Abstract
Nocardiosis is an uncommon infection caused by the aerobic actinomycete nocardia. Identification of the pathogen is essential for the definitive diagnosis and for an effective treatment. This report describes a case of chronic granulomatous pleuritis caused by nocardia. A 59 year old Japanese man had a history of repeated pyothorax. Right pleural decortication and thoracic drainage were performed. Microbiological examinations of the drained fluid failed to identify a pathogen. Pathological examinations revealed Gram positive filamentous and branching bacilli in the granulomatous lesion of the pleura. Sequencing of the 971 bp 16S ribosomal DNA extracted and amplified from paraffin wax embedded sections identified the microorganism as Nocardia sp. IFM 0860. The patient received sulfamethoxazol/trimethoprim and minocycline. Although the presence of a brain abscess was disclosed by systemic examination, the clinical course has been favourable. In this patient, polymerase chain reaction analysis of 16S ribosomal DNA in pathological specimens was useful in making an accurate diagnosis of nocardiosis and in determining the appropriate treatment.
Keywords: nocardiosis, 16S rDNA, polymerase chain reaction, paraffin wax section
Nocardiosis is an uncommon infection caused by several species of the aerobic actinomycete nocardia.1 The microorganisms are present in house dust and soil. Infection usually causes acute purulent lesions in the lung as a result of inhalation of contaminated dust and soil. A definitive diagnosis of nocardiosis is dependent on isolation of the microorganism from the clinical samples. Because there is a difference in susceptibility to antibiotics among species of nocardia, identification of the species is necessary to determine the appropriate antibiotics. However, the isolation of the microorganism is often difficult by conventional culture methods.2,3 Recently, we encountered a case of chronic granulomatous pleuritis caused by nocardia. The diagnosis of nocardiosis was made by histopathological examination, and the species was identified by polymerase chain reaction (PCR) analysis of 16S ribosomal DNA (rDNA) using paraffin wax embedded sections.
“Because there is a difference in susceptibility to antibiotics among species of nocardia, identification of the species is necessary to determine the appropriate antibiotics”
CASE REPORT
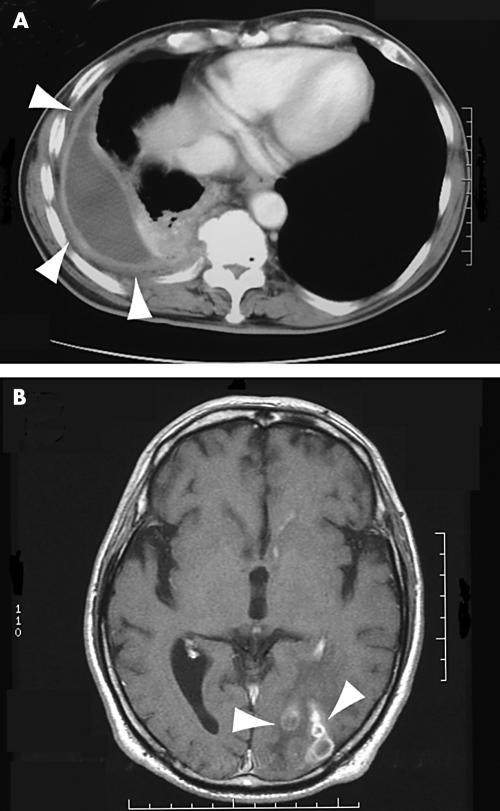
A 59 year old Japanese plumber was referred to the Hirosaki Central Hospital because of pyothorax and sustained high fever. He had a history of repeated bouts of pyothorax, but no possible causative agent was discovered. He had been treated with wide spectrum antibiotics and drainage. Previously, he was once diagnosed as having diabetes, but no medical treatment was undertaken. His family history was not remarkable. The peripheral white blood cell count was 9070/mm3 (neutrophils, 84.3%; eosinophils, 2.6%; basophils, 0.2%; monocytes, 5.2%; and lymphocytes, 7.7%). Red blood cell and platelet counts were normal. The erythrocyte sedimentation rate was increased to 38 mm at one hour, and C reactive protein was increased to 3.99 (normal, < 3.0 mg/litre). The blood glucose concentration was normal, as was the oral glucose tolerance test. A skin test for tuberculin was negative. There was no evidence of immunocompromised status. Computed tomography of the chest revealed thickening of the pleura with effusion in the right thorax (fig 1A). Cytological smears of the pleural effusion demonstrated numerous neutrophils and reactive mesothelial cells. Repeated microbiological examinations by smears and culture of the pleural effusion were negative. The patient was treated with piperacillin at a dose of 1 g/day for five days, but his high temperature continued. The patient then underwent pleural decortication and drainage. The resected pleura was thickened with fibrosis, and there was no apparent neoplastic component. After the operation the patient was treated with flomoxef at a dose of 1 g/day for two weeks. The resected pleura was subjected to pathological examinations, and a diagnosis of nocardiosis was made. At this point, the pleural effusion was cultured in Sabouraud’s dextrose agar for two weeks, but nocardia was not isolated. Treatment with minocycline (200 mg/day) and sulfamethoxazole (1600 mg/day)/trimethoprim (320 mg/day) was then begun. Thereafter, the patient started to complain of a headache. Computed tomography and magnetic resonance imaging of the brain revealed multiple foci suggestive of abscess lesions (fig 1B). Microbiological examinations of the cerebrospinal fluid were again negative for nocardiae. Three weeks later, treatment with sulfamethoxazole/trimethoprim was stopped, because of the appearance of a drug induced skin eruption. The patient is currently being treated with minocycline, and the brain lesions on the image analysis appear to have reduced in size.
