Abstract
Aims: Because of the observation of an abundance of leukaemia/lymphoma cell microparticles in the bone marrow aspiration sample of a patient with Burkitt’s leukaemia at diagnosis, the occurrence of this phenomenon in leukaemia/lymphoma samples with available immune phenotyping data was investigated retrospectively.
Methods: Flow cytometric immune phenotyping and spontaneous apoptosis analysis of the bone marrow mononuclear cell preparation of the index case were performed. Microparticles isolated form the bone marrow sample were also studied for the presence of leukaemia/lymphoma cell microparticles. List mode analysis of 225 cases of acute leukaemia or lymphoma with previously performed immune phenotyping was also carried out.
Results: The presence of leukaemia/lymphoma cell microparticles could be detected by flow cytometry and they were found to be different from apoptotic bodies. Leukaemia/lymphoma cell microparticles were released in all cases of mature B cell neoplasms studied, although this phenomenon was rare in precursor B cell disorders and acute myeloid leukaemia.
Conclusions: The generation of leukaemia/lymphoma cell microparticles in mature B cell neoplasms appears to be a common phenomenon. The pathogenesis and clinical implications must be investigated.
Keywords: mature B cell neoplasm, leukaemia/lymphoma microparticles, apoptotic bodies
The release of cytoplasmic microparticles (membrane covered cytoplasmic particles) from different cell types under physiological conditions has been well described. During the maturation of erythroid cells, such particles are generated and contribute to the elimination of transferrin receptors. Such physiological processes are reminiscent of the production of platelets. In recent years, the in vitro production of endothelial cell microparticles has been demonstrated and the mechanism of generation of these particles has been elucidated. 1 Microparticles developing as a result of endothelial cell activation differ from particles that are generated as a consequence of apoptotic processes. 2
MATERIALS AND METHODS
An index case of Burkitt’s leukaemia and Epstein-Barr virus (EBV) transformed normal B cells were studied prospectively. List mode analysis of 225 cases of acute leukaemia or lymphoma with previously performed immune phenotyping as part of an institutional review board approved study was also carried out.
Bone marrow cells were separated on Ficoll and any remaining red blood cells lysed. Immune phenotypic staining of these cells, in addition to lymph node and tissue culture suspension cells, was performed according to previously published methods. 3, 4 Briefly, mononuclear cells were resuspended in phosphate buffered saline (PBS) containing 30% adult bovine serum (to block Fc receptors) and stained at room temperature for 30 minutes using the antibody concentrations recommended by the manufacturers. After staining, samples were washed with PBS and resuspended in PBS + 0.5% formaldehyde before flow cytometric analysis. Events were collected on the immature blast population using forward scatter (FS)/side scatter (SS) analysis as inferred from initial SS/CD45 analysis. There was a second group of events recognised with the platelet or erythrocyte population on FS/SS histograms, so these populations were analysed separately, and were shown to include leukaemia/lymphoma cell microparticles. The antibody panel included antibodies to: CD45, CD19, CD10, CD20, CD38, HLA-DR, surface IgG, λ chain, κ chain, CD2, CD3, CD4, CD5, CD7, CD8, CD11, CD13, CD33, CD34, CD41, and glycophorin A, in addition to CD32, CD21, CD49d, CD80, and CD86 in the index case and the EBV transformed normal B cells.
The presence of apoptotic bodies was investigated using annexin V (AnnV)–propidium iodide (PI) colabelling by flow cytometry. 5 Two colour histograms of the leukaemia/lymphoma cell microparticle gated populations were analysed. The AnnV negative (−)/PI negative (−) population represents viable cells, the AnnV+/PI− population represents early apoptotic cells, and the AnnV+/PI+ population represents late apoptotic or dead cells.
Density centrifugation of the bone marrow specimen to isolate cellular microparticles was carried out using a published method, with slight modifications. 1 Briefly, the bone marrow specimen in sodium citrate was spun down at 160 ×g for 10 minutes using an IEC PR-6000 centrifuge to obtain platelet rich plasma. The upper layer of platelet rich plasma was spun down at 1500 ×g for six minutes to obtain platelet poor plasma. The platelet poor plasma was stained with combinations of conjugated CD42a, glycophorin A, CD20, and P1H12 in the dark on a shaker for 20 minutes and run on an EPICS-XL flow cytometer. Gating was based on the isotype control staining patterns.
