Abstract
Background: Vascular endothelial growth factor (VEGF) mediates endothelial cell mitogenesis and enhances vascular permeability. The existence of single or multiple VEGF isoforms and receptors suggests that these proteins may have overlapping but distinct functions, which may be reflected in their cell expression and distribution.
Methods: The localisation of VEGFs A–C and their receptors (VEGFRs 1–3, respectively) in 30 fresh human atherosclerotic arteries, 15 normal uterine arteries, and 15 saphenous veins using immunohistochemistry and western blotting.
Results: Saphenous veins showed no staining for VEGF-B or VEGFR-2. Smooth muscle cells (SMCs) showed the strongest staining for VEGF-A, VEGF-B, VEGFR-1, and VEGFR-2 in all specimens. Conversely, VEGFR-3 and VEGF-C were predominately localised to the endothelial vasa vasorum in normal arteries, whereas medial SMCs showed the strongest staining in atherosclerotic arteries. Western blotting showed variations in VEGF protein localisation, with lower amounts of VEGF-B and VEGF-C in saphenous veins, compared with arterial tissue. Amounts of VEGF-C were lower than those of VEGF-A and VEGF-B in all specimens.
Conclusion: This study provides direct evidence of the presence of VEGF proteins and receptors in human physiology and pathology, with variations in both the amounts of VEGF proteins expressed and their cellular distribution in normal arteries compared with atherosclerotic arteries. The presence of VEGFs A–C and their receptors in normal arterial tissue implies that VEGF functions may extend beyond endothelial cell proliferation. Reduced VEGFR-2 staining in atherosclerotic arteries may have implications for the atherosclerosis process and the development of vascular disease and its complications.
Keywords: VEGF, atherosclerosis, angiogenesis, cardiovascular disease
Vascular endothelial growth factor (VEGF) is a vital determinant of the rate and extent of angiogenesis. Initially thought to be an endothelial cell specific mitogen, it is now clear that the angiogenic properties of VEGF extend beyond the mitogenesis of endothelial cells.1–3 Five VEGF isoforms (A–E) have currently been characterised, differing in their molecular mass and biochemical properties. VEGF-A is secreted by tumour cells and may promote collateral formation in ischaemic cardiac tissue4–5; it is also present in human kidney, lung, liver, and placenta.5–7 VEGF-B mRNA has been detected in a variety of human tissues but is highly expressed in heart and skeletal muscle, in common with VEGF-C, which may be a reflection of the specialist functions of these two VEGF proteins.8,9 In addition, VEGF-C is present in low amounts in ovarian and placental tissue and is released by platelets.9,10 VEGF-C also seems to induce lymphatic (but not vascular) endothelial cell proliferation and vessel enlargement upon interacting with its receptor VEGFR-3.11–12
“It has recently been recognised that angiogenesis may be involved in the pathogenesis of atherosclerosis, thus providing a possible role for vascular endothelial growth factor”
The biological actions of VEGF-A are mediated through two tyrosine receptor kinases, VEGFR-1 and VEGFR-2, and studies of knockout mice have revealed that these receptors are vital for normal embryonic vasculogenesis.13–15 VEGFR-2 is believed to act as a signal transducing molecule upon binding of the appropriate VEGF ligand. The action of VEGFR-2 is thought to be distinct from that of VEGFR-1, as demonstrated by the variation seen in the interaction of ligands with both receptors.13,16 A third receptor, VEGFR-3, is present in mouse embryonic and adult tissues, and mRNA for this receptor is highly expressed in active vascularised tissue.17–19 The exact role of VEGFR-3 in embryonic vasculature is still unknown, and its activities in adult tissue seem to be limited to lymphatic angiogenesis.11,12
It has recently been recognised that angiogenesis may be involved in the pathogenesis of atherosclerosis, thus providing a possible role for VEGF.20–24 For example, increased vasa vasorum in atherosclerotic arteries compared with normal arteries, possibly in response to hypoxia, suggests the possible involvement of angiogenic growth factors such as VEGF,20 and animals treated with VEGF exhibit increased plaque development.24 Nevertheless, the specific biological effect(s) of VEGF and its receptors in atherosclerosis in humans are yet to be clarified, especially in non-coronary vessels, although these probably extend beyond endothelial cell proliferation and vascular angiogenesis. Staining for VEGF-A has been reported in postmortem samples of human coronary atherosclerotic tissue, and normal arteries and veins,21–23 but there are few firm data on VEGF-B and VEGF-C and the VEGFR-3 receptor in these or peripheral arteries burdened by atheroma. Furthermore, we are concerned that (necrotic) postmortem changes may be important artefacts in the tissue localisation of growth factors and their receptors.
To investigate this further, we hypothesised differences in the patterns of cell surface staining of VEGF-A, VEGF-B, and VEGF-C, together with their receptors VEGFR-1, VEGFR-2, and VEGFR-3, respectively, in human atherosclerotic peripheral arteries from patients undergoing elective surgery, compared with the staining pattern in normal arteries and veins. As stated, in wishing to avoid the possible difficulties in extrapolating data from postmortem tissues to “live” tissues (for example, the possibility of necrotic changes to tissues after death), we tested our hypothesis with fresh human tissue obtained at surgery using both immunohistochemistry and western blotting.
