Abstract
Aims: To assess the accuracy and precision of measuring haemoglobin A2 by high performance liquid chromatography (HPLC) in the presence and absence of sickle cell trait, with or without α thalassaemia trait.
Methods: The haemoglobin A2 percentage and the haemoglobin A2 plus S percentages were determined by HPLC on 82 normal controls and 78 patients with sickle cell trait, respectively; the α thalassaemia status of each patient was determined by polymerase chain reaction. Red cell indices and haemoglobin A2 and S percentages were compared in patients with two, three, or four α genes.
Results: Of the 78 patients with sickle cell trait, 17 were heterozygous for α+ thalassaemia (−α3.7/αα) and 13 were homozygous (−α3.7/−α3.7). Microcolumn chromatography showed that the haemoglobin A2 percentage was slightly, but significantly, higher than normal in sickle cell trait. HPLC determinations of haemoglobin A2 percentage in patients with sickle cell trait are precise but inaccurate, the percentage being appreciably overestimated. The measured haemoglobin A2 percentage is stable for one week, but inaccuracy increases by two weeks in most samples. Despite this inaccuracy, there are significant differences in the HPLC “haemoglobin A2 percentage” between groups of individuals with two, three, and four α genes.
Conclusions: Haemoglobin A2 determinations by HPLC are precise but inaccurate. Nevertheless, there are significant differences in the haemoglobin A2 percentage in subjects with two, three, and four α genes. Although there is some overlap between groups, this can be useful, together with the red cell indices, in predicting the likelihood of coexisting α thalassaemia.
Keywords: high performance liquid chromatography, haemoglobin A2, haemoglobin S, α thalassaemia
Haemoglobin A2 can be measured by several laboratory methods of varying accuracy and precision. The principal reason for measuring A2 is for the diagnosis of β thalassaemia trait and, for this purpose, methods that are considered sufficiently precise for diagnosis include cation exchange high performance liquid chromatography (HPLC), microcolumn chromatography, and cellulose acetate electrophoresis with elution. Cellulose acetate electrophoresis followed by scanning densitometry is thought to be too imprecise for this purpose. Although the diagnosis of β thalassaemia trait is the main reason for the measurement of haemoglobin A2, the A2 percentage is also affected by other factors. Both iron deficiency and α thalassaemia trait are known to lower the percentage, whereas the percentage has been reported to be increased in individuals with sickle cell trait,1 and in compound heterozygotes for βS and β0 thalassaemia.2 In contrast to patients with βS/β0 thalassaemia, homozygotes for βS have generally been reported to have a normal haemoglobin A2 percentage,2 and it is thought that this may be of some use in diagnosis.
“The degree of inaccuracy of haemoglobin S and A2 percentages by high performance liquid chromatography and whether this interferes with their diagnostic usefulness has not been determined previously”
The haemoglobin S percentage is usually measured to distinguish between sickle cell anaemia or sickle cell/β0 thalassaemia (haemoglobin S usually greater than 90%), sickle cell/β+ thalassaemia (haemoglobin S more than 50%), and sickle cell trait (haemoglobin S less than 50%); making these distinctions does not require a particularly precise assay because differences are usually clear cut. In addition, the percentage of haemoglobin S may be useful in predicting whether an individual with sickle cell trait has coexisting α thalassaemia trait. A lower haemoglobin S percentage is seen if one of the four α genes is deleted and lower still if two of the four α genes are deleted. This results from the negatively charged βA having a greater affinity than the positively charged βS for the reduced amount of positively charged α chain present. For indicating the likely presence of α thalassaemia trait, the assay should be more precise because otherwise increasing overlap between groups will probably occur.
Previous observations on haemoglobin S and A2 percentages have generally been based on cellulose acetate electrophoresis and microcolumn chromatography. However, increasingly, normal and variant haemoglobins are being measured by HPLC. This technique has been shown, in a small number of patient samples, to overestimate the haemoglobin A2 percentage in the presence of haemoglobin S.3 This is because some haemoglobin S that has undergone post-translational modification has the same retention time as haemoglobin A2, and thus is measured along with it. In addition, glycosylated haemoglobin S has the same retention time as haemoglobin A, so that it is also measured with haemoglobin A when both haemoglobins are present. The degree of inaccuracy of haemoglobin S and A2 percentages by HPLC and whether this interferes with their diagnostic usefulness has not been determined previously. Therefore, we studied the accuracy and precision of estimates of the haemoglobin A2 percentage by three methods in patients with haemoglobin S, and also studied the effect of aging of the sample on any inaccuracy.
