Abstract
Aims: To investigate the presence of iron in biopsy and resection specimens from the stomach of patients with hepatic cirrhosis of various aetiologies.
Methods: Among 753 patients who had been admitted to the hospital with liver cirrhosis from 1984 to 2002, and 723 patients who underwent liver biopsy or liver resection from 1990 to 2003, 426 patients with concomitant gastric biopsy or gastrectomy were selected for study. Formalin fixed, paraffin wax embedded tissues of the stomach and the liver (when available) were retrieved from the pathology files of Kariya General Hospital, Japan. Haematoxylin and eosin staining and Perls’ stain were performed for all the available tissues and haemosiderin and its localisation were examined.
Results: In total, 78 patients—72 of those with cirrhosis (26%) and six without cirrhosis (4%)—showed accumulation of haemosiderin. Regardless of aetiology, patients with clinical varices showed more frequent haemosiderin accumulation (40%) than patients without varices (19%). For patients with cirrhosis, there were no significant differences in the positive rate between those with (28%) or without (23%) hepatocellular carcinoma.
Conclusion: The significant increase in haemosiderin deposition in the gastric glands of patients with cirrhosis suggests that the assessment of iron deposition in gastric biopsy specimens may have predictive value in controlling patients with cirrhosis.
Keywords: oesophagogastric varices, haemosiderosis, iron overload, stomach, portal hypertensive gastropathy
Haemosiderin deposition is a rare, incidental finding in gastric biopsy specimens, and may prompt the pathologist to alert the clinician to the possible presence of occult primary haemochromatosis. Alternatively, it can be a secondary result of alcohol abuse, hepatic cirrhosis, or spontaneous portacaval shunt with oesophagogastric varices, in addition to transfusion or iron medication. Unfortunately, to date, there have been no definitive data concerning the presence of iron in the gastric mucosa of patients with cirrhosis other than those with an alcoholic aetiology. We investigated retrospectively the presence of iron in biopsy and resection specimens from the stomach of patients with hepatic disease of various degrees and aetiologies.
MATERIALS AND METHODS
Selection of cases
Among 753 patients who had been admitted to our hospital with a diagnosis of liver cirrhosis from 1984 to 2002, 271 underwent gastric biopsy or gastrectomy. These patients made up the first set of cases. The diagnosis in set 1 had been based on the symptoms, history, physical examination, liver biochemistry, and liver histology (when available), depicted from a database constructed from the clinical summary of all patients who had been admitted to our hospital. Among 723 patients who underwent liver biopsy or liver resection from 1990 to 2003, after most of the patients with metastatic liver tumours had been excluded, we had 235 patients who underwent synchronous or metachronous gastric biopsy or gastrectomy. These patients made up the second set of cases. Eighty patients were in both set 1 and set 2, so that our study population consisted of 426 patients, with most of the patients with hepatitis being from set 2. All the patients were Japanese. Among 426 cases, 415 were gastric biopsy specimens and 11 were resection specimens. Only one block was studied for each patient, and if both biopsy and resected specimens existed for the same patient the biopsy specimen was selected. If repeat a biopsy had been performed, selection was made according to the following criteria: if a liver biopsy or resection had also been performed, the specimen that had been taken at the time nearest to the date of liver biopsy was used, but if no liver specimen had been taken the most recent sample was used. Patients were grouped according to the status of liver disease at the time of gastric biopsy. Patients without hepatitis or cirrhosis were grouped as miscellaneous; patients in this group included those with cholangiocarcinoma, fatty liver, and hepatocellular carcinoma (HCC) without hepatitis or cirrhosis. The hepatitis group included those with acute hepatitis and chronic hepatitis with various aetiologies. Patients with cirrhosis were subdivided into cirrhosis only, cirrhosis with varices, and cirrhosis with ruptured varices. We could not obtain sufficient data on the degree of hepatic synthetic dysfunction, so that the influence of this factor on gastric iron could not be studied. A Banti liver with oesophageal varices was included in the cirrhosis with varices group. The influence on gastric iron of sex, aetiology, presence or absence of HCC, iron overload of the liver, and history of transfusion was studied. Patients with acute hepatitis, primary biliary cirrhosis, autoimmune hepatitis, and non-B and non-C viral hepatitis were too small in number to study the aetiological influences on gastric iron deposition. In assessing the influence of HCC, patients who had been diagnosed as having HCC at the time of gastric biopsy were defined as HCC positive. In set 2, 200 cases could be assessed histologically for iron in the liver also. In many instances, the gastric and liver biopsies were almost synchronous, but in some cases one preceded the other, with a substantial lapse of time in between. The history of transfusion was assessed in 153 patients who underwent gastric biopsy after 2000. Presence or absence of a history of transfusion was ascertained from the computerised register of transfusion, which dates back to 1998. Details of gastric disease and the specific site of the stomach that was biopsied were available from the clinical information in the pathology report.
