Abstract
Background: Hirschsprung’s disease (HD) is a congenital disorder characterised by the absence of ganglion cells in the large bowel, leading to functional obstruction and colonic dilatation proximal to the affected segment. A subclass of nerve cell bodies in both submucosa and myenteric ganglia of the human gastrointestinal tract were found to show immunopositivity for calretinin, a calcium binding protein, which plays an important role in the organisation and functioning of the central nervous system.
Aim: To investigate calretinin immunostaining in ganglionic and aganglionic HD colon specimens, and compare it with staining for S100, neurone specific enolase, and c-kit.
Methods: Ten large bowel, full thickness specimens from patients with classic rectosigmoid HD were selected from the pathology repository. In total, 54 paraffin wax blocks—24 from the ganglionic zone, 17 from the aganglionic zone, and 13 from the transitional zone—were processed.
Results: Calretinin was not expressed in aganglionic segments of HD and associated nerve fibres, whereas in ganglionic HD segments and in normal colon both ganglion cells and nerve fibres were immunopositive. In addition, c-kit showed an altered distribution in the interstitial cells of Cajal. The transitional zone showed a broad spectrum of histomorphological and immunohistochemical patterns of both calretinin and c-kit expression.
Conclusion: The absence of calretinin expression may serve as a diagnostic aid in identifying aganglionic segments in HD.
Keywords: Hirschsprung’s disease, calretinin, S100, neurone specific enolase, c-kit
Hirschsprung’s disease (HD) is a congenital disorder characterised by the absence of ganglion cells in the large bowel, leading to functional obstruction and colonic dilatation proximal to the affected segment. The diagnosis of HD is usually based on a combination of the presenting symptoms, the radiological appearance of the bowel, rectal manometry, and histological features of rectal biopsies. Typical histological features of HD include the absence of ganglion cells and increased numbers of hypertrophic nerves.1–4
The traditional surgical treatment for HD consists of two operations. However, recently, several centres have moved towards a one step approach, making diagnostic accuracy even more crucial.5 Although most patients with HD have a satisfactory outcome after definitive corrective surgery, some have persistent bowel dysfunction including enterocolitis, constipation, and incontinence.6
“Typical histological features of Hirschsprung’s disease include the absence of ganglion cells and increased numbers of hypertrophic nerves”
The functions of the gastrointestinal tract are controlled by the enteric nervous system,7 the smooth muscle layers, and the interstitial cells of Cajal (ICCs).8 The ganglionated plexuses of the enteric nervous system are composed of neurones that subserve various functions, including motor neurones to the circular and longitudinal muscle layers, sensory neurones, ascending and descending interneurones, and secretomotor neurones.9 ICCs are pacemaker cells that generate slow waves and facilitate active propagation of electrical events and neurotransmission in the bowel wall.10 In the normal bowel, ICCs form a dense network surrounding the myenteric plexus, but in the aganglionic bowel of HD, these cells are sparse or absent.11
A subclass of nerve cell bodies in both submucosal and myenteric ganglia of the human gastrointestinal tract shows immunopositivity for calretinin, a calcium binding protein, which plays an important role in the organisation and functioning of the central nervous system.9
The aim of our study was to investigate and describe the characteristic calretinin immunostaining patterns seen in both ganglionic and aganglionic HD colon specimens, and compare this expression with that of S100, neurone specific enolase (NSE), and c-kit, thereby exploring its potential as a diagnostic method for HD.
MATERIALS AND METHODS
Ten large bowel, full thickness specimens from patients with classic rectosigmoid HD were selected from the pathology repository of the department of pathology at Sheba Medical Centre, Israel. The patients’ ages ranged from 3 days to 4 years, with a single patient who underwent surgery at the age of 22 years. The median age was 6 months. Three of the patients underwent two operations, and samples from both operations were included in our study. In total, 54 paraffin wax blocks or specimens were examined and processed. These comprised 17 from the aganglionic zone, 24 from the ganglionic zone, and 13 from the transitional zone. The transitional zone was defined as the segment between the contracted aganglionic segment and the normal or dilated ganglionated bowel, either as designated by the surgeon in cases submitted to frozen section, or as described in the postoperative gross macroscopic description of resection specimen. In addition, five colon specimens, received as surgical margins of the resected tumours, were used as normal colon.
Antibodies
Primary antibodies to the following antigens were used in our immunohistochemical study:
calretinin: polyclonal antibody (Zymed Laboratories Inc, South San Francisco, California, USA)
c-kit: CD117 polyclonal antibody (Biocare Medical, Walnut Greek, California, USA)
S100: polyclonal antibody (Dako, Glostrup, Denmark)
NSE: polyclonal antibody (Dako).
Immunohistochemical staining
Paraffin wax embedded, 4 μm thick sections of all tissues were thaw mounted on to Fisherbrand Super Frost Plus slides. After air drying at 37°C for 16 hours, incubation for 30 minutes at 60°C, dewaxing, and rehydration, slides were separated into two groups depending on the need for antigen retrieval. Slides for calretinin and c-kit were placed in jars containing 10mM citrate buffer (pH = 6.0), which were placed into a plastic pressure cooker filled with 1 litre of water. The pressure cooker was placed in a microwave oven, which was set at 750 W for 20 minutes to achieve boiling and for another 10 minutes to maintain boiling. The slides for S100 and NSE did not undergo antigen retrieval. To reduce background signals, all slides were incubated at room temperature with 10% non-immune goat serum for 15 minutes, followed by CAS block (Zymed Laboratories Inc) for 30 minutes. For S100 and NSE staining, the antibody was applied to the slides and incubated at room temperature for 30 minutes and for calretinin and c-kit for 60 minutes. Staining was performed with labelled avidin–biotin.12,13
Control sections consisted of serial sections from the same blocks that were similarly processed and analysed during the experiment except that no primary antibodies were used.
Histological and immunohistochemical analysis
Original and deeper sections stained with haematoxylin and eosin were carefully examined for the presence of ganglion cells in the submucosal and in the myenteric plexus. S100 immunostaining was positive in nerve fibres and NSE was positive in prominent ganglion cells. Positive calretinin immunostaining was seen in ganglion cells and nerve fibres in both the submucosal and myenteric plexus. Altered c-kit immunoexpression was defined as reduced numbers of positive cells or total loss of immunostaining. In addition, altered organisation and formation of small clusters was also considered abnormal.
All slides were evaluated by two pathologists, and the results were also compared with the original pathological report.
RESULTS
Normal colon
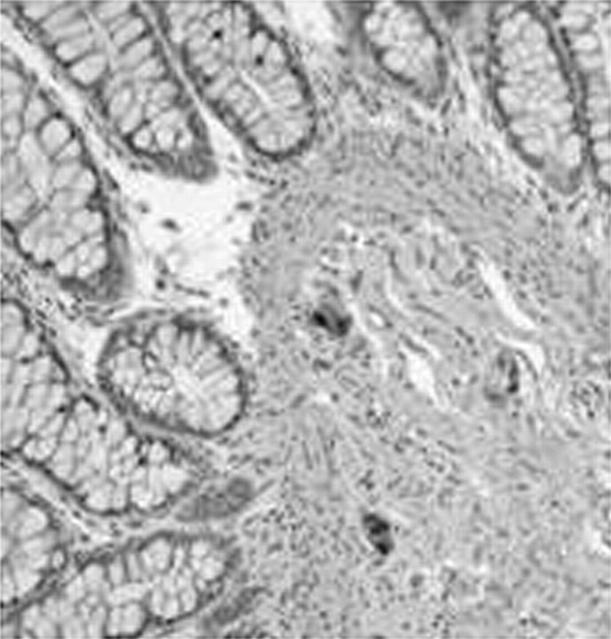
Haematoxylin and eosin staining and immunostaining for S100, NSE, calretinin, and c-kit demonstrated normal submucosal and myenteric plexus and normal intramuscular innervation. Intense immunostaining for NSE was seen in the ganglia. S100 immunostaining highlighted ganglion cells as prominent negatively stained cells surrounded by positive Schwann cells and nerve fibres. c-kit positive ICCs formed a dense network surrounding the myenteric plexus. More than 80% of the ganglion cells were immunopositive for calretinin, both in the submucosal (fig 1) and in the myenteric plexus, and the supporting Schwann cells and nerve fibres were also immunopositive.
Figure 1.
Normal colon. Positive immunostaining for calretinin in submucosal ganglion cells and Schwann cell elements and nerve fibres.
Aganglionic bowel of patients with HD
Features typical of aganglionic bowel were found in all HD specimens, including absence of ganglia in the submucosal and myenteric plexus and hypertrophic nerve fibres. NSE was weakly positive in the proliferating nerve fibres, and c-kit positive ICC cells were absent, sparse, or disorganised.
