Abstract
Approximately 20% of patients with systemic mastocytosis (SM) have an associated haematological, clonal, non-mast cell lineage disease, and most exhibit an associated myelogenous neoplasm. This report describes a 48 year old man with acute myeloid leukaemia (AML) and a type t(8;21) cytogenetic abnormality. Associated bone marrow mastocytosis (a defined subtype of SM) was only detected after successful polychemotherapy in the state of bone marrow aplasia, and persisted after complete remission of AML. The diagnosis of mastocytosis was based on the demonstration of a multifocal dense mastocytic infiltrate. The atypical mast cells showed prominent spindling and an aberrant immunophenotype, with coexpression of tryptase, chymase, KIT, and CD25—which is expressed only on neoplastic (not normal) mast cells. In addition, the transforming somatic mutation D816V of the c-kit gene was detected. Re-examination of the pretherapeutic (initial) bone marrow revealed a slight diffuse increase in partially spindle shaped mast cells also exhibiting an abnormal immunophenotype, with CD25 expression, although compact mastocytic infiltrates were not detected. Because the D816V mutation was detected in the initial bone marrow specimen, strict application of three minor diagnostic criteria (spindling, CD25, D816V) enabled a diagnosis of SM-AML to be confirmed retrospectively in the initial bone marrow tissue.
Keywords: mastocytosis, bone marrow, CD25, acute myeloid leukaemia, mast cell tryptase, occult, mastocytosis, c-kit mutation
Systemic mastocytosis (SM) is a neoplasm of multilineage, myelomastocytic, or mast cell committed haemopoietic progenitor cells. In a large proportion of cases, these progenitor cells have the capacity to transform not only into SM, but also into a haemopoietic clonal non-mast cell lineage disease (AHNMD). In approximately 20% of all SM cases, an associated AHNMD is diagnosed (SM-AHNMD). In most patients, myelogenous neoplasms such as myelodysplastic syndromes or acute myeloid leukaemia (AML) occur. It is particularly important for the patient that full diagnosis is established by applying reliable disease criteria. The World Health Organisation (WHO) has recently proposed a new classification for AML and for mastocytosis, which includes disease criteria that may be very helpful in this regard. Here, we present a patient with AML and t(8;21), in whom a coexisting mastocytosis was not detectable at initial diagnosis because of an excessive accumulation of AML blasts obviously masking the compact mast cell infiltrates. After successful chemotherapy, however, a few compact (diagnostic) mast cell infiltrates were detected in the aplastic bone marrow. Applying the full range of diagnostic criteria, a final diagnosis of AML with coexisting systemic (bone marrow) mastocytosis (SM-AML) could be established.
“It is particularly important for the patient that full diagnosis is established by applying reliable disease criteria”
CASE REPORT AND METHODS
Case report
A 48 year old man presented with AML, cytologically classified on bone marrow smears as AML M2 according to FAB criteria, and AML with t(8;21) according to the WHO classification. The complete karyotype was 46, XY, t(8;21;12)(q22; q22; q24), suggesting a secondary aberration in the setting of a t(8;21) translocation. His previous medical history was unremarkable. He received three cycles of chemotherapy with idarubicin and cytosinarabinosid and one cycle of high dose chemotherapy with cytosinarabinosid, administered within a period of six months. The high dose chemotherapy cycle was accompanied by a pseudomembranous colitis, with subsequent surgical resection of the caecum. At the end of the first cycle of chemotherapy, complete remission of the AML could be detected in a bone marrow biopsy specimen. However, an increased number of strongly metachromatic mast cells, sometimes forming dense clusters, was seen in this second biopsy specimen. Accordingly, the diagnosis of bone marrow (systemic) mastocytosis was established. Reviewing the pretherapeutic bone marrow biopsy, the final diagnosis of SM-AML could be established retrospectively. Follow up revealed a stable complete morphological remission of AML, although mastocytosis persisted morphologically for about 18 months. About 20 months after initial biopsy, however, compact mast cell infiltrates were no longer detectable, and there was only a slight increase in loosely scattered mast cells. Only very few of these mast cells expressed CD25. Thus, according to the WHO criteria, a definitive diagnosis of SM was not longer possible.