MATERIALS AND METHODS
Tissue specimens
After informed consent and ethical committee approval, surgical specimens were obtained from 30 patients (22 men; mean age, 66 years; SD, 5). Fifteen patients had elective surgical repair of abdominal aortic aneurysms (AAA), and from these the tissue obtained was a cross section across the atherosclerotic artery from intima to adventitia. A further 15 patients had carotid endarterectomy: tissue provided was the excised atheroma. Fifteen uterine arteries (mean age, 53 years; SD, 16) and saphenous veins (five men; mean age, 57 years; SD, 11) were obtained from patients after total abdominal hysterectomy (TAH) and varicose vein stripping (VVS) procedures, respectively. This control tissue provided a full cross section of the particular vessel—we do acknowledge the possible artefact of the origin of these normal tissues (from (largely) postmenopausal women). Nevertheless, patients undergoing TAH and VVS procedures were free of arterial disease, as confirmed by careful history and examination in the outpatient clinic. Human full term placenta was used as the control tissue for the immunohistological studies. Immediately after surgical removal, tissue specimens were rinsed in phosphate buffered saline (PBS) and fixed in 4% formalin (Surgipath UK Ltd, Peterborough, UK) for routine paraffin wax embedding. Other tissue was snap frozen in liquid nitrogen. Tissues were serially sectioned at 5 μm on to poly L-lysine coated slides (Surgipath UK Ltd), wrapped in tin foil, and stored at room temperature until required.
Grading
Routine histological staining with haematoxylin and eosin and elastic Van Gieson permitted grading of the tissue independently by two pathologists. The tissue samples were classed as either “normal” (no apparent pathological changes to the arterial or venous wall), “uncomplicated lesions” (fibromuscular intimal thickening with or without fatty deposition, possibly accompanied by some leucocyte infiltration), or “complicated lesions” (features associated with the uncomplicated lesions in addition to pronounced leucocyte infiltration, calcification, plaque rupture, and/or haemorrhage). Only grossly normal uterine arteries and saphenous veins were used in our study. Seven of the carotid artery tissues were classed as being uncomplicated and the remaining eight and all the AAAs were classed as complicated lesions.
Antibodies
Polyclonal rabbit and goat and mouse monoclonal antihuman VEGF-A (manufacturers designation A-20), VEGF-B (C-19), VEGF-C (H-190), VEGFR-1 (Flt-1, H-225), VEGFR-2, Flk-1, C-20), and VEGFR-3 (Flt-4, sc-637) antisera and control peptide were purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA). These antibodies have been used by others.22 Immunostaining of endothelial cells, smooth muscle cells (SMCs), and monocyte/macrophages within the specimens was carried out using mouse monoclonal antihuman Von Willebrand factor (MO616), anti-α actin (MO635), and antihuman CD68 (PG-M1) antibodies, respectively (Dako, Ely, Cambridgeshire, UK). Primary antibodies were visualised with detection systems such as a peroxidase labelled streptavidin biotin secondary detection kit (Dako; LSAB KO679), detecting rabbit, goat, and mouse primary antibodies, bound to the corresponding antigenic cellular protein. Individual antibody titres are given below.
Immunohistochemistry
Paraffin wax embedded sections were dewaxed and dehydrated in graded alcohol (Surgipath Europe Ltd, Peterborough, UK) and endogenous peroxidase activity was quenched with 3% hydrogen peroxide (Sigma Aldrich, Poole, Dorset, UK). Antigenic proteins were unmasked by microwave antigen retrieval using 10mM sodium citrate (Merck UK Ltd, Nottingham, UK). Slides were allowed to cool and non-specific antigenic proteins were blocked with 5% dried milk (Marvel™) in PBS. Primary antibody at a 1/100 dilution was added to the sections and incubated overnight at 4°C. The rabbit or mouse primary antibody reactions were amplified using the Dako LSAB kit (according to the manufacturer’s instructions), or rabbit antigoat peroxidase secondary antibody (PO160; Dako) at a 1/1000 dilution. Slides were rinsed in PBS and the streptavidin–biotin peroxidase complex was visualised using diaminobenzidine (DAB) or 3-amino-9-ethylcarbazole (AEC) (Sigma Aldrich), which resulted in brown or red/pink reaction products, respectively. Sections were counterstained in haematoxylin (Surgipath UK Ltd) and mounted with a coverslip in DPX (Surgipath UK Ltd), or covered with aquaperm (Thermo Shandon Ltd, Runcorn, UK), without dehydrating in alcohol. In each experiment, human placental tissue was used as a positive control, whereas negative controls were achieved by substituting the primary antibody with PBS. The specificity of each antibody was confirmed using serial sections labelled with the same antibody preabsorbed for two hours with a 10-fold concentration of the specific peptide.