In addition, we determined the α gene status of the patients with sickle cell trait and related the S and A2 percentages, as determined by different techniques, to the number of α genes present.
MATERIALS AND METHODS
All investigations were performed on EDTA anticoagulated venous blood samples, either from healthy volunteers or from patients in whom haemoglobinopathy investigations had been requested. In the case of patient samples, that part of the blood sample remaining when all necessary diagnostic tests had been performed was used for our study.
A full blood count and HPLC analysis were performed on the samples from healthy volunteers, mainly of northern European origin. Data derived from volunteers with normal haemoglobin concentrations and red blood cell indices were used to establish a reference range for the haemoglobin A2 percentage.
Patient samples were studied by HPLC on a Bio-Rad Variant 2 instrument (Bio-Rad Laboratories, Hemel Hempstead, Hertfordshire, UK) using the β thalassaemia short programme. Haemoglobins A, A2, and S were measured by microcolumn chromatography using a Helena Sickle-Thal QUIK column kit. Cellulose acetate electrophoresis at pH 8.2–8.6 was performed on Helena Titan III cellulose acetate plates and haemoglobins A, S, and A2 were then measured by scanning densitometry. A sickle solubility test was performed on all samples with variant haemoglobin, using Lorne Sicklechek. A full blood count was performed on a Coulter Gen S automated counter. Serum ferritin was estimated when red blood cell indices suggested possible coexisting iron deficiency. Patient samples were considered to contain haemoglobin S when there was a variant haemoglobin with an appropriate retention time and the sickle solubility test was positive. A diagnosis of sickle cell trait was made if patients had both haemoglobin A and haemoglobin S, with the proportion of haemoglobin A being greater than that of haemoglobin S. A diagnosis of sickle cell anaemia was made only if haemoglobins S, F, and A2 were present and if the mean cell volume and mean cell haemoglobin were normal or near normal, thus excluding S/β0 thalassaemia compound heterozygosity.
All patients with sickle cell trait were investigated by the polymerase chain reaction (PCR)4 to identify heterozygosity (−α3.7/αα) or homozygosity (−α3.7/−α3.7) for −α3.7, the α+ thalassaemia determinant which is present in about 25–30% of Afro-Caribbeans (1–2% being homozygotes). A similar or higher frequency of this variant is seen in West Africans, who comprised most of our African patients.
Haemoglobin S and A2 percentages and red blood cell indices were compared in patients with sickle cell trait with and without α thalassaemia trait, and an algorithm was devised to help distinguish those with α thalassaemia trait from those with four α genes.
RESULTS
We studied 82 healthy volunteers and 78 patients with sickle cell trait, with or without α thalassaemia trait. PCR analysis showed that 17 of the patients were heterozygous for α+ thalassaemia (−α3.7/αα) and 13 were homozygous (−α3.7/−α3.7). One patient with red blood cell indices strongly suggestive of thalassaemia trait did not have either β thalassaemia or −α3.7, and further investigation did not disclose α0 thalassaemia; it was thought that he probably had some type of α thalassaemia, possibly non-deletional, but because this could not be determined he was excluded from further analysis. The remaining 47 patients were assessed as likely to have four α genes because other α thalassaemia determinants are uncommon in this population. Four of the patients with sickle cell trait and normal α genes were found to have coexisting iron deficiency anaemia.
The data for haemoglobin A2 percentages in 82 healthy volunteers with normal red cell indices showed a Gaussian distribution. The observed range of haemoglobin A2 was from 2.4% to 3.3%, with a mean of 2.8%, and a 95% range of 2.4–3.2%. This compares with a range of 2.3–3.5% previously derived in our laboratory using microcolumn chromatography.
The precision of the estimates of the haemoglobin A2 percentage in the presence of haemoglobin S for the three methods studied was determined by means of duplicate observations; table 1 shows the results. Precision was best with HPLC, intermediate with microcolumn chromatography, and (as expected) considerably worse with scanning densitometry.
Table 1.
Precision of duplicate estimates of haemoglobin A2 percentage by HPLC, MC, and scanning densitometry
| Method | Number of samples | CV |
| HPLC | 5 | 1.0% |
| MC | 5 | 2.4% |
| Scanning densitometry | 10 | 10.5% |
CV, coefficient of variation; HPLC, high performance liquid chromatography; MC, microcolumn chromatography.
Samples were assayed in duplicate.