Detection of haemosiderin in the stomach and liver
Formalin fixed and paraffin wax embedded tissues from all the patients were retrieved from the pathology files of Kariya General Hospital, Japan. All the sections under study were stained with haematoxylin and eosin (H&E) and by Perls’ method. For the stomach, specimens with very few gastric glands were excluded from our study at the beginning. Only the stainable iron that was seen within the cytoplasm of gastric glands was counted as positive. This is because in the various conditions studied, bleeding can occur in the stomach, so that interstitial haemosiderin deposition might have no specific link with portal hypertension. Haemosiderin was first assessed by H&E slides only. The severity of gastric haemosiderin deposition was then assessed by Perls’ stain and tentatively graded from 0 to 3. For the liver, we assessed the degree of haemosiderin deposition according to the histological grading system. 1
Statistical analysis
The χ2 test was used to assess significant differences between gastric iron deposition in each group using Microsoft Excel. Because the only factor influencing gastric iron turned out to be the presence or absence of cirrhosis, multivariate logistic analysis was not performed.
RESULTS
Of the 426 specimens, 78 (76 biopsies and two resection specimens) showed iron deposition. In grade 1 deposition, iron accumulation was visible only after carefully searching all of the high power field (fig 1A ). In grade 3, it was visible even under low power (fig 1B ). Grade 2 was intermediate between grade 1 and grade 3. Sections showing only interstitial deposition were graded as 0, however strong the staining was. Glandular deposition was usually accompanied by varying degrees of interstitial deposition. Table 1 summarises all the results.
Figure 1.
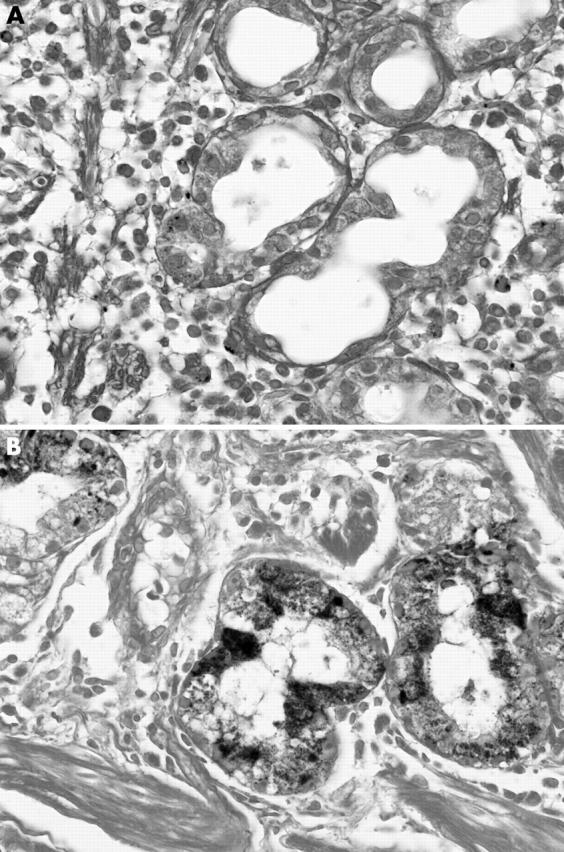
Haemosiderin was seen in the cytoplasm of parenchymal cells of the gastric glands. (A) In this example of grade 1 deposition, both interstitial deposition and glandular deposition are scant. (B) In this example of grade 3 deposition, stainable iron is almost exclusively distributed within the glands (Perls’ stain).