RESULTS
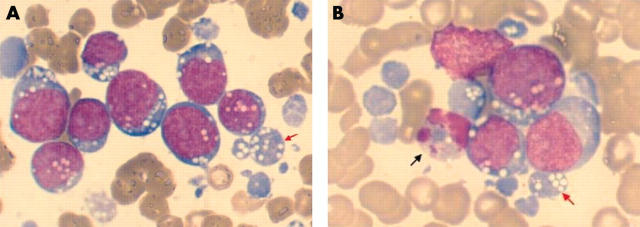
Microparticles were present in abundance in the bone marrow of the index case at the time of diagnosis. The patient was a child with acute lymphoblastic leukaemia (ALL) with L3 morphology (Burkitt’s type) according to the French–American–British (FAB) classification and a mature B cell neoplasm according to the World Health Organisation classification. EBV late membrane protein-1 was demonstrated in the tumour cells. The small blue cellular particles were recognised as microparticles because of the multiple cytoplasmic vacuoles characteristic of L3 morphology on the Wright/Giemsa stained bone marrow aspiration smears under light microscopy (fig 1 ).
Figure 1.
Leukaemia cell microparticles in the bone marrow. (A) Abundant leukaemia/lymphoma cell microparticles on the Wright/Giemsa stained bone marrow aspiration smear in a patient with Burkitt’s leukaemia. (B) Presence of apoptotic figures along with the leukaemia/lymphoma cell microparticles. The red arrowheads point to leukaemia/lymphoma cell microparticles and the black arrowhead points to an apoptotic cell.
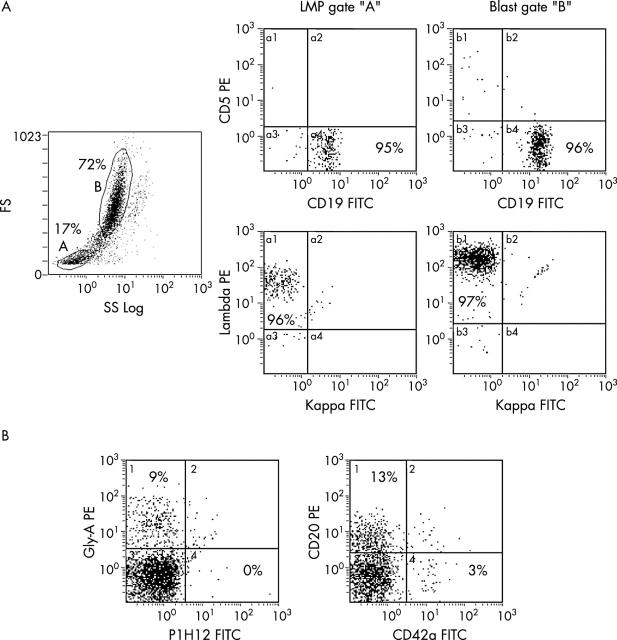
Flow cytometric immune phenotyping of bone marrow cells revealed a small population of events with FS and SS characteristics of the platelet or erythrocyte population on FS/SS histograms. The staining pattern of this population was the same as, but less intense (because of the small size) than, the leukaemia population, revealing high CD19, CD10, λ chain, CD38, and DR expression; in addition, this population was negative for platelet and erythrocyte associated markers, such as CD41 and glycophorin A, respectively (fig 2 ). Density centrifugation isolated microparticles revealed a population of events positive for CD20, along with other smaller populations consistent with platelets and erythrocytes (fig 2 ).
Figure 2.
Surface characteristics of leukaemia cell microparticles. (A) Surface immune phenotyping staining patterns in leukaemia/lymphoma cell microparticle (LMP) (gated as A) and leukaemic blast (gated as B) populations in a patient with Burkitt’s leukaemia. (B) Presence of LMPs (CD20 positive) along with platelets (CD42a positive) and erythrocytes (glycophorin A positive), but without endothelial cell particles (P1H12 positive) in a platelet poor plasma sample. The numbers on the histograms represent the percentage of events. FITC, fluorescein isothiocyanate; FS, forward scatter; PE, phycoerythrin; SS, side scatter.
These microparticles did not appear to be a result of the apoptotic process for the following reasons: (a) there were very few apoptotic figures identifiable on the Wright/Giemsa stained bone marrow aspiration smears (fig 1 ); (b) leukaemia/lymphoma cell microparticles contained vacuoles, as did the intact leukaemic blasts, and there were no morphological signs of apoptosis, consistent with direct release; and (c) many of the particles were negative for AnnV and PI, characteristics of apoptosis. Furthermore, the particles also partially expressed adhesion and costimulatory molecules including CD32, CD21, CD49d, CD80, and CD86, as did the leukaemic clone. Flow cytometric analysis of EBV transformed B cells from a subject without a malignant disorder revealed leukaemia/lymphoma cell microparticles with similar characteristics to the case discussed above including CD32, CD21, CD49d, CD80, and CD86 expression.
A review of flow cytometric immune phenotyping results performed previously on three patients with mature B cell ALL and lymph node samples on two patients with Burkitt’s lymphoma revealed a similar population, with staining patterns similar to the malignant clone. Upon further review of the results on leukaemia samples at our institution (220 cases), one acute myeloid leukaemia (AML)-M7 and four B precursor ALL cases were found to have identifiable potential leukaemia/lymphoma cell microparticle populations. List mode analysis of that population (leukaemia/lymphoma cell microparticle gate) revealed small percentages of microparticles sharing the characteristics of the leukaemic clone.