The haemoglobin A2 percentage was measured by HPLC, microcolumn chromatography, and scanning densitometry in 73 patients with sickle cell trait without iron deficiency. Table 2 shows the results. As expected, the standard deviation was greatest when scanning densitometry was used, but the mean values by scanning densitometry and microcolumn chromatography were very similar, 3.3% and 3.6%, respectively (p > 0.05), supporting the accuracy of the microcolumn chromatography methodology. In contrast, the mean value by HPLC was 4.4%. HPLC measurements were significantly higher than microcolumn chromatography (p < 0.001) or scanning densitometry (p < 0.01) measurements, whereas the last two methods did not differ from one another (p > 0.1). Not surprisingly, the degree of overestimation of haemoglobin A2 by HPLC, in comparison with microcolumn chromatography, correlated significantly (albeit weakly) with the percentage of haemoglobin S in the specimen (r = 0.28; p < 0.05).
Table 2.
Mean, standard deviation, and 95% range of measurements of haemoglobin A2 percentage in 73 patients with sickle cell trait when measured by HPLC, MC, and scanning densitometry
| Method | Mean (SD) | 95% range |
| HPLC | 4.4% (0.34) | 3.7−5.1% |
| MC | 3.3% (0.36) | 2.6−4.0% |
| Scanning densitometry | 3.6% (0.70) | 2.2−5.0% |
HPLC, high performance liquid chromatography; MC, microcolumn chromatography.
When measured by microcolumn chromatography, the mean value of haemoglobin A2 in the 73 patients with sickle cell trait without iron deficiency was significantly higher than that in normal individuals, whose haemoglobin A2 values had been estimated by HPLC (3.3% in comparison with 2.8%; p < 0.05). A similar difference from normal was seen for the mean haemoglobin A2 by scanning densitometry (3.6% in comparison with 2.8%) but, because of the greater scatter, this was not significant (p > 0.1). In contrast to these slightly higher mean values, haemoglobin A2 as measured by HPLC was considerably higher in subjects with sickle cell trait than in normal individuals, reflecting the inaccuracy of measurements in the patients with sickle cell trait (mean values 4.4% and 2.8%, respectively; p < 0.01).
The degree of overestimation of haemoglobin A2 in 22 samples from subjects with sickle cell trait was stable for one week, but in 15 of these samples increased between one and two weeks; mean values when fresh and at two weeks were 4.5% and 5.1%, respectively (p < 0.05). Samples from four patients with sickle cell anaemia showed an even greater increase in apparent haemoglobin A2 by two weeks, whereas in 12 normal subjects there was no change in haemoglobin A2 estimates (data not shown). In contrast to the increasing inaccuracy of HPLC estimates of haemoglobin A2 with aging in samples containing haemoglobin S, estimates in 12 such samples by microcolumn chromatography were stable for two weeks (data not shown).
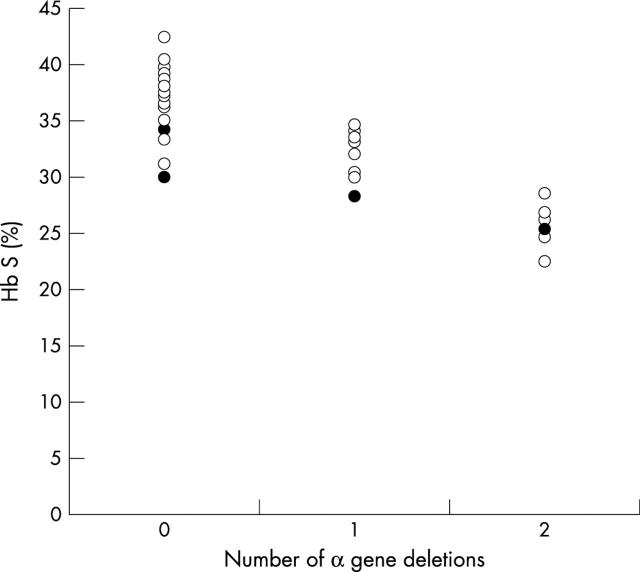
Figure 1 shows the haemoglobin S percentage in groups with two, three, and four α genes and tables 3–5 summarise the results (excluding the four patients with iron deficiency) in comparison with previously published data.
Figure 1.
Haemoglobin S (Hb S) percentage in individuals with four, three, and two α genes respectively; filled circles represent those with coexisting iron deficiency.
Table 3.