MATERIAL AND METHODS
Bone marrow smears were routinely stained with Pappenheim stain, myeloperoxidase, non-specific esterase, and naphthol AS-D chloroacetate esterase. The bone marrow specimens were fixed in 5% neutral formalin, mildly decalcified overnight in edetic acid, and embedded in paraffin wax. All slides were routinely stained with haematoxylin and eosin, Giemsa, Prussian blue, and Gömöri’s silver impregnation. Immunohistochemical studies were performed using the avidin–biotin complex (ABC) method with antibodies directed against the antigens listed in table 1. Molecular biological studies were performed by peptide nucleic acid (PNA) mediated polymerase chain reaction clamping and mutation detection with light cycler hybridisation probes, as described previously.1
Table 1.
SM-AML with t(8;21). Immunhistochemical phenotype of mast cells and AML blast cells
| Antigen | CD | Blast cells | Mast cells |
| Chloroacetate esterase | NC | + | + |
| Myeloperoxidase | NC | + | − |
| 3-Fal/Le | 15 | −/+ | − |
| HPCA-1 | 34 | −/+ | − |
| Macro-Si | 68 | −/+ | − |
| Kit | 117 | − | + |
| Tryptase | NC | − | + |
| Chymase | NC | − | + |
| IL-2Rα | 25 | − | +/− |
| LFA-2 | 2 | − | − |
AML, acute myeloid leukaemia; NC, not clustered; SM, systemic mastocytosis.
Bone marrow histology
The initial bone marrow trephine biopsy specimen before treatment showed extreme hypercellularity, with diffuse infiltration by blast cells expressing chloroacetate esterase and myeloperoxidase, whereas antibodies against CD34, CD68, and terminal deoxynucleotidyl transferase were negative (fig 1). Normal blood cell precursors and fat cells were nearly absent. The findings were regarded as consistent with the diagnosis of AML. The second bone marrow biopsy obtained after successful induction polychemotherapy showed bone marrow aplasia, with pronounced stromal oedema and subtotal depletion of the neutrophilic granulocytopoiesis. Blast cells were virtually absent now. However, an increased and focal accumulation of atypical metachromatic cells coexpressing tryptase, CD117, and CD25 was a most remarkable finding, now enabling the diagnosis of bone marrow mastocytosis (fig 2). On re-examination of the pretherapeutic bone marrow biopsy specimen, some inconspicuous, loosely scattered mast cells coexpressing tryptase and KIT (CD117) were detected. Dense (diagnostic) mast cell infiltrates were not present. Furthermore, immunohistochemical analysis revealed an additional strong expression of CD25 on these mast cells, which emphasised their neoplastic nature. Obviously, mastocytosis had already been present in the initial biopsy, but was obscured by the dense, diffuse, blastic infiltrates of AML. The diagnosis of SM-AHNMD, namely SM-AML M2 with t(8;21), could be established retrospectively, even in the initial bone marrow sample. Table 1 depicts the complete immunophenotypic characteristics of mast cells and myeloid blasts. In total, seven subsequent bone marrow trephine biopsy specimens were analysed during a follow up period of 20 months. All these bone marrow biopsies showed a continuing complete morphological remission of AML, although some compact dense aggregates of atypical mast cells expressing CD25 were detectable in the first five follow up specimens. In contrast to AML, bone marrow mastocytosis persisted for about 18 months. However, the last two consecutive trephine biopsies contained no compact mast cell aggregates, but exhibited a slight increase in loosely scattered mast cells, which were mostly negative for CD25. Hence, the diagnosis of morphologic remission of both AML and mastocytosis was then established.
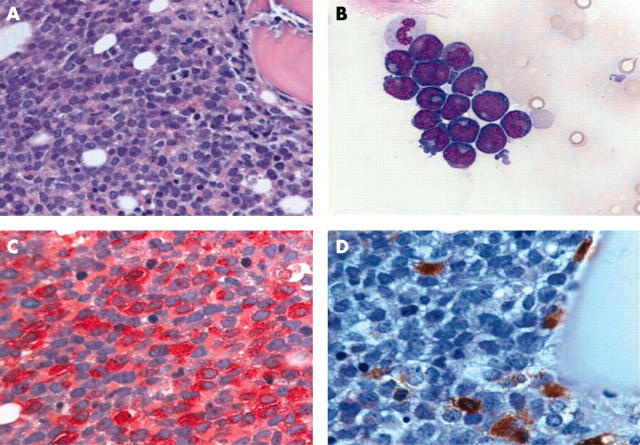
Figure 1.
