Abstract
Waterhouse–Friderichsen syndrome—massive adrenal haemorrhage in the setting of overwhelming clinical sepsis—is usually taken at necropsy to indicate meningococcal infection, and may be the only evidence of this pathogen. This report describes three fatal cases of the syndrome in which the causative organism proved to be a streptococcus. The organisms were detected during routine coroners’ autopsies with histology and microbiological investigations. In two cases, the syndrome followed Streptococcus pneumoniae infection and in a third β haemolytic streptococcus group A. Thus, adrenal haemorrhage alone cannot be taken to indicate meningococcal disease and other pathogens, particularly streptococcus, must be considered.
Keywords: Waterhouse-Friderichsen syndrome, adrenal haemorrhage, streptococcus, septicaemia, necropsy
Massive adrenal haemorrhage (Waterhouse–Friderichsen syndrome)1 is an uncommon, but usually fatal, consequence of overwhelming sepsis. In the UK, it is most frequently seen as a result of meningococcal infection,2 and at necropsy often leads to a presumptive diagnosis of meningococcal septicaemia. Despite the predominant association with meningococcal infection, there are numerous other well recognised aetiologies, including sepsis resulting from other organisms, and non-infectious causes, such as anticoagulant treatment, antiphospholipid syndrome, trauma, and spontaneously occurring or postoperative adrenal haemorrhage.3 We report three cases of adrenal haemorrhage as a result of streptococcal infection.
Case 1
Case 1 was a 4 year old, previously healthy boy. There was a short history of cough and malaise, which had also affected other family members. On attending the accident and emergency department he was found to have a fever of 39°C, an erythematous, blanching skin rash, mild pharyngitis, and cervical lymphadenopathy. A diagnosis of viral infection was made and he was sent home. Five days later his condition worsened, with shock and a confluent haemorrhagic rash. His temperature remained high and he was noted to be tachypnoeic. Clotting parameters, including D dimers, were abnormal and his platelet count was low, consistent with disseminated intravascular coagulation. Despite resuscitation, he died.
At necropsy there were signs of upper airway infection and bilateral basal bronchopneumonia, with consolidation. Massive haemorrhage was present in the right adrenal gland, but not the left. There was no evidence of meningitis or haemorrhage elsewhere. Microvascular thrombi were not seen on histology.
The cause of death was given as acute adrenal haemorrhage as a result of meningococcal septicaemia. Family members were given antibiotic prophylaxis and the consultant in communicable diseases was informed. Blood cultures and skin scrapings taken before death were unhelpful. Blood and pleural fluid taken aseptically at necropsy grew a heavy pure growth of β haemolytic streptococcus group A. Other surface swabs also grew streptococcus group A. The isolates typed as the M1 strain and contained genes for toxins A and B (the cause of streptococcal toxic shock syndrome). Polymerase chain reaction for meningococcal DNA was negative.
Case 2
Case 2 was a 64 year old man who died suddenly and unexpectedly at home, with no known preceding illness. He had undergone a laparotomy following abdominal trauma at age 14 years, with splenectomy, and had a history of rheumatoid arthritis treated with methotrexate.
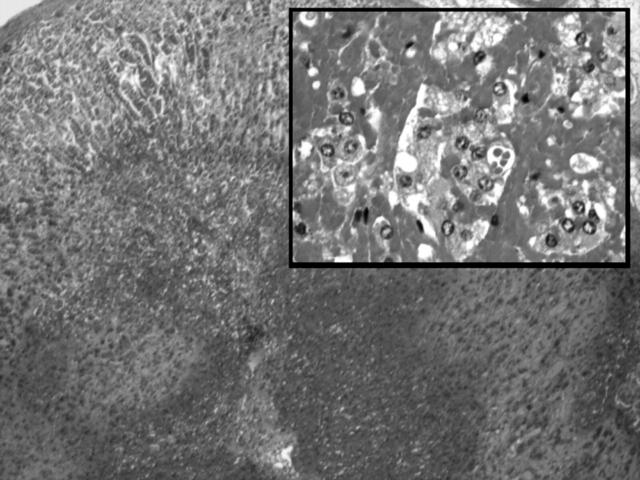
At necropsy a skin rash was noted. The lungs were congested and massive bilateral adrenal haemorrhages were present (fig 1). The spleen was absent and the upper peritoneum was studded with multiple soft splenunculi. The brain showed severe vascular congestion within the choroid plexus, with mild cerebral oedema. There was no evidence of meningitis or haemorrhage elsewhere and microvascular thrombi were not seen on histology.
Figure 1.
Postmortem histology from case 2 showing massive adrenal haemorrhage, low power and (inset) high power. Haematoxylin and eosin stain.
Postmortem blood cultures, taken aseptically, grew a pure growth of S pneumoniae.
Case 3
Case 3 was a 65 year old man, who was previously healthy, had no history of recent illness, but was found dead at home.
Necropsy revealed a large gastric carcinoma with liver metastases, the largest of which had undergone central necrosis and contained pus. The lungs were essentially clear. Bilateral adrenal haemorrhages were present macroscopically and were confirmed by histology. Histology also confirmed metastatic gastric carcinoma. There was no evidence of meningitis or haemorrhage elsewhere and microvascular thrombi were not seen on histology.
A pure heavy growth of S pneumoniae was obtained from pus aspirated aseptically from the liver.
DISCUSSION
In the first of these cases the clinical picture was consistent with Friderichsen’s description of Waterhouse–Friderichsen syndrome, namely: sudden onset shock, pyrexia, cyanosis, dyspnoea, and purpura, with adrenal haemorrhage.4 The other two cases presented as sudden deaths, but both showed evidence of infection and adrenal haemorrhage, for which no other cause could be found.