Observed range of haemoglobin S percentage in individuals with sickle cell trait divided into three groups by inspection of the data, with of without globin chain synthesis data, but without analysis of globin genes
| N | Postulated genotype | Method | Ref | ||
| αα/αα | −α/αα | −α/−α | |||
| Electrophoresis on polyacrylamide gel or cellulose acetate followed by elution | 5 | ||||
| NS | 37–50 | 30–36 | 20–29 | NS | 6 |
| 75 | >38 | 31–38 | <31 | Column chromatography | 7 |
| 56 | >38 | 31–38 | <31 | Column chromatography | 8 |
| 171 | 35–45 | 28–35 | <28 | Cellulose acetate electrophoresis and elution | 9 |
NS, not specified.
Table 5.
Haemoglobin A2 percentage, red cell indices, and haemoglobin S percentage in patients with sickle cell trait with or without α thalassaemia (the four patients with iron deficiency have been excluded)
| α Gene status | αα/αα (group A) | −α/αα (group B) | −α/−α (group C) | Significance of difference | ||
| A v B | B v C | A v C | ||||
| Hb (g/l) | 125.1 (19.1) | 114.9 (19.6) | 119.5 (13.1) | >0.1 | >0.1 | >0.1 |
| RBC ×1012/l | 4.48 (0.67) | 4.45 (0.61) | 5.54 (0.67) | >0.1 | <0.001 | <0.001 |
| MCV (fl) | 84.88 (8.26) | 79.08 (5.80) | 67.77 (5.81) | <0.001 | <0.001 | <0.001 |
| MCH (pg) | 28.05 (5.49) | 25.79 (2.10) | 21., 65 (1.84) | <0.001 | <0.001 | <0.001 |
| Hb S % by MC | 37.24 (3.14) | 32.61 (2.33) | 25.53 (2.08) | <0.001 | <0.001 | <0.001 |
| Hb S % by HPLC | 37.13 (2.51) | 32.61 (1.91) | 25.52 (1.44) | <0.001 | <0.001 | <0.001 |
| Hb A2 % by MC | 3.23 (0.31) | 3.41 (0.43) | 3.54 (0.45) | >0.1 | >0.1 | <0.01 |
| Hb A2 % by SD | 3.44 (0.62) | 3.78 (0.7) | 3.85 (0.72) | >0.1 | >0.1 | <0.01 |
| Hb A2 % by HPLC | 4.33 (0.37) | 4.41 (0.35) | 4.49 (0.76) | >0.1 | >0.1 | >0.1 |
A, subjects with no α gene deletion; B, subjects with one α gene deletion; C, subjects with two α gene deletions.
Values are mean (SD).
HPLC, high performance liquid chromatography; MC, microcolumn chromatography; MCH, mean cell haemoglobin; MCV, mean cell volume; RBC, red blood cells; SD, scanning densitometry.
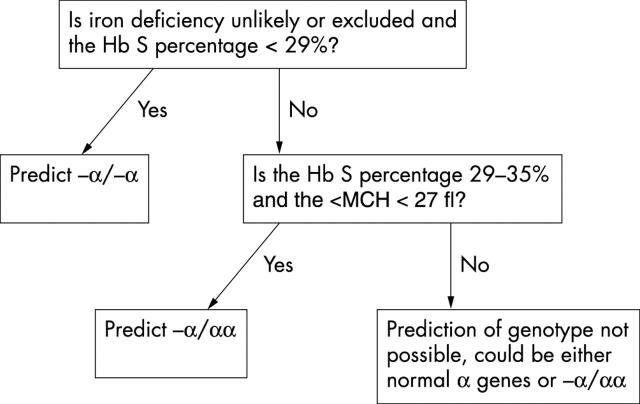
An algorithm was devised to aid in separating those with two or three α genes from those with a full complement of four α genes (fig 2). Using this algorithm, all 12 patients with −α/−α were identified and no other individuals were misassigned to this group. Of 16 individuals with −α/αα, 11 were correctly identified, with only four of 45 individuals with αα/αα being misassigned.
Figure 2.
An algorithm based on haemoglobin S percentage and mean cell haemoglobin (MCH) to aid in distinguishing individuals with homozygosity or heterozygosity for α+ thalassaemia (−α/−α and −α/αα, respectively) from those with four α genes. Hb, haemoglobin.
DISCUSSION
The reference range for haemoglobin A2 by HPLC using the Biorad Variant 2 instrument is comparable with our previous reference range derived by microcolumn chromatography. However, it appears to differ appreciably from a reference range derived from 104 subjects with normal red blood cell indices studied on the Biorad Variant; the mean in that study was 2.4% and the 95% range 1.9–3.0%,11 emphasising the importance of each laboratory deriving a reference range for the specific instrument and method used.