Acute myeloid leukaemia (AML) with “occult” mastocytosis (SM-AML). (A) Bone marrow section with extreme hypercellularity and subtotal depletion of normal blood cell precursors and fat cells. Sheets of blast cells dominate the picture. Infiltrates of mastocytosis cannot be detected (haematoxylin and eosin stain). (B) Bone marrow smear with a large cluster of medium sized to large blast cells exhibiting pleomorphic, sometimes slightly indented, nuclei and prominent nucleoli (Pappenheim stain). (C) Bone marrow section with chloroacetate esterase staining showing strong expression of this enzyme by blast cells. Note the bright granular cytoplasmic reactivity of almost all the blast cells (naphthol AS-D chloroacetate esterase). (D) Immunostaining with an antibody against tryptase reveals loosely scattered mast cells in the immediate vicinity of a trabeculum, possibly remnants of a distorted dense infiltrate. Note that the blast cells were non-reactive with anti-tryptase (AA1; avidin–biotin complex method).
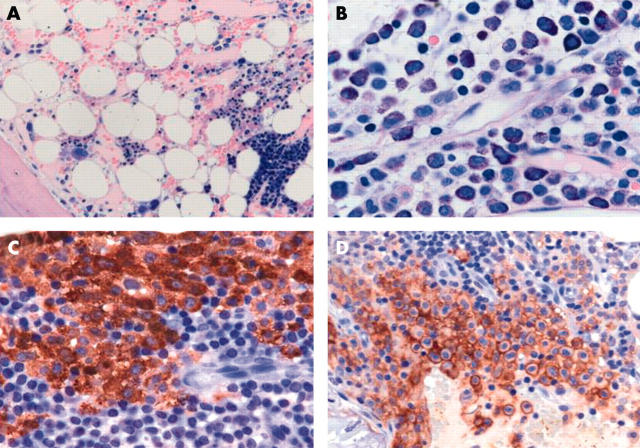
Figure 2.
Acute myeloid leukaemia (AML) with “overt” mastocytosis (SM-AML). (A) Bone marrow section after first cycle of chemotherapy shows overall pronounced stromal oedema, with subtotal aplasia of blood cell precursors. Note a giant erythron in the right lower quadrant (haematoxylin and eosin stain). (B) At this time a few dense infiltrates of strongly metachromatic mast cells intermingled with lymphocytes and macrophages were easily detected (Giemsa stain). (C) One year later, the bone marrow still showed complete remission of the AML, but contained some dense mast cell infiltrates, which were immunopositive using the antibody against tryptase (AA1; avidin–biotin complex (ABC) method). (D) The neoplastic nature of the mast cells is underlined by the demonstration of aberrant coexpression of the antigen CD25, which is not seen on normal/reactive mast cells, even in states of extreme hyperplasia. Note the specific anular (membrane associated) staining of the mast cells (anti-CD25; ABC method).
Molecular findings
Table 2 summarises the results of the molecular analyses. It is of major importance that the initial bone marrow biopsy contained the transforming point mutation at codon 816 (Asp816→Val) of the c-kit protooncogene, a finding that supports the diagnosis of mastocytosis. Although amplifiable DNA was not available from the second and fifth bone marrow biopsy specimens, the specific mutation could be demonstrated in biopsy specimens numbers 3 and 4. Although bone marrow biopsy specimens 6 and 7 both contained compact (diagnostic) mast cell infiltrates, the wild-type configuration of c-kit was found. Moreover, the last trephine biopsy specimen again contained the specific mutation of c-kit (fig 3C), but morphologically diagnostic compact mast cell infiltrates were not detected, although a few mast cells were found to express CD25. The persistent morphological disappearance of AML blast cells after chemotherapy was accompanied by the repeated demonstration of a low number of t(8;21) transcripts (table 2).
Table 2.