Assessment of positive staining
The antibodies used were specific for the various VEGF isoforms and receptors, and crossreactivity between the antibodies was minimal. The staining intensity varied from one section to another and with the different antibodies used. However, because immunohistochemistry is a non-quantitative technique, it is difficult to express the staining intensity as a unit or on a scale as a result of interindividual variations. Consequently, “positive staining” was assessed as the definite and unambiguous presence of brown or red/pink colour (the DAB or AEC reaction product) compared with the negative control. Negative staining was assessed as “absence of colouration” (no visible brown or red/pink colouration) or “no apparent difference” when compared with the negative control, allowing for background and non-cellular staining. This approach to measure tissue staining is widely used.22 In general, positive staining was standardised using human placental tissue as the positive control. Tables 1 and 2 show the proportions of sections staining positively for the different VEGF proteins and receptors.
Table 1.
Sections stained positive for the various vascular endothelial growth factor (VEGF) isoforms
| Tissue type | N | VEGF-A (95% CI) | VEGF-B (95% CI) | VEGF-C (95% CI) | Overall p value |
| Uterine arteries | 15 | 40% (15% to 65%) | 73% (55% to 91%) | 60% (35% to 85%) | 0.18 |
| Saphenous veins | 15 | 33% (9% to 57%) | 0 | 40% (15% to 65%) | 0.02 |
| Atherosclerotic arteries* | 30 | 60% (43% to 77%) | 50% (41% to 59%) | 46% (28% to 64%) | 0.56 |
| Overall p value | 0.18 | <0.001 | 0.53 |
Percentages and 95% confidence intervals (CI) shown in the table correspond to the proportion of sections where positive staining for the various VEGF isoforms was detected. The χ2 test was used to calculate the p value.
Table 2.
Sections stained positive for the various vascular endothelial growth factor receptors (VEGFRs)
| Tissue type | N | VEGFR-1 (95% CI) | VEGFR-2 (95% CI) | VEGFR-3 (95% CI) | Overall p value |
| Uterine arteries | 15 | 27% (5% to 49%) | 53% (28% to 78%) | 80% (60% to 100%) | 0.02 |
| Saphenous veins | 15 | 93% (80% to 100%) | 0 | 80% (60% to 100%) | <0.001 |
| Atherosclerotic arteries* | 30 | 20% (7% to 34%) | 20% (7% to 34%) | 83% (70% to 96%) | <0.001 |
| Overall p value | <0.001 | 0.02 | 0.95 |
Percentages and 95% confidence intervals shown in the table correspond to the proportion of sections where positive staining for the various VEGF receptors was detected. The χ2 test was used to calculate the p value.
Western blotting analysis
Five specimens each of snap frozen uterine arteries, saphenous vein, and complicated and uncomplicated atherosclerotic carotid artery and AAA, trimmed of extraneous connective tissue, were washed in ice cold PBS, then homogenised in lysis buffer (50mM Tris/HCl, pH 7.1, containing 1% Igepal, 0.25% sodium deoxylate, 150mM sodium chloride, 1mM EGT, 1mM PMSF, 1 μg/ml aprotinin, leupeptin, and pepstatin, 1mM activated sodium vanadate, 1mM sodium fluoride; all Sigma Aldrich) with a serrated pestle. Homogenates were left on ice for 30 minutes, and then centrifuged at 1500 ×g for 10 minutes at 4°C. Supernatant protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad, Hemel Hempstead, UK). Equal volumes of transfer buffer (Sigma Aldrich) were added to 200 μg of total protein/tissue type and loaded on to non-reducing 10% sodium dodecyl sulfate polyacrylamide gels for electrophoresis at 50 V, building to 100 V, for two hours. Gels were transferred on to nitrocellulose membranes and the membranes were immunoblotted using the Western Breeze chromogenic detection system, (Novex Electrophoresis, Oxford, UK). This kit consisted of ready to use BCIP/NBT alkaline phosphatase substrate, and a secondary antibody to the rabbit primary antibody. Rabbit primary antibodies to VEGF-A and VEGF-C were used at 1/500 dilutions and the immunoreactive bands were visualised using the Western Breeze detection system, according to the manufacturer’s recommendations. Immunoreactive bands for goat anti-VEGF-B (1/500 dilution) were visualised using goat anti-immunoglobulin alkaline phosphatase (Dako) instead of antirabbit secondary antibody.
Statistical analysis
We are unable to provide a fully quantified hypothesis and thus a power calculation. However, the number of subjects studied (30 patients with atherosclerosis, 15 healthy arteries, 15 varicose veins) is equivalent to, or exceeds, other histological studies of this nature.21,22 Because our data were categorical, we used the χ2 test with two or three degrees of freedom (Minitab Inc, State College, Pennsylvania, USA) to determine significant differences in the proportion of positively stained sections.
