein A-I- and A-II-containing lipoproteins in a new case of high-density lipoprotein deficiency resembling Tangier disease and their effects on intracellular cholesterol efflux. J Clin Invest 1993;91:522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang CS, Alaupovic P, Gregg RE, et al. Studies on the mechanism of hypertriglyceridemia in Tangier disease. Determination of the plasma lipolytic activities, k1 values and apolipoprotein composition of the major lipoprotein density classes. Biochim Biophys Acta 1987;920:9–19. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz G, Fischer H, Beuck M, et al. Dysregulation of lipid metabolism in Tangier-monocyte derived macrophages. Arteriosclerosis 1990;10:1010–19. [DOI] [PubMed] [Google Scholar]
- 32.von Eckardstein A, Chirazi A, Schuler-Lüttmann S, et al. Plasma and fibroblasts of Tangier disease patients are disturbed in transferring phospholipids onto apolipoprotein A-I. J Lipid Res 1998;39:987–98. [PubMed] [Google Scholar]
- 33.Assmann G, Adler ES, Capurso A, et al. The lipoprotein abnormality in Tangier disease. J Clin Invest 1977;59:565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tall AR, Wang N. Tangier disease as a test of the reverse cholesterol transport hypothesis. J Clin Invest 2000;106:1205–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizos E, Mikhailidis DP. Are high density lipoprotein (HDL) and triglyceride levels relevant in stroke prevention? Cardiovasc Res 2001;52:199–207. [DOI] [PubMed] [Google Scholar]
- 36.Rizos E, Mikhailidis DP. Are high-density lipoprotein and triglyceride levels important in secondary prevention—impressions from the BIP and VA-HIT trials. Int J Cardiol 2002;82:199–207. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz G, Langmann T. Structure, function and regulation of the ABC1 gene product. Curr Opin Lipidol 2001;12:129–40. [DOI] [PubMed] [Google Scholar]
- 38.Van Eck M, Bos IST, Kaminski WE, et al. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc Natl Acad Sci U S A 2002;99:6298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wierzbicki AS, Mikhailidis DP. Beyond LDL-C—the importance of raising HDL-C. Curr Med Res Opin 2002;18:36–44. [DOI] [PubMed] [Google Scholar]
- 40.Kolovou GD, Cokkinos DV. Low serum high-density lipoprotein cholesterol and hypolipidemic treatment. Curr Med Res Opin 2002;18:265–8. [DOI] [PubMed] [Google Scholar]
- 41.Sacks FS for the Expert Group on HDL Cholesterol. The role of high-density lipoprotein (HDL) cholesterol in the prevention and treatment of coronary heart disease: expert group recommendations. Am J Cardiol 2002;90:139–43. [DOI] [PubMed] [Google Scholar]
- 42.Cohn JS, McNamara JR, Cohn SEM, et al. Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res 1988;29:469–79. [PubMed] [Google Scholar]