SM-AML with t(8;21). Follow up at 20 months, with histological and molecular/cytogenetic findings
| Sample (bone marrow trephine) | AML | Mastocytosis | ||
| Morphological | Cytogenetic t(8;21)* | Morphological | Molecular (c-kit mutation) | |
| 1 (pretherapeutic) | + | 2.47 | +† | + |
| 2 (after induction therapy) | − | 8.25×10−3 | + | No amplification |
| 3 (follow up) | − | 1.01×10−3 | + | + |
| 4 (follow up) | − | 4.98×10−4 | + | + |
| 5 (follow up) | − | 2.11×10−4 | + | No amplification |
| 6 (follow up) | − | 3.57×10−4 | + | WT |
| 7 (follow up) | − | 4.06×10−4 | + | WT |
| 8 (follow up) | − | NT | − | WT |
| 9 (follow up) | − | NT | −/+‡ | + |
*Ratio of AML1-ETO/G6PDH (housekeeping gene); †according to minor World Health Organisation criteria; ‡not sufficient for definite SM diagnosis.
AML, acute myeloid leukaemia; NT, not tested; WT, wild type; SM-AHNMD, systemic mastocytosis with haemopoietic clonal non-mast cell lineage disease; +, present; −, absent.
Figure 3.

c-kit D816V mutation detection by peptide nucleic acid (PNA) mediated polymerase chain reaction clamping and melting point analysis in representative bone marrow samples. (A) Analysis of the pretherapeutic bone marrow trephine (table 2, sample 1). Melting point analysis of total DNA amplified without PNA (PNA–) generated a prominent wild-type specific peak at about 57.5°C. In addition, a strong mutation specific melting peak appears (broken black line). This two peak melting curve is very similar to the HMC-1 control, which is heterozygous for the c-kit mutation D816V, indicating that a large number of cells carry this mutation (broken grey line) in the pretherapeutic sample. Amplification with PNA (PNA+) completely suppresses the wild-type specific peak (continuous black line). (B) A follow up sample (table 2, sample 6) was investigated. Melting point analysis of PNA– amplified DNA generated only the wild-type specific melting curve (broken black line). PNA+ amplification resulted in complete suppression of all amplification products, indicating the virtual absence of mutated cells in this sample (continuous black line). (C) A follow up sample (table 2, sample 9) was investigated. Again the PNA– amplified sample only showed the wild-type specific melting peak (broken black line). In this case, however, melting curve analysis of PNA+ amplification products demonstrated the presence of a few mutated cells in the sample, which were below the detection limit of the PNA– analysis. Amplification of wild-type alleles is again completely suppressed (continuous black line).
DISCUSSION
The revised WHO classification on mastocytosis defines four major variants of systemic mast cell proliferative diseases.2 These entities strongly differ with respect to clinical course, extent of organ involvement, and clinical outcome. In general, systemic variants of mastocytosis are characterised by an abnormal accumulation of mast cells in one or more extracutaneous organs. Urticaria pigmentosa-like skin lesions may be present and are often associated with an indolent course of the disease. In about 20% of patients with SM, an associated clonal haemopoietic disorder is diagnosed.3–9 This observation led to the definition of a new entity termed systemic mastocytosis with associated clonal haemopoietic non-mast cell lineage disorder (SM-AHNMD). Quantitatively, SM-AHNMD assumes an intermediate position between the relatively common indolent SM and the rare aggressive SM. The clinical presentation and outcome in the setting of SM-AHNMD is often more related to the associated haematological neoplasm than to the mastocytosis. In most patients with SM-AHNMD, a myeloid neoplasm can be diagnosed. All major subtypes of myeloid malignancies including myelodysplastic and myeloproliferative syndromes have been described.7,9 The AHNMD should be strictly classified according to the WHO or FAB criteria. Even pure cutaneous forms of mastocytosis (urticaria pigmentosa) have been reported to be associated with haematological malignancies, in particular AML.10,11 To the best of our knowledge, approximately 20 cases of SM-AML have been published. In six of these cases, a t(8;21) translocation is reported.3,8,9,12–18
“Our present case validates the usefulness of the diagnostic guidelines included in the WHO system of classification for mastocytosis, even to detect and define a so called occult mastocytosis”
Here, we present an unusual case in which the final diagnosis of SM-AML could be established only in the second (control) bone marrow biopsy specimen after chemotherapy for AML with t(8;21). Apparently, the complete diagnosis was obscured by a diffuse, compact infiltration of an extremely hypercellular bone marrow by myeloblasts. The cytoreductive treatment disclosed multifocal dense infiltrates of mastocytosis in an aplastic bone marrow. The morphological diagnosis in this trephine specimen is that of an isolated bone marrow mastocytosis, which, by definition, belongs to the SM group.