RESULTS
VEGF isoforms
There was no overall difference in the general staining of VEGF-A by the three tissue types (table 1), although there was a trend to a difference between saphenous vein and atherosclerotic artery expression (33% v 60%; p = 0.092). The saphenous veins failed to stain for VEGF-B (but did stain for isoforms A and C) and there was no overall difference in VEGF-C staining. Within the arterial tissues, there was also no clear pattern, although the difference in staining for VEGF-A and VEGF-B (40% and 73%, respectively) by uterine arteries just failed to reach significance (p = 0.065).
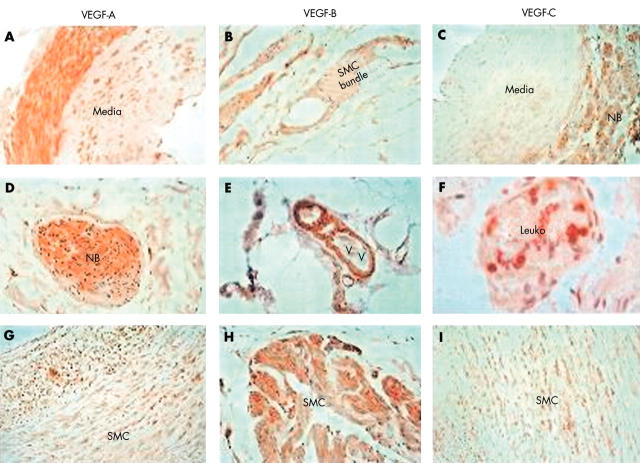
VEGF-A staining by uterine arteries, where present, was greatest in the adventitia (fig 1A), with some staining of the media. Positive morphological identification of the different regions of the tissues (media, intima, and adventitia) was confirmed by low power microscopy. There was also some staining in the adventitia/extracellular matrix (ECM), and occasionally leucocytes and nerve bundles in the adventitia, but very little positive staining of the vasa vasorum or luminal endothelium. Varying degrees of staining were detected in seven of the 15 AAAs and 12 of the 15 carotid atheroma samples (p = 0.136). Staining was also localised to SMCs within the arterial media of the uncomplicated carotid atheroma samples (fig 1G). Conversely, in the AAAs, positive VEGF-A staining was less cellular, with the occasional staining of nerve bundles in the adventitia (fig 1D).
Figure 1.
Immunohistology for vascular endothelial growth factor (VEGF) isoforms. (A–C) Sections of uterine artery. (A) Staining for vascular endothelial growth factor A (VEGF-A): positive staining is localised predominantly to the adventitia, with sporadic staining of the media (original magnification, ×400). (B) Staining for VEGF-B: positive staining in smooth muscle cells (SMCs) within the collagenous tissue in the extracellular matrix (original magnification, ×400). (C) Staining for VEGF-C: localisation of VEGF-C to tissues in the adventitia (original magnification, ×200). (D–F) Sections of abdominal aortic aneurysm (AAA) tissue. (D) Localisation of VEGF-A to a nerve bundle (NB) (surrounded by the perineural sheath) within the adventitia (original magnification, ×400). (E) Localisation of VEGF-B to SMCs around a vasa vasorum (VV) in the adventitia (original magnification, ×400). (F) Localisation of VEGF-C to leucocytes within a vasa vasorum (original magnification, ×1000) but no staining of endothelium or SMCs. (G–I) Sections of carotid atheroma. (G) Localisation of VEGF-A to medial SMCs in diffusely thickened intima excised from an atherosclerotic artery (original magnification, ×200). (H) Localisation of VEGF-B to a bundle of SMC in the media of a complicated atheroma (original magnification, ×400). (I) Diffuse localisation of VEGF-C to the medial SMCs in an uncomplicated atheroma in a similar pattern to VEGF-A (original magnification, ×200).
VEGF-B positive staining was detected in the adventitia and ECM in the normal arterial tissue; SMCs were the major cell type to which VEGF-B was localised, in common with VEGF-A (fig 1B). In the carotid tissue, VEGF-B was detected in 66% of the sections, the staining was prominent in SMCs in the atheromatous tissue excised from complicated atherosclerotic carotid arteries (fig 1H). In contrast, staining was detected in less than 40% of the AAA samples (p = 0.143 compared with carotid artery tissue) and this was mostly within the ECM. However, there was intermittent staining of SMCs surrounding the vasa vasorum in the adventitia (fig 1E) but, unlike the carotid artery atheroma, there was no positive staining of medial SMCs.
VEGF-C staining was not consistent in the uterine artery sections and, when present, was seen mainly in the adventitia and nerve bundles (fig 1C), in addition to the SMC layer and vasa vasorum endothelium. Very little positive staining was detected within the arterial media or on leucocytes, as with VEGF-A. Conversely, VEGF-C staining was localised to medial SMCs of the uncomplicated carotid atheromata (fig 1I), leucocytes, and SMCs of the vasa vasorum but not the endothelial lining, again, as with VEGF-A. Clear positive staining was localised to leucocytes in the blood within the vasa vasorum of AAAs (fig 1F).