Figure 1.
(A) Chest computed tomography scan; (B) brain computed tomography scan.
Pathological findings
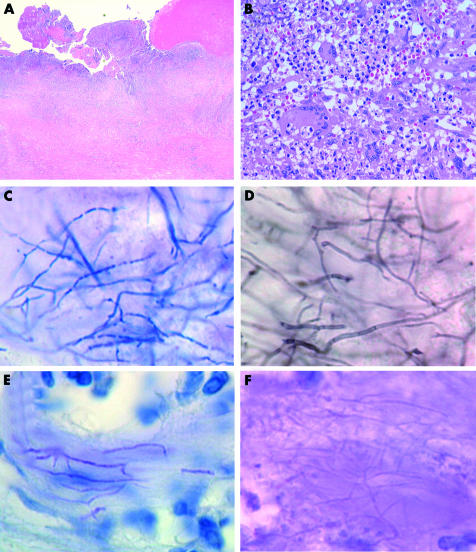
The pleura of the right thorax was fibrous and thickened (fig 2A). There were no neoplastic mesothelial cells. The tissue was granulomatous and there was massive infiltration of lymphocytes, neutrophils, and epithelioid cells with occasional giant cells (fig 2B). Small foci of microabscesses were also present. Gram staining clearly depicted Gram positive bacilli in the granulomatous tissues (fig 2C). They showed filamentous growth with occasional branching. A beaded appearance was also noted. The bacilli were positive using Grocott’s methenamine silver staining, appearing as thin branching hyphae with septae (fig 2D). They were negative on routine acid fast staining by the Ziehl-Neelsen method, but they showed weak acid fast reactivity when stained by the Fite-Faraco method (fig 2E). They were only weakly stained by the periodic acid Schiff method (fig 2F).
Figure 2.
(A) Low power magnification of the pleura (haematoxylin and eosin stain). (B) High power magnification of the granulomatous tissue, depicting the infiltration of giant cells, lymphocytes, and neutrophils (haematoxylin and eosin stain). (C) Gram staining revealed the thin microorganisms with a filamentous growth pattern. (D) The bacilli were positively stained with Grocott’s methenamine silver. (E) The bacilli exhibited weak acid fast reactivity by the Fite-Faraco staining method. (F) The microorganism exhibited only a weak reaction with the periodic acid Schiff stain.
PCR analysis of pathological specimens
Nocardial 16S rDNA genes in the paraffin wax embedded sections were amplified by PCR. To ensure the effective retrieval of bacterial DNA from the paraffin wax embedded sections, extraction was carried out according to the method described by Laurent et al,4 using a modification for the paraffin wax sections. A surgical specimen of bullous emphysema from the same hospital was used as a positive control. Briefly, five 4 µm thick sections were put into 1.5 ml tubes and dewaxed with xylene and ethanol. They were lysed in 500 µl of 10mM Tris/HCl (pH 7.5), 1mM EDTA, 150mM NaCl, 1% sodium dodecyl sulfate, and 800 µg/ml proteinase K and incubated at 56°C. Next, NP-40 and Tween-80 were added to a final concentration of 1%, and the samples were incubated at 100°C for 30 minutes. DNA was purified by phenol/chloroform and precipitated in ethanol.