DISCUSSION
The generation of leukaemia/lymphoma cell microparticles may not be a rare phenomenon in childhood leukaemias and it appears to be substantially more pronounced in mature B cell tumours than in precursor B cell neoplasms. The degree of leukaemia/lymphoma cell microparticle production may be related to the cellular activation status, as shown by costimulatory molecule expression, or may just be a reflection of the characteristics of the leukaemia type and/or the cell type that the leukaemia has originated from, such as increased mechanical fragility. This last notion is consistent with the known rapid tumour cytolysis seen during chemotherapy in mature B cell neoplasms. The generation of such particles was reported in an AML-M7 cell line in vitro. 6 Despite reviewing more than 20 cases of AML-M7 bone marrow immune phenotyping results, we identified only one clearcut case with a leukaemia/lymphoma cell microparticle population. This could partially be attributed to loss of particles through mononuclear cell isolation techniques that are not designed specifically to enrich low density particles, and it may be more common than we have detected. Nevertheless, observing leukaemia/lymphoma cell microparticle generation in AML-M7 cases may be a reflection of megakaryocyte biology, namely platelet production.
“Are there any implications for the presence of leukaemia/lymphoma cell microparticles in leukaemia or lymphoma?”
It is important to be aware of this phenomenon and not to confuse leukaemia/lymphoma cell microparticles with platelets. More importantly, are there any implications for the presence of leukaemia/lymphoma cell microparticles in leukaemia or lymphoma? Increased numbers of endothelial cell microparticles were isolated from the blood of patients with systemic lupus erythematosus compared with healthy individuals. 7 In addition, it has been postulated that microparticles can contribute to thrombotic disorders. 7, 8 Apoptotic bodies originating from human immunodeficiency virus infected T cells have been shown to transfer the virus into other cells. 9 However, because leukaemia/lymphoma cell microparticles are not apoptotic bodies, they may not be good targets for cellular uptake but, rather, may circulate in the blood and lymph vessels. Further investigations are needed to determine whether leukaemia/lymphoma cell microparticle generation is related to cellular activation associated with EBV infection, with potential clinical consequences.
Take home messages .
The generation of leukaemia/lymphoma cell microparticles appears to be common in mature B cell neoplasms
The pathogenesis and clinical implications of this phenomenon need to be investigated
Abbreviations
ALL, acute lymphoblastic leukaemia
AML, acute myeloid leukaemia
AnnV, annexin V
EBV, Epstein-Barr virus
FS, forward scatter
PBS, phosphate buffered saline
PI, propidium iodide
SS, side scatter
REFERENCES
- 1. Jimenez JJ, Jy W, Mauro LM, et al. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol 2001;112:81–90. [DOI] [PubMed] [Google Scholar]
- 2. Jimenez JJ, Jy W, Mauro LM, et al. Endothelial cells (EC) release phenotypically distinct endothelial microparticles (EMP) in activation vs. apoptosis: findings in TTP patients [abstract]. Blood 2001;98:249a. [Google Scholar]
- 3. Willman CL, Stewart CC. General principles of multiparameter flow cytometric analysis: applications of flow cytometry in the diagnostic pathology laboratory. Semin Diagn Pathol 1989;6:3–12. [PubMed] [Google Scholar]
- 4. Terstappen LWMM, Johnson D, Mickaels RA, et al. Multidimensional flow cytometric blood cell differentiation without erythrocyte lysis. Blood Cells 1991;17:585–602. [PubMed] [Google Scholar]
- 5. Ozgen U, Savasan S, Buck S, et al. Comparison of DiOC6 uptake and annexin V labeling for quantification of apoptosis in leukemia cells and non-malignant T lymphocytes from children. Cytometry 2000;42:74–8. [DOI] [PubMed] [Google Scholar]
- 6. Takeuchi K, Ogura M, Saito H, et al. Production of platelet-like particles by a human megakaryoblastic leukemia cell line (MEG-01). Exp Cell Res 1991;193:223–6. [DOI] [PubMed] [Google Scholar]
- 7. Combes V, Simon AC, Grau GE, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest 1999;104:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jy W, Jimenez JJ, Mauro L, et al. Interaction of endothelial microparticles (EMP) with leukocytes: potential roles of EMP in thrombosis and inflammation [abstract]. Blood 2001;98:226a. [Google Scholar]
- 9. Spetz AL, Patterson BK, Lore K, et al. Functional gene transfer of HIV DNA by an HIV receptor-independent mechanism. J Immunol 1999;163:736–42. [PubMed] [Google Scholar]