VEGF receptors
There was a significant difference in the staining for VEGFR-1 in the three different tissues, with it being seen most frequently in the venous tissue (93%) (table 2). Staining for VEGFR-2 was also very different, with no staining seen in venous tissue, and less staining in atherosclerotic than normal arteries (p = 0.023). Staining for VEGFR-3 was consistent across the tissues. Within the arterial tissue, both uterine and atherosclerosis arteries expressed more VEGFR-3 than VEGFR-1 (p < 0.001 and p = 0.009, respectively).
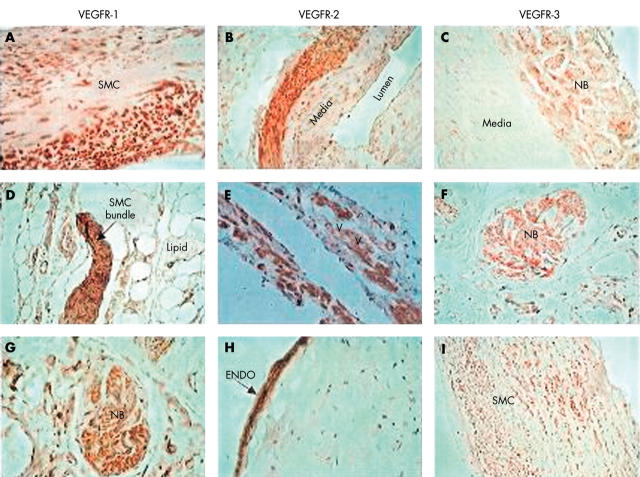
VEGFR-1 staining in the uterine arteries was localised to SMCs in the media, with no staining of luminal endothelium (fig 2A). Faint staining was present in the adventitia, with more intense staining localised to the SMCs of the vasa vasorum. Six of the 15 atherosclerotic carotid artery tissues were positive for VEGFR-1, and it was primarily detected in nerve bundles and ECM in the adventitia, but not the endothelium lining of the vasa vasorum (fig 2G). VEGFR-1 staining was seen in only two of the AAA samples (p = 0.206 compared with carotid tissue), and where present it was predominantly seen in bundles of SMCs in the adventitia (fig 2D).
Figure 2.
Immunohistology for vascular endothelial growth factor receptors (VEGFRs). (A–C) Sections of uterine artery. (A, B) Localisation of VEGFR-1 and VEGFR-2 to medial smooth muscle cells (SMCs) in the arterial wall (original magnification, ×200) with no staining of luminal endothelium. (C) Localisation of VEGFR-3 to small nerve bundles (NB), SMCs, and extracellular matrix (ECM) in the adventitia (original magnification, ×200). (D–F) Sections of abdominal aortic aneurysm (AAA) tissue. (D) Staining for VEGFR-1 is localised to a bundle of SMCs within the adventitia (original magnification, ×400) and (E) localisation of VEGFR-2 to SMC surrounding the vasa vasorum in the adventitia, but no staining of the endothelium (original magnification, ×400). (F) Staining for VEGFR-3 in AAA, red positive staining is localised to a nerve bundle in the adventitia but no staining of the nearby vasa vasorum (original magnification, ×400). (G–I) Sections of carotid atheroma. (G) A section of atheroma from an atherosclerotic artery and localisation of VEGFR-1 to a nerve bundle with some ECM staining but no staining of nearby vasa vasorum (original magnification, ×400). (H, I) Sections of a carotid atheroma stained for VEGFR-2 and VEGFR-3, respectively. Highly positive staining for VEGFR-2 is localised to the luminal endothelial (ENDO) (original magnification, ×400), whereas VEGFR-3 is localised to SMCs in the media and adventitia, with no luminal endothelial staining (original magnification, ×200). In all figures, positive staining by antibodies is indicated by brown or red/pink. The haematoxylin counterstain stains pale blue.
When present, staining for VEGFR-2 in uterine arteries was localised mainly to the adventitial SMCs (fig 2B), with poor medial staining, but the luminal endothelium was not stained. Conversely, strong staining in uncomplicated carotid atheroma was seen in the luminal endothelium (fig 2H) and in the SMCs lining the vasa vasorum within AAA samples (fig 2E).
VEGFR-3 staining in the uterine arteries was localised to the endothelial lining of the vasa vasorum and nerve bundles (fig 2C) in the adventitia/ECM. There was no positive staining of the medial SMCs or luminal endothelium, and very scarce staining of the smooth muscle layer of the vasa vasorum, despite positive staining of the endothelial lining. VEGFR-3 positivity in the atherosclerotic arteries was localised to medial SMCs, with no luminal endothelial staining (fig 2I), and to SMCs and nerve bundles within the adventitia of the AAA samples (fig 2F). Notably, there was staining of the SMCs and the endothelium of the vasa vasorum and the lumen (fig 2F and I).
Costaining of VEGF isoforms and receptors
The most striking pattern in the uterine arteries was the non-overlapping localisation of VEGF-A to the adventitia (fig 1A) and VEGFR-1 to the media (fig 2A). Conversely, both VEGF-C and VEGFR-3 stained the adventitia, but not medial tissues (figs 1C and 2C). Staining of luminal or vasa vasorum endothelium for any of the VEGF isoforms or receptors was rare. Both types of atherosclerotic arteries were characterised by diffuse staining for various VEGF isoforms and receptors in the media or adventitia (for example, fig 1G and I, fig 2I). The only cells that were consistently positive for any of the receptors were the luminal cells, which were always positive for VEGFR-2 (fig 2H). Nerves bundles stained for both VEGF isoforms (fig 1D) and receptors (fig 2F and G).
Western blotting studies
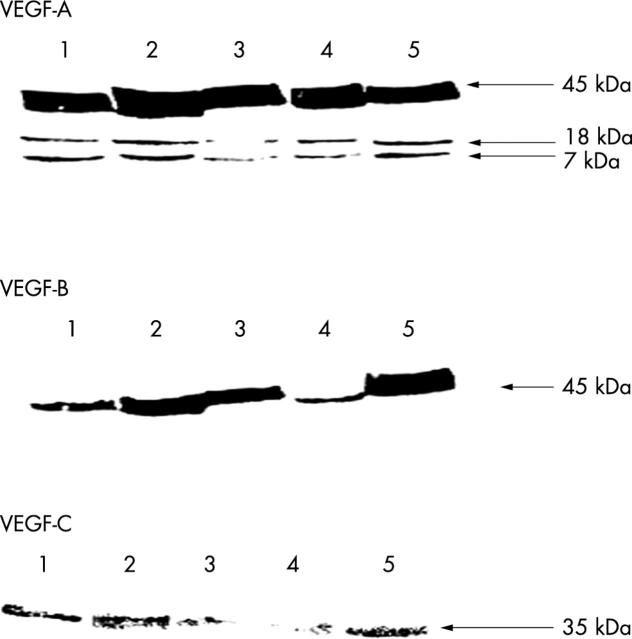
These studies verified the presence of VEGF-A, VEGF-B, and VEGF-C protein in normal vascular tissue and demonstrated variations in the amounts of cellular VEGF-A present, compared with VEGF-B and VEGF-C (fig 3). The antibody to VEGF-A recognised three bands at 45, 18, and 7 kDa. The last two bands may be minor cleaved products. Overall, amounts of VEGF-C protein were considerably lower than that seen for VEGF-A and VEGF-B. VEGF-B and VEGF-C signals were much lower in the saphenous vein samples than in the arterial samples. None of the VEGF receptors was detected by western blotting in the normal or atherosclerotic tissue. This is not because of a failure of the technique because signal was detected in placental tissue (data not shown).
Figure 3.
Western blotting for the vascular endothelial growth factor (VEGF) isoforms. Lanes 1 and 2, complicated carotid atheroma and abdominal aortic aneurysm, respectively; lane 3, uncomplicated carotid atheroma; lane 4, saphenous vein; and lane 5, uterine artery. Aliquots of 200 μg/well of total protein from each tissue type were loaded.
DISCUSSION
Changes in staining for VEGF and its receptors in the tissues, and changes in concentrations in the plasma, are clearly present in atherosclerosis, but it is unclear whether or not tissue changes are important in the pathophysiology of this disease or are postmortem artefacts.20–25 Our study provides additional and confirmatory information about staining for VEGFs and their receptors in normal and atherosclerotic human vascular tissue. Broadly speaking, and using crude quantitative terms, we were unable to find unequivocal distinctions between the overall staining for the VEGF isoforms in the different blood vessels, except the clear absence of VEGF-B in saphenous veins. However, staining for the receptors did show differences, with lower overall staining for VEGFR-1 compared with VEGFR-3 in both normal and atherosclerotic arteries. The only clear distinction between normal and atherosclerotic arteries was the reduced staining for VEGFR-2 by atherosclerotic arteries, and its absence in veins.
VEGF-A was found on SMCs in all the samples examined but VEGF-B was only detected in the arterial tissue. A more specific function of VEGF-B, which shares a receptor with VEGF-A, might be regulating the activity of VEGF-A by competing for receptor binding. This may partly explain the predominance of VEGF-B in uterine arteries and perhaps its low concentrations in saphenous veins, which are unaffected by atherosclerosis. VEGF-A was found only on endothelial cells in uterine arteries, not on atherosclerotic endothelium: antibodies to VEGF-B failed to stain endothelial cells in all the tissues studied. VEGF-C was predominately detected on/in the SMCs and endothelial lining of the vasa vasorum of uterine arteries, with very little positive staining by cells of the media. In contrast, VEGF-C stained leucocytes and SMCs in the media and vasa vasorum in both types of atherosclerotic arteries, but there was no luminal or vasa vasorum endothelial cell staining.