We have shown that estimates of haemoglobin A2 percentage by microcolumn chromatography are precise and accurate in samples from patients with sickle cell trait, and have confirmed previous observations that haemoglobin A2 percentage is slightly but significantly higher in sickle cell trait. We have also confirmed that estimates of haemoglobin A2 by HPLC are inaccurate in the presence of haemoglobin S, even though the precision is high. The mean observed difference between microcolumn chromatography and HPLC was 1.1%, and the mean observed difference between scanning densitometry and HPLC was 0.8%. Because the degree of inaccuracy was related to the amount of haemoglobin S present, it can be predicted that the degree of inaccuracy will be greater in patients with sickle cell anaemia or sickle cell/β thalassaemia than in those with sickle cell trait.
“Estimates of haemoglobin A2 by high performance liquid chromatography are inaccurate in the presence of haemoglobin S, even though the precision is high”
The degree of inaccuracy of haemoglobin A2 estimates in patients with sickle cell trait does not increase if the sample is stored for up to one week but, after two weeks of storage, an increase is seen. In the absence of haemoglobin S, HPLC estimates of haemoglobin A2 are stable for at least two weeks. Our observations of a storage effect in the presence of haemoglobin S confirm the observations of Suh et al, which were based on only three samples.3
If the haemoglobin A2 percentage, as measured by HPLC, were to be used to predict whether a patient had sickle cell anaemia or sickle cell/β0 thalassaemia it would be necessary to determine the percentage of apparent haemoglobin A2 seen in these two conditions, rather than extrapolating from percentages obtained by more accurate methods. In addition, it would be important to measure the percentage within one week of obtaining the sample.
A corollary of the overestimation of haemoglobin A2 by HPLC in individuals with sickle cell trait is that haemoglobin S is underestimated. However, because the error is proportionately much lower, this is of no practical importance. The trimodal distribution of haemoglobin S percentages, attributable to the presence of two, three, or four α genes is still apparent when the haemoglobin S percentage is measured by HPLC, and the overlap between the three groups is similar to that seen with more accurate techniques.
Normally, the α chain is present in excess. However, when α thalassaemia coexists with sickle cell trait, the amount of α chain available becomes rate limiting, and the positively charged α chain combines preferentially with the negatively charged βA chain, rather than with the positively charged βS chain, with a consequent reduction in the haemoglobin S percentage. The δ chain is also positively charged, so that the α chain is likely to combine preferentially with βA, rather than with δ. Haemoglobin A2 has been reported to be higher in patients with sickle cell anaemia who also have α thalassaemia than in those who do not,12,13 suggesting that, when the amount of α chain is rate limiting, combination with the δ chain is favoured over combination with βS. These observations suggest that the affinity of non-α chains for the α chain is βA > δ > βS.
Data from two previous studies, those of Whitten and Rucknagel1 and of Wong et al,8 suggest that haemoglobin A2 is higher in patients with sickle cell trait and α thalassaemia than in those with sickle cell trait without α thalassaemia, whereas El-Hazmi9 found no differences. Our data confirm the first two studies because we found a significantly higher haemoglobin A2 percentage in patients with two deleted α genes than in those with four α genes. This suggests that, when there are insufficient α chains but βA, δ and βS chains are all present, the lower affinity of α chains for βS than for βA means that there are more α chains available to combine with δ chains, and that there is preferential combination with δ chains rather than with βS. However, our data show that haemoglobin A2 as a percentage of haemoglobin A plus A2 falls when α thalassaemia coexists with sickle cell trait, supporting the above evidence that the affinity of non-α chains for α chains is βA > δ > βS. It could be postulated that the slightly higher percentage of haemoglobin A2 in individuals with sickle cell trait, in comparison with normal individuals, results from the fact that the lower affinity of βS for the α chain leaves more free α chain to combine with βA and δ; however, because of the higher affinity of βA than δ for the α chain, there is only a slight increase in the amount of α chain available to combine with the δ chain, and there is only a modest increase in haemoglobin A2.
Take home messages.