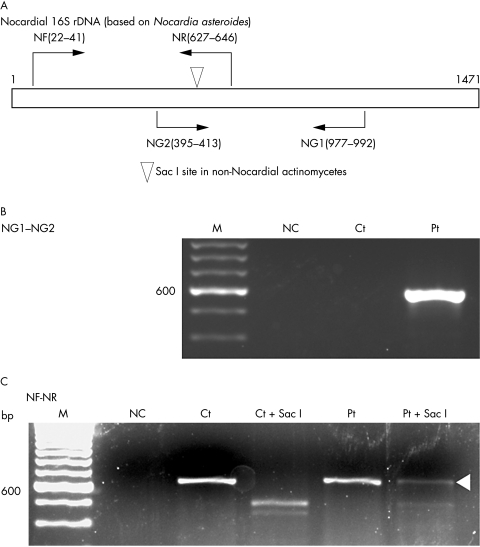
Nocardial 16S rDNA was amplified in two segments (fig 3A). The primers specific for the 16S rDNA of genus nocardia were forward (NG1): 5′-ACC GAC CAC AAG GGG G-3′ and reverse (NG2): 5′-GGT TGT AAA CCT CTT TCG A-3′. The sequences of these primers were reported by Laurent et al.4 They also reported the presence of a Sac I restriction site in this fragment of 16S rDNA of non-nocardial aerobic actinomycetes (fig 3A).4 Another set of primers was designed by the authors based on the sequence of Nocardia asteroides (×80606), and the PCR products of these primers also include the Sac I restriction site (fig 3A). The constructs of these primers were forward (NF): 5′-CGT GCT TAA CAC ATG CAA GT-3′ and reverse (NR): 5′-TTC ACC GCT ACA CCA GGA AT-3′. Two rounds of PCR were carried out. In the first round of the PCR, 200 ng of extracted DNA was used as template and in the second round, 1 µl aliquots of the first round PCR products were used as template. The amplified PCR products were sequenced by the dye terminator direct sequencing method. To differentiate nocardial 16S rDNA, the PCR products amplified with NF and NR were digested with Sac I (New England BioLabs, Beverly, Maryland, USA). Undigested products were also purified and sequenced.
Figure 3.
Polymerase chain reaction (PCR) analyses of 16S ribosomal DNA (rDNA) in pathological specimens. (A) Primer sets used for the amplification of nocardial 16S rDNA. The Sac I restriction site in 16S rDNA from non-nocardial actinomycetes is indicated by the arrow head. The sequence is based on Nocardia asteroides 16S rDNA (GenBank ×80606). (B) PCR amplification with the NG1 and NG2 primers. (C) PCR amplification with the NF and NR primers. M, marker; NC, no template control; Ct, control case; Ct + Sac I, digested with Sac I; Pt, the current patient; Pt + Sac I, digested with Sac I. The white arrowhead indicates the product not digested by Sac I.
The NG1 and NG2 primers, which are specific for nocardial 16S rDNA, amplified single bands of approximately 600 bp in our patient. There was no product in the control case (fig 3B). Sequencing revealed that the products were 598 bp long. The NF and NR primers yielded products of approximately 620 bp, both in our patient and the controls (fig 3C). The products from the control case were completely digested with the Sac I restriction enzyme, whereas non-digested products were present in our patient (fig 3C; white arrowhead). The length of the remaining products was 625 bp. The combined sequence of the above two products was 971 bp in length and matched the sequence from base 28 to 998 of Nocardia sp. IFM 0860 16S rRNA (GenBank/EMBL/DDBJ; Accession number, AB092570).
DISCUSSION
Chronic granulomatous inflammation of the pleura caused by nocardia is rare, although up to a quarter of cases of pulmonary nocardiosis involve the pleura, often presenting as empyema.5–7 In immunocompetent subjects, the infection may run a chronic course and show a granulomatous reaction.8–10 Because tuberculosis, fungal and bacterial infections, and mesothelioma also cause granulomatous lesions in the pleura, a careful differential diagnosis should be made with the aid of histopathological staining. Both Gram staining and Grocott’s methenamine silver staining are useful for the demonstration of nocardia in pathological specimens. In addition, nocardiae show weak acid fast reactivity in the Kinyoun or Fite-Faraco methods.2,11
Take home messages.
We report a case of chronic granulomatous pleuritis caused by nocardia in which an accurate diagnosis was made by polymerase chain reaction analysis of 16S ribosomal DNA in pathological specimens
This enabled the most appropriate and effective antibiotic treatment to be determined and instituted
A definitive diagnosis of nocardiosis is usually dependent on the microbiological isolation of nocardia from clinical samples, such as pleural effusions or pus discharge. Nocardia are not human commensals, and isolation from clinical samples should be regarded as evidence of active infection.3,9 The growth of the microorganism is very slow, and it may be overlooked by overgrowth of other rapidly growing aerobic bacteria in mixed flora.2,3 Prolonged treatment with various antibiotics may make it difficult to culture the microorganism. When isolation of the microorganism is not feasible, alternative means to identify pathogenic nocardia are required.