Overall, all VEGF receptors were found on SMCs from all tissues, with minor staining of nerve bundles and ECM. However, the only consistent and strong endothelial cell staining was for VEGFR-2 by the luminal cells of arteries burdened by atheroma. The variation in the staining of VEGFR-3 in the diseased arteries compared with normal tissue suggests that the distribution and possibly function of this receptor can be altered in pathological conditions.26 It is known that conditions such as hypoxia alter the expression of certain VEGF species and VEGFR-2. Because only small amounts of this receptor are found in resting endothelium, upregulation results in increased receptor phosphorylation and, hence, an increase in VEGF activity.27,28 This may account for the presence of VEGFR-2 on the arterial endothelium of atherosclerotic vessels but not normal arteries and, possibly, increased SMC staining of VEGFR-3 by atherosclerotic arteries (compare fig 2C with 2I). Interestingly, VEGF-C binds and activates both VEGFR-2 and VEGFR-3,9,24 so that pathological events that affect receptor properties might also influence that of the receptor ligand, justifying the co-localisation of VEGF-C and VEGFR-3 to SMCs rather than endothelium in atherosclerotic arterial tissue. Because VEGFR-2 is believed to be a major participant in VEGF induced endothelial cell proliferation,5 detection of this receptor on the luminal endothelial in diseased arteries is in agreement with this hypothesis.
“The only clear distinction between normal and atherosclerotic arteries was the reduced staining for vascular endothelial growth factor receptor type 2 by atherosclerotic arteries, and its absence in veins”
Very little is known about the roles of VEGF-C and VEGFR-3 in adult tissue, although preliminary studies indicate that binding of VEGFR-3 to VEGF-C initiates a paracrine regulatory mechanism that may be important in angiogenesis of the lymphatic vasculature.9,11,23,26 In the arterial tissue used in our study, VEGF-C and VEGFR-3 were colocalised to the endothelium in normal tissue, implying that the VEGF-C–VEGFR-3 complex might be involved in more than the coordination of lymphatic angiogenesis, as previously thought.
A limitation of our work is the origin of the normal (healthy) tissues, namely: uterine arteries from women undergoing hysterectomy and saphenous veins removed because of varicosation. It is almost impossible to obtain normal tissues in in vivo studies (rather than postmortem specimens, as in previous work21,23). Thus, despite the normal anatomical appearance of these tissues, we remain cautious in our interpretation. Nonetheless, clear differences in VEGF-B and VEGFR-2 staining are unequivocal, as is the relatively poor staining of endothelium, with the clear exception of VEGFR-2 in atherosclerotic arteries. Notably, we found far fewer sections staining positive for VEGF (up to 60%) than did Couffinhal et al,21 who reported 97% positive staining of atherosclerotic coronary arteries. Some of this difference may result from necrotic changes occurring after death. However, like this group, and Nakagawa et al,23 we also found SMCs to be the predominant cell type staining for VEGF. We also did not quantify neovascularisation within the atherosclerotic plaque pattern of VEGF, or receptor staining in these newly formed blood vessels and how this staining pattern may be related to plaque rupture. Furthermore, we did not attempt to correlate our findings with the severity of the plaque, and indeed, it would be interesting to hypothesise that lesions expressing less VEGFR-2 are more prone to rupture. These limitations reflect working with tissue obtained from elective, stable patients undergoing vascular surgery, rather than from unstable ischaemic syndromes (where plaque rupture is more likely). Indeed, although the atherosclerotic lesions were graded for severity, the results of immunostaining were not broken down by grades of severity, because this was not in our original hypothesis and much larger numbers would have been required. If the pattern of staining for VEGF and its receptors is related to the pathophysiology of progression and complications of atherosclerosis, one might expect a change in pattern and intensity of staining in different degrees of atherosclerosis.
Our present descriptive study offers direct evidence of the presence of VEGF proteins and their receptors in human physiology and pathology. Furthermore, we have shown differences in the degree of cellular staining for these VEGF proteins and variations in their cellular distribution in normal arteries compared with atherosclerotic arteries. This discrepancy may have significant implications for the process of atherosclerosis and the development of vascular disease and its complications. Indeed, a greater understanding of the differential expression of VEGF and its receptors in diseased and normal arteries may allow targeted therapeutic approaches to be developed.
Take home messages.
Vascular endothelial growth factors (VEGFs) type A–C and their receptors were found in normal arterial tissue so that the functions of these factors may extend beyond endothelial cell proliferation
Both the amounts of VEGF proteins expressed and their cellular distribution varied in normal arteries compared with atherosclerotic arteries
Reduced VEGF receptor type 2 staining in atherosclerotic arteries may have implications for the atherosclerosis process and the development of vascular disease and its complications
Acknowledgments
We acknowledge the support of the City Hospital NHS Trust Research and Development Programme for the Haemostasis Thrombosis and Vascular Biology Unit. We thank Mr Z Mzimba and Mr SH Silverman for help with the collection of tissue specimens.