At necropsy, adrenal haemorrhage with no other predisposing cause is usually recorded as Waterhouse–Friderichsen syndrome, and is often regarded as pathognomonic of meningococcal septicaemia.1 The prudent practice of giving antibiotics as soon as possible in suspected meningococcal disease often means that the causative organism is not isolated. Because the presence of adrenal haemorrhage may be the only indication of meningococcal infection, antibiotic prophylaxis may also be given to close contacts, including the pathologist and mortuary staff.
“It has been proposed that Waterhouse–Friderichsen syndrome is a subtype of severe sepsis and any organism that can induce disseminated intravascular coagulation can lead to adrenal haemorrhage”
However, other Gram negative organisms, including klebsiella,5 pasturella,6 and Haemophilus influenzae,7 have all been reported to precipitate the syndrome. There are fewer reports of Waterhouse–Friderichsen syndrome following infection with Gram positive bacteria. A case of adrenal haemorrhage in an asplenic patient with S pneumoniae infection has been described previously.8 Friderichsen himself described Waterhouse-Friderichsen syndrome after infection with S aureus, and was able to induce adrenal haemorrhage in guinea pigs with anthrax, tetanus, and diphtheria, in addition to S pneumoniae. The association with β haemolytic streptococcus group A is much more unusual, reported in three other cases in the literature.3,9,10
The blood supply of the adrenal gland makes it particularly vulnerable to haemorrhage if there is an increase in adrenal venous pressure. Adrenal haemorrhage can be induced in rabbits by intravenous injection of endotoxin following adrenocorticotrophin priming.11 Adrenocorticotrophin increases adrenal blood flow and adrenalin induces platelet aggregation in the adrenal veins. Therefore, it has been proposed that Waterhouse–Friderichsen syndrome is a subtype of severe sepsis and any organism that can induce disseminated intravascular coagulation can lead to adrenal haemorrhage.8
Our three cases, occurring in two district general hospitals over a three year period, demonstrate that Waterhouse–Friderichsen syndrome may be precipitated by S pneumoniae and β haemolytic streptococcus group A infection. Adrenal haemorrhage alone cannot be taken to indicate meningococcal disease, but rather sepsis, which can potentially be caused by a range of Gram positive or Gram negative pathogens. It is important that this is borne in mind by both histopathologists and microbiologists investigating such deaths. Microbiological sampling at necropsy, for culture or polymerase chain reaction, may prove particularly useful in these cases.
Take home messages.
We report three cases of Waterhouse–Friderichsen syndrome caused by Streptococcus pneumoniae and β haemolytic streptococcus group A infection, not meningococcal infection
Thus, adrenal haemorrhage can be caused by organisms other than meningococcus, and is indicative of sepsis, which can potentially be caused by a range of Gram positive or negative pathogens
This should be borne in mind by both histopathologists and microbiologists investigating such deaths
Microbiological sampling at necropsy, for culture or polymerase chain reaction, may prove particularly useful in these cases
REFERENCES
- 1.Varon J, Chen K, Sternbach GI. Rupert Waterhouse and Carl Friderichsen: adrenal apoplexy. J Emerg Med 1998;16:643–7. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira JG, Borri ML, Menasce S, et al. Acute adrenal haemorrhage: diagnosis, treatment and follow-up. Int Urol Nephrol 1996;28:735–41. [DOI] [PubMed] [Google Scholar]
- 3.Karakousis PC, Page KR, Varello MA, et al. Waterhouse–Friderichsen syndrome after infection with group A streptococcus. Mayo Clin Proc 2001;76:1167–70. [DOI] [PubMed] [Google Scholar]
- 4.Friderichsen C. Waterhouse–Friderichsen syndrome (W.–F. S.). Acta Endocrinol 1955;18:482–92. [DOI] [PubMed] [Google Scholar]
- 5.Hori K, Yasoshima H, Yamada A, et al. Adrenal haemorrhage associated with Klebsiella oxytoca bacteraemia. Intern Med 1998;37:990–4. [DOI] [PubMed] [Google Scholar]
- 6.Ip M, Teo JG, Cheng AF. Waterhouse–Friderichsen syndrome complicating primary biliary sepsis due to Pasturella multocida in a patient with cirrhosis. J Clin Pathol 1995;48:775–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison U, Taylor M, Sheahan, et al. Waterhouse–Friderichsen syndrome without purpura due to Haemophilus influenzae group B. Postgrad Med J 1985;61:67–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccioli A, Chini G, Mannelli M, et al. Bilateral massive adrenal hemorrhage due to sepsis: report of two cases. J Endochrinol Invest 1994;17:821–4. [DOI] [PubMed] [Google Scholar]
- 9.Gertner M, Rodriguez L, Barnett SH, et al. Group A beta-hemolytic streptococcus and Waterhouse–Friderichsen syndrome. Pediatr Infect Dis J 1992;11:595–6. [DOI] [PubMed] [Google Scholar]
- 10.Givner LB. Invasive disease due to group A beta-hemolytic streptococci: continued occurrence in children in North Carolina. South Med J 1998;91:333–7. [DOI] [PubMed] [Google Scholar]
- 11.Levin J, Cluff LE. Endotoxaemia and adrenal hemorrhage: a mechanism for the Waterhouse–Friderichsen syndrome. J Exp Med 1965;121:247–9. [DOI] [PMC free article] [PubMed] [Google Scholar]