Ideally, the correct diagnosis of SM-AML would have been possible even in the initial biopsy diffusely infiltrated by AML, although compact mast cell infiltrates as the one and only major diagnostic criterion for mastocytosis were not detected. However, two minor diagnostic criteria were fulfilled morphologically, namely spindling in more than 25% of the loosely scattered mast cells and the proof of CD25 expression by mast cells, suggesting their neoplastic nature. Because the transforming point mutation D816V was also detected, three of a total of four minor diagnostic criteria were met, thus enabling the definitive diagnosis of mastocytosis. Our present case validates the usefulness of the diagnostic guidelines included in the WHO system of classification for mastocytosis, even to detect and define a so called occult mastocytosis.
Take home messages.
We describe a 48 year old man with acute myeloid leukaemia (AML) and a type t(8;21) cytogenetic abnormality who had associated bone marrow mastocytosis (SM-AML) that was only detected after successful polychemotherapy in the state of bone marrow aplasia, and persisted after complete remission of AML
The diagnosis of mastocytosis was based on the dense infiltrate of atypical mast cells, which expressed CD25 (expressed only on neoplastic (not normal) mast cells) and exhibited the transforming somatic mutation D816V of the c-kit gene
Re-examination of the initial biopsy enabled a diagnosis of SM-AML to be confirmed retrospectively in the initial bone marrow tissue
According to Pullarkat et al, patients with associated SM and AML t(8;21) have a worse prognosis with standard chemotherapy protocols than patients with AML t(8;21).9 However, our patient is still in complete haematological remission after two years of follow up, although the polychemotherapy was much more effective (and gave a much faster response) on the AML than the mastocytosis, which persisted morphologically for a period of at least 18 months, whereas the AML showed immediate complete remission after induction phase chemotherapy. In this respect, our case confirms the experiences reported by others,17–19 and underlines the resistance of neoplastic mast cells to conventional chemotherapy. The delayed decrease of mast cell infiltration in response to chemotherapy may be explained by the fact that treatment resulted in the depletion of mast cell progenitors but not of mature mast cells. In fact, mast cells are long lived cells and any effect of chemotherapy on their immature progenitors must be expected to lead to a reduction in mast cell numbers only after several months. In this regard, it is also of interest that the last bone marrow biopsy specimen to be analysed contained the transforming D816V mutation, whereas the morphological findings were regarded as not sufficient for a diagnosis of persistent mastocytosis. In fact, this last bone marrow biopsy showed a nearly normal microarchitecture, with intact haemopoiesis and a diffuse moderate increase in loosely scattered mast cells, about 15% of which had a spindle shape appearance and less than 5% of which expressed CD25. These findings may best be interpreted as minimal residual mastocytosis.
Acknowledgments
This work was supported in part by a grant to KS from the University of Tübingen (fortüne 1067-0-0).
Abbreviations
AHNMD, associated haematological clonal non-mast cell lineage disease
AML, acute myeloid leukaemia
PNA, peptide nucleic acid
WHO, World Health Organisation
REFERENCES
- 1.Sotlar K, Escribano L, Landt O, et al. One-step detection of c-kit point mutations using peptide nucleic acid-mediated polymerase chain reaction clamping and hybridization probes. Am J Pathol 2003;162:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valent P, Horny HP, Li CY, et al. Mastocytosis. In: Jaffe ES, Harris NL, Stein H, et al, eds. World Health Organisation classification of tumours. Pathology and genetics of haematopoietic and lymphoid tissues. Lyon: IARC Press, 2001:293–302.