“Our current case is possibly the first case of disseminated infection of this species, suggesting its high virulence in humans”
PCR analysis of 16S rDNA is a useful tool to identify the species of nocardia.4,12 The application of this method has enabled the identification of nocardia in pathological specimens. The sequencing of 16S rDNA determined the species of nocardia in our current patient. The 971 bp sequence of two segments matched the 16S rDNA gene sequence of Nocardia sp. IFM 0860. The 16S rDNA sequence of this species was recently registered by Kageyama and Mikami. The microorganism was isolated from soil. Based on these sequence structures, our current case is possibly the first case of disseminated infection of this species, suggesting its high virulence in humans. Obviously, further cases are needed to confirm our conclusion and to clarify the pathogenicity of this strain.
Identification of the species of nocardia by 16S rDNA analysis is useful for determining the most appropriate and effective treatment. There are differences in antibiotic susceptibility among species of nocardia.2,13Nocardia sp. IFM 0860 has no significant resistance to antibiotics except for the third generation of cephalosporins (A Kageyama and Y Mikami, personal communication, 2003). Our patient was treated with minocycline after the diagnosis was made and has made an uneventful recovery. It usually takes about two weeks for the microbiological isolation and antibiotic susceptibility test in cases of nocardiosis, but PCR analysis of 16S rDNA can be completed in a few days.
Acknowledgments
The authors are grateful to Miss M Tsujii and Y Nakata for their skilful assistance. We also thank Dr Y Mikami, The Research Centre for Pathogenic Fungi and Microbial Toxicoses, Chiba University, Japan, for providing us with valuable information concerning Nocardia sp. IFM 0860.
Note in proof It has been proposed that Nocardia sp. IFM0860 should be named Nocardia asiatica by Kageyama et al (Int J Syst Evol Microbiol, in press).
Abbreviations
PCR, polymerase chain reaction
rDNA, ribosomal DNA
REFERENCES
- 1.Sorrell TC, Iredell JR, Mitchell DH. Nocardia species. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases, 5 th ed. Philadelphia: Churchill Livingstone, 2000:2637–45.
- 2.McNeil MM, Brown JM. The medically important aerobic actinomycetess: epidemiology and microbiology. Clin Microbiol Rev 1994;7:357–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerner PI. Nocardiosis. Clin Infect Dis 1996;22:891–903. [DOI] [PubMed] [Google Scholar]
- 4.Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevant nocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol 1999;37:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George RB, Penn RL, Kinasewitz GT. Mycobacterial, fungal, actinomycotic, and nocardial infections of the pleura. Clin Chest Med 1985;6:63–75. [PubMed] [Google Scholar]
- 6.Scully RE, Mark EJ, McNeely WF, et al. Case records of the Massachusetts General Hospital. Case 42-1991. N Engl J Med 1991;325:1155–65.1891025 [Google Scholar]
- 7.La Civita L, Battiloro R, Celano M. Nocardia pleural empyema complicating anti-Jo1 positive polymyositis during immunoglobulin and steroid therapy. J Rheumatol 2001;28:215–17. [PubMed] [Google Scholar]
- 8.Ben-Bassat M. Destructive granulomatous lesion of the lung due to Nocardia asteroides in patient with leukemia. Dapim Refuiim 1965;24:372–5. [PubMed] [Google Scholar]
- 9.Rolfe MW, Strieter RM, Lynch JP. Nocardiosis. Semin Respir Med 1992;13:216–33. [Google Scholar]
- 10.Apisarnthanarak A, Razavi B, Bailey T. Disseminated Nocardia asteroides presenting as pulmonary non-caseating granulomas in a patient with Waldenstrom macroglobulinemia. Infection 2002;30:38–40. [DOI] [PubMed] [Google Scholar]
- 11.Robboy SJ, Vickery AL Jr. Tinctorial and morphologic properties distinguishing actinomycosis and nocardiosis. N Engl J Med 1970;282:593–6. [DOI] [PubMed] [Google Scholar]
- 12.Conville PS, Fischer SH, Cartwright, et al. Identification of nocardia species by restriction endonuclease analysis of an amplified portion of the 16S rRNA gene. J Clin Microbiol 2000;38:158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wellinghausen N, Pietzcker T, Kern WV, et al. Expanded spectrum of Nocardia species causing clinical nocardiosis detected by molecular methods. Int J Med Microbiol 2002;292:277–82. [DOI] [PubMed] [Google Scholar]