High performance liquid chromatography (HPLC) provides precise but inaccurate estimations of haemoglobin A2 in the presence of haemoglobin S
Despite this, HPLC estimates of the percentages of haemoglobins A, A2, and S in patients with sickle cell trait can be used, together with the red blood cell indices, to predict the presence of coexisting α thalassaemia trait
We have devised an algorithm that correctly identified all patients with deletion of two of four α genes without misassigning other individuals to this group
“Our algorithm enables most cases to be recognised, with only a minority of those with four α genes being misassigned”
In conclusion, our data show that despite the inaccuracy inherent in the measurement of haemoglobin A2 by HPLC, the percentages of haemoglobins A, A2, and S in patients with sickle cell trait can nevertheless be used, together with the red blood cell indices, to predict the presence of coexisting α thalassaemia trait, with the overlap between groups being similar to that seen with more accurate techniques. The algorithm we have devised correctly identified all patients with deletion of two of four α genes without misassigning other individuals to this group. No algorithm based on haematological variables is likely to permit the recognition of all individuals with deletion of a single α gene because some of these individuals have completely normal red blood cell indices and, as we have shown, their haemoglobin S percentages show some overlap with the values seen in individuals with four α genes. However, our algorithm enables most cases to be recognised, with only a minority of those with four α genes being misassigned. This permits the haematology laboratory to give useful advice to clinical staff as to the probable cause of microcytosis.
Table 4.
Observed range of haemoglobin S percentage in individuals with sickle cell trait with four, three, or two α globin genes (the four patients with iron deficiency in our present study were excluded)
| N | αα/αα | −α/αα | −α/−α | Method | Ref |
| 23 | 36–43 | 29–37 | 22–28 | Cellulose acetate electrophoresis | 6 |
| 31 | 32.5–38 | 28–35 | 24–27 | Cellulose acetate electrophoresis and elution | 10 |
| 74 | 31–43 | 29–35 | 22–29 | HPLC | Present study |
HPLC, high performance liquid chromatography.
Abbreviations
HPLC, high performance liquid chromatography
REFERENCES
- 1.Whitten WJ, Rucknagel DL. The proportion of Hb A2 is higher in sickle cell trait than in normal homozygotes. Hemoglobin 1981;5:371–8. [DOI] [PubMed] [Google Scholar]
- 2.Sergeant G. Sickle cell disease. Oxford: Oxford University Press, 2001.
- 3.Suh DD, Krauss JS, Bures K. Influence of haemoglobin S adducts on hemoglobin A2 quantification by HPLC. Clin Chem 1996;42:1113–14. [PubMed] [Google Scholar]
- 4.Baysal E, Huisman THJ. Detection of common deletional α-thalassemia-2 determinants by PCR. Am J Hematol 1994;46:208–13. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg MH, Adams HG, Dreiling BJ. Alpha thalassaemia in adults with sickle cell trait. Br J Haematol 1975;30:31–7. [DOI] [PubMed] [Google Scholar]
- 6.Brittenham G, Lozoff B, Harris JW, et al. Alpha globin gene number: population and restriction endonuclease studies. Blood 1980;55:706–8. [PubMed] [Google Scholar]
- 7.Felice A, Altay G, Milner PF, et al. The occurrence and identification of α-thalassemia-2 among hemoglobin S heterozygotes. Am J Clin Pathol 1981;76:70–3. [DOI] [PubMed] [Google Scholar]
- 8.Wong SC, Ali MAM, Boyadjian SE. Sickle cell traits in Canada: trimodal distribution of Hb S as a result of interaction with α-thalassemia gene. Acta Haematol 1981;65:157–63. [DOI] [PubMed] [Google Scholar]
- 9.El-Hazmi MAF. Studies on sickle cell heterozygotes in Saudi Arabia—interaction with α-thalassaemia. Acta Haematol 1986;75:100–4. [DOI] [PubMed] [Google Scholar]
- 10.Higgs DR, Clegg JB, Weatherall DJ, et al. GR Interaction of the ααα globin gene haplotype and sickle haemoglobin. Br J Haematol 1984;57:671–8. [PubMed] [Google Scholar]
- 11.Tan GB, Aw TC, et al. Evaluation of high performance liquid chromatography for routine estimation of haemoglobins A2 and F. J Clin Pathol 1993;46:852–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg MH, Embury SH. α-Thalassemia in blacks: genetic and clinical aspects and interactions with the sickle cell hemoglobin gene. Blood 1986;68:985–90. [PubMed] [Google Scholar]
- 13.de Ceulaer K, Higgs DR, Weatherall DJ, et al. α-Thalassemia reduces the hemolytic rate in homozygous sickle-cell disease. N Engl J Med 1983;309:189–90. [DOI] [PubMed] [Google Scholar]