- 3.Travis WD, Li CY, Yam LT, et al. Significance of systemic mast cell disease with associated hematologic disorders. Cancer 1988;62:965–72. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence JB, Friedman BS, Travis WD, et al. Hematologic manifestations of systemic mast cell disease: a prospective study of laboratory and morphologic features and their relation to prognosis. Am J Med 1991;91:612–24. [DOI] [PubMed] [Google Scholar]
- 5.Horny HP, Ruck M, Wehrmann M, et al. Blood findings in generalized masto-cytosis: evidence of frequent simultaneous occurrence of myeloproliferative disorders. Br J Haematol 1990;76:186–93. [DOI] [PubMed] [Google Scholar]
- 6.Sperr WR, Horny HP, Lechner K, et al. Clinical and biologic diversity of leukemias occurring in patients with mastocytosis. Leuk Lymphoma 2000;37:473–86. [DOI] [PubMed] [Google Scholar]
- 7.Sperr WR, Horny HP, Valent P. Spectrum of associated clonal hematologic non-mast cell lineage disorders occurring in patients with systemic mastocytosis. Int Arch Allergy Immunol 2002;127:140–2. [DOI] [PubMed] [Google Scholar]
- 8.Pullarkat VA, Pullarkat ST, Calverley DC, et al. Mast cell disease associated with acute myeloid leukemia: detection of a new c-kit mutation Asp816His. Am J Hematol 2000;65:307–9. [DOI] [PubMed] [Google Scholar]
- 9.Pullarkat VA, Bueso-Ramos C, Lai R, et al. Systemic mastocytosis with associated clonal hematological non-mast-cell lineage disease: analysis of clinicopathologic features and activating c-kit mutations. Am J Hematol 2003;73:12–17. [DOI] [PubMed] [Google Scholar]
- 10.Cooper AJ, Winkelmann RK, Wiltsie JC. Hematologic malignancies occurring in patients with urticaria pigmentosa. J Am Acad Dermatol 1982;7:215–20. [DOI] [PubMed] [Google Scholar]
- 11.Shaw DW, Hocking W, Ahmed AR. Generalized cutaneous mastocytosis and acute myelogenous leukemia. Int J Dermatol 1983;22:109–12. [DOI] [PubMed] [Google Scholar]
- 12.Horny HP, Parwaresch MR, Lennert K. Bone marrow findings in systemic mastocytosis. Hum Pathol 1985;16:808–14. [DOI] [PubMed] [Google Scholar]
- 13.Sperr WR, Walchshofer S, Horny HP, et al. Systemic mastocytosis associated with acute myeloid leukaemia: report of two cases and detection of the c-kit mutation Asp-816 to Val. Br J Haematol 1998;103:740–9. [DOI] [PubMed] [Google Scholar]
- 14.Beghini A, Cairoli R, Morra E, et al. In vivo differentiation of mast cells from acute myeloid leukemia blasts carrying a novel activating ligand-independent C-kit mutation. Blood Cells Mol Dis 1998;24:262–70. [DOI] [PubMed] [Google Scholar]
- 15.Safyan EL, Veerabagu MP, Swerdlow SH, et al. Intrahepatic cholestasis due to systemic mastocytosis: a case report and review of literature. Am J Gastroenterol 1997;92:1197–2000. [PubMed] [Google Scholar]
- 16.Aravindan KP, Jose L. Ovarian cancer, acute leukemia and systemic mastocytosis. A case report. Indian J Pathol Microbiol 1990;33:76–8. [PubMed] [Google Scholar]
- 17.Wong KF, Chan JKC, Chan JCW, et al. Concurrent acute myeloid leukemia and systemic mastocytosis. Am J Hematol 1991;38:243–44. [DOI] [PubMed] [Google Scholar]
- 18.Chen TY, Chen JS, Huang WT, et al. Rapid engraftment of mast cells of donor origin in a case of acute myeloid leukemia with mast cell leukemia after allogeneic stem cell transplantation. Bone Marrow Transplant 2003;32:111–14. [DOI] [PubMed] [Google Scholar]
- 19.Worobec AS, Semere T, Nagata H, et al. Clinical correlates of the presence of the Asp816Val c-kit mutation in the peripheral blood mononuclear cells of patient with mastocytosis. Cancer 1998;83:2120–9. [DOI] [PubMed] [Google Scholar]