Table 1.
Comparison of the prevalence of gastric iron in various conditions
| Iron | ||||||
| + | − | p Value | ||||
| Hepatic disease | ||||||
| MI | 0 | 28 | ] 0.2 | |||
| HE | 6 | 113 | ] 0.0003 | |||
| CI | 38 | 157 | ] 0.006 | |||
| VX | 29 | 44 | ] 0.7 | |||
| R | 5 | 6 | ||||
| MI, HE | 6 | 141 | ] <0.0001 | |||
| CI, VX, R | 72 | 207 | ||||
| Sex | ||||||
| MI, HE | ||||||
| Female | 1 | 59 | ] 0.2 | |||
| Male | 5 | 82 | ||||
| CI, VX, R | ||||||
| Female | 22 | 65 | ] 0.8 | |||
| Male | 50 | 142 | ||||
| Aetiology | ||||||
| CI, VX, R | ||||||
| Alcoholic | 8 | 18 | ] 0.9 | ] 0.33 | ||
| HBV | 7 | 17 | ] 0.44 | |||
| HCV | 29 | 103 | ||||
| HCC | ||||||
| MI, HE | ||||||
| HCC+ | 3 | 10 | ] 0.002 | |||
| HCC− | 3 | 131 | ||||
| CI, VX, R | ||||||
| HCC+ | 29 | 95 | ] 0.4 | |||
| HCC− | 43 | 112 | ||||
| Transfusion | ||||||
| CI, VX, R | ||||||
| Yes | 3 | 9 | ] 0.9 | |||
| No | 19 | 53 | ||||
| Liver iron | ||||||
| Grade 0 | 8 | 72 | ] 0.004 | |||
| Grade 1 | 8 | 44 | ] 0.01 | |||
| Grade 2 | 3 | 32 | ] 0.002 | |||
| Grade 3 | 2 | 16 | ] 0.05 | |||
| Grade 4 | 7 | 8 | ||||
CI, cirrhosis only; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HE, hepatitis; MI, miscellaneous; R, ruptured varices; VX, cirrhosis with varices.
Hepatic cirrhosis
In total, 78 patients—72 of 279 patients with cirrhosis (26%) and six of 147 patients without cirrhosis (4%)—showed accumulation of haemosiderin, suggesting that cirrhosis has a significant positive influence on gastric iron.
Oesophagogastric varices
In patients with cirrhosis, regardless of aetiology, 34 of 84 (40%) patients with varices and 38 of 195 (19%) patients with cirrhosis only showed haemosiderin accumulation. Thus, the presence of oesophagogastric varices had a significant influence on gastric iron, although there was no significant difference between patients with varices and those with ruptured varices.
Sex
Among the patients with cirrhosis, there were 192 men and 87 women. Fifty men (26%) and 22 women (25%) showed gastric iron deposition, so that sex had no significant effect on gastric iron overload among patients with and also without cirrhosis.
Aetiology
Because only a few patients with hepatitis had accumulation of gastric iron the significance of different aetiologies could be investigated only for patients with cirrhosis. Aetiologies other than alcohol abuse, hepatitis B, and hepatitis C could not readily be assessed because the number of such cases in our series was too small. There were no significant differences between gastric iron accumulation in patients with the above mentioned aetiologies.
Presence of HCC
Among the patients with cirrhosis, 29 of the 124 patients with HCC (23%) and 43 of the 155 patients without HCC (28%) showed gastric iron deposition, suggesting that the presence of HCC had no significant effect on gastric iron in this group of patients. However, in patients with hepatitis HCC had a positive significant effect on gastric iron accumulation.
Correlation with transfusion
Because the turnover of gastric glands is believed to be one to three years, 2 transfusions more than three years before the date of gastric biopsy should theoretically have no significant influence on gastric iron. We investigated 84 patients with hepatic cirrhosis who underwent gastric biopsy relatively recently. For this particular group of patients, information on transfusion within the previous three years was available from the computer registration files on transfusion. It was confirmed that, for patients with cirrhosis, there were no significant differences in the rate of gastric iron accumulation between those with and without a history of transfusion within the previous three years. Within this particular group, no patients without cirrhosis were positive for iron accumulation.