Abbreviations
AAA, abdominal aortic aneurysm
AEC, 3-amino-9-ethylcarbazole
DAB, diaminobenzidine
ECM, extracellular matrix
PBS, phosphate buffered saline
SMC, smooth muscle cell
TAH, total abdominal hysterectomy
VEGF, vascular endothelial growth factor
VEGFR, vascular endothelial growth factor receptor
VVS, varicose vein stripping
REFERENCES
- 1.Risau W. Mechanisms of angiogenesis. Nature 1997;386:671–4. [DOI] [PubMed] [Google Scholar]
- 2.Battegay EJ. Angiogenesis: mechanistic insight, neovascular diseases, and therapeutic prospects. J Mol Med 1995;73:333–46. [DOI] [PubMed] [Google Scholar]
- 3.Dvorak HF, Brown LF, Detmar M, et al. Vascular permeability factor/vascular endothelial growth, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995;146:1029–39. [PMC free article] [PubMed] [Google Scholar]
- 4.Banai S, Jaklitsch MT, Shou M, et al. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation 1994;89:2183–9. [DOI] [PubMed] [Google Scholar]
- 5.Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999;13:9–22. [PubMed] [Google Scholar]
- 6.Ahmed A, Li XF, Duck C, et al. Colocalisation of vascular endothelial growth factor and its Flt-1 receptor in human placenta. Growth Factors 1995;12:235–43. [DOI] [PubMed] [Google Scholar]
- 7.Simon M, Grone HJ, Johren O, et al. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and adult kidney. Am J Physiol 1995;268:F240–50. [DOI] [PubMed] [Google Scholar]
- 8.Olofsson B, Pajusola K, Kaipainen A, et al. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci U S A 1996;93:2576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joukov V, Kaipainen A, Jeltsch M, et al. Vascular endothelial growth factors VEGF-B and VEGF-C. J Cell Physiol 1997;173:211–15. [DOI] [PubMed] [Google Scholar]
- 10.Wartiovaara U, Salven P, Mikkola H, et al. Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost 1998;80:171–5. [PubMed] [Google Scholar]
- 11.Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt-4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996;15:290–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Kukk E, Lymboussaki A, Taira S, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development 1996;122:3829–37. [DOI] [PubMed] [Google Scholar]
- 13.Keyt BA, Nguyen HV, Berleau LT, et al. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem 1996;271:5638–46. [DOI] [PubMed] [Google Scholar]
- 14.Shalably F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in FLK-1 deficient mice. Nature 1995;376:62–6. [DOI] [PubMed] [Google Scholar]
- 15.Fong G, Rossant J, Gartsenstein M, et al. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995;376:67–70. [DOI] [PubMed] [Google Scholar]
- 16.Davis-Smyth T, Chen H, Park J, et al. The second immunoglobulin-like domain of the VEGF tyrosine kinase receptor Flt-1 determines ligand binding and may initiate a signal transduction cascade. EMBO J 1996;15:4919–27. [PMC free article] [PubMed] [Google Scholar]
- 17.Borg JP, deLapeyriere O, Noguchi T, et al. Biochemical characterisation of two isoforms of FLT-4, a VEGF receptor-related tyrosine kinase. Oncogene 1995;10:973–84. [PubMed] [Google Scholar]
- 18.Lee J, Gray A, Yuan J, et al. Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt-4. Proc Natl Acad Sci U S A 1996;93:1988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pajusola K, Aprelikova O, Korhonen J, et al. Flt-4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res 1992;52:5738–43. [PubMed] [Google Scholar]
- 20.Barger AC, Beeuwkes R III, et al. Hypothesis: vasa vasorum and neovascularisation of human coronary arteries. N Engl J Med 1984;310:175–7. [DOI] [PubMed] [Google Scholar]
- 21.Couffinhal T, Kearney M, Witzenbichler B, et al. Vascular endothelial growth factor (VEGF/VPF) in normal and atherosclerotic human arteries. Am J Pathol 1997;150:1673–85. [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue M, Itoh H, Ueda M, et al. Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions. Possible pathophysiological significance of VEGF in the progression of atherosclerosis. Circulation 1998;98:2108–16. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa K, Chen YX, Ishibashi H, et al. Angiogenesis and its regulation: roles of vascular endothelial growth factor. Semin Thromb Haemost 2000;26:61–6. [DOI] [PubMed] [Google Scholar]
- 24.Celletti FL, Waught JM, Amabile PG, et al. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med 2001;7:425–9. [DOI] [PubMed] [Google Scholar]
- 25.Blann AD, Belgore FM, McCollum CN, et al. Vascular endothelial growth factor and its receptor, Flt-1, in the plasma of patients with coronary or peripheral atherosclerosis or type II diabetes. Clin Sci 2002;102:187–94. [PubMed] [Google Scholar]
- 26.Kaipainen A, Korhonen J, Mustonen T, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A 1995;92:3566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandner P, Wolf K, Bergmaier U, et al. Induction of VEGF and VEGF receptor gene expression by hypoxia: divergent regulation in vivo and in vitro. Kidney Int 1997;51:448–53. [DOI] [PubMed] [Google Scholar]
- 28.Walterenberger J, Mayr U, Hombach V. Functional upregulation of vascular endothelial growth factor receptor KDR by hypoxia. Circulation 1996;94:1647–54. [DOI] [PubMed] [Google Scholar]