Predictive value of gastric iron for the development of oesophagogastric varices
We followed a limited number of patients with cirrhosis without varices. Twelve of the 38 haemosiderin negative patients and five of the 12 haemosiderin positive patients developed varices, with the follow up interval varying from one to five years. There was no significant difference between these data.
Correlation with hepatic iron
Hepatic iron was assessed in 182 patients in set 2.
Only grade 4 hepatic iron overload had a significant effect on gastric iron (seven positive cases out of 15). There was no significant difference between the groups with lower grade hepatic iron.
Clinical and endoscopic diagnosis, and histological features of the stomach of iron positive cases
The endoscopic features of the haemosiderin positive samples were classified according to the site of the stomach, in addition to whether the lesion was localised, such as an ulcer, or diffuse. Among the localised lesions, ulcers or erosion comprised nearly half of the haemosiderin positive group (36 of 76). There were two cases of fundic gland polyp with haemosiderin deposition. Diffuse changes included redness, giant fold, and gastritis. There were seven biopsies taken to assess the presence of Helicobacter pylori. The site of the stomach included 38 body, 15 angle, nine antrum, and 14 diffuse. A specific diagnosis of portal hypertensive gastropathy was made in only one specimen.
Degree of gastric iron and false negative results by the observation of H&E stained slides only
All of the 25 grade 3 specimens were detectable from H&E stained slides. In 15 of the 33 grade 2 cases and 24 of 30 grade 1 cases, haemosiderin was detected only after Perls’ stain. There was no correlation between the grade of gastric iron deposition and absence, presence, or rupture of varices.
DISCUSSION
Iron deposition in the gastric mucosa has long been recognised in patients with primary haemochromatosis. More recently, Conte et al reported the presence of iron in half of the alcoholics that they studied. 3 The incidental finding of iron in gastric biopsies is a rare event. In a study of gastric injury caused by iron medication, Abraham et al found haemosiderin deposition in 0.7% of gastric biopsy specimens. 4
We found that this rate is much higher in patients with cirrhosis, the aetiology of which was not restricted to alcoholic liver disease. Moreover, the presence of varices had a significantly positive influence on gastric iron deposition. We found that, except for patients with grade 4 liver iron overload, liver iron has no significant effect on gastric iron overload. Although primary haemochromatosis with distinct genetic abnormalities is extremely rare in Japan, 5 some of the patients with grade 4 iron overload in our series could have had primary haemochromatosis with yet unknown genetic abnormalities. However, in most cases haemosiderin deposition in gastric glands is a primary phenomenon caused directly by portal hypertension and not secondary to the deposition of iron in the cirrhotic liver. 6– 8 Deposition would occur as a result of spontaneous portacaval shunt caused by hepatic cirrhosis, followed by intermittent microhaemorrhage in the gastric mucosa, together with long turnover time of gastric glands.
“We found that, except for patients with grade 4 liver iron overload, liver iron has no significant effect on gastric iron overload”
Some believe that gastric iron could be the result of transfusion. However, haemosiderosis caused by transfusion presents first in the reticuloendothelial system, and parenchymal organs are affected only if the transfusion is heavy and persistent. 9 It is unlikely that predominantly parenchymal deposition in the gastric glands would be a result of transfusion alone. Our data also show that the transfusion history has no significant effect on gastric iron, suggesting that gastric iron is not a result of transfusion to treat the rupture of oesophageal varices. Portal hypertensive gastropathy (PHG) is an endoscopically defined entity. 10 Among 78 patients with gastric iron, only one had been diagnosed as having PHG. In part, this could be because PGH had not been considered as a differential diagnosis by the endoscopist, who had no special concern with PGH. Both PHG and gastric iron accumulation could be separate results of a common cause, namely increased portal flow as a result of hepatic cirrhosis. In contrast to PGH, which spontaneously waxes and wanes, gastric iron accumulation would be a progressive change mainly because of the long turnover time of gastric glands. However, there could be a close link between PGH and gastric iron accumulation, namely the iron rich microenvironment caused by PGH related microhaemorrhage could lead to the excess absorption of iron by cells of gastric glands. In fact, glandular deposition is sometimes accompanied by interstitial deposition of haemosiderin. Haemosiderin accumulation in the gastric glands is likely to be overlooked if the pathologist does not deliberately search for this phenomenon. In fact, haemosiderin accumulation was mentioned in the pathology reports of only two of the 78 positive cases. By a careful search of H&E slides more than half of the positive cases could be detected.
The high rate of haemosiderin accumulation in patients with cirrhosis suggests that caution is needed when interpreting the incidental finding of haemosiderin accumulation in gastric glands—this phenomenon should be regarded as not necessarily associated with primary haemochromatosis, but as a result of portal hypertension caused by cirrhosis itself. Alternatively, it could suggest the presence of a chronic increase in portal flow in the gastroenteric microvasculature, which may precede the evolution of oesophagogastric varices. Because iron deposition in the stomach is diffuse to patchy and has a predilection for the body mucosa, mucosal sampling in a similar fashion to that used for assessing H pylori infection, followed by histological evaluation of iron by Perls’ stain, might be sufficient. Gastric iron may have predictive value for the development of varices in patients with cirrhosis. A case controlled study would be necessary to test this hypothesis.
Take home messages .
Accumulation of haemosiderin was higher in patients with cirrhosis (26%) than those without cirrhosis (4%)
Regardless of aetiology, patients with clinical varices showed more frequent haemosiderin accumulation (40%) than those without (19%)
Thus, the assessment of iron deposition in gastric biopsies may have predictive value for the development of varices in patients with cirrhosis
Abbreviations
H&E, haematoxylin and eosin
HCC, hepatocellular carcinoma
PHG, portal hypertensive gastropathy
REFERENCES
- 1. Searl J, Kerr JFR, Halliday JW, et al. Iron storage disease. In: MacSween RNM, Anthony PP, Scheuer PJ, eds. Pathology of the liver, 3rd ed. Edinburgh: Churchill Livingston, 1994:219–41.
- 2. Owen DA, Stomach. In: Sternberg SS, ed. Histology for pathologist, 2nd ed. Philadelphia, Lippincott-Raven, 1997:481–93.
- 3. Conte D, Velio P, Brunelli L, et al. Stainable iron in gastric and duodenal mucosa of primary haemochromatosis patients and alcoholics. Am J Gastroenterol 1987;82:237–40. [PubMed] [Google Scholar]
- 4. Abraham SC, Yardley JH, Wu TT. Erosive injury to the upper gastrointestinal tract in patients receiving iron medication: an underrecognized entity. Am J Surg Pathol 1999;23:1241–7. [DOI] [PubMed] [Google Scholar]
- 5. Merryweather-Clarke AT, Pointon JJ, Shearman JD, et al. Global prevalence of putative haemochromatosis mutations. J Med Genet 1997;34:275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludwig J, Hashimoto E, Porayko MK, et al. Hemosiderosis in cirrhosis: a study of 447 native livers. Gastroenterology 1997;112:882–8. [DOI] [PubMed] [Google Scholar]
- 7. Deugnier Y, Turlin B, le Quilleuc D, et al. A reappraisal of hepatic siderosis in patients with end-stage cirrhosis: practical implications for the diagnosis of hemochromatosis. Am J Surg Pathol 1997;21:669–75. [DOI] [PubMed] [Google Scholar]
- 8. Haque S, Chandra B, Gerber MA, et al. Iron overload in patients with chronic hepatitis C: a clinicopathologic study. Hum Pathol 1996;27:1277–81. [DOI] [PubMed] [Google Scholar]
- 9. Ley TJ, Griffith P, Nienhuis AW. Transfusion haemosiderosis and chelation therapy. Clin Haematol 1982;11:437–64. [PubMed] [Google Scholar]
- 10. McCormack TT, Sims J, Eyre-Brook I, et al. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut 1985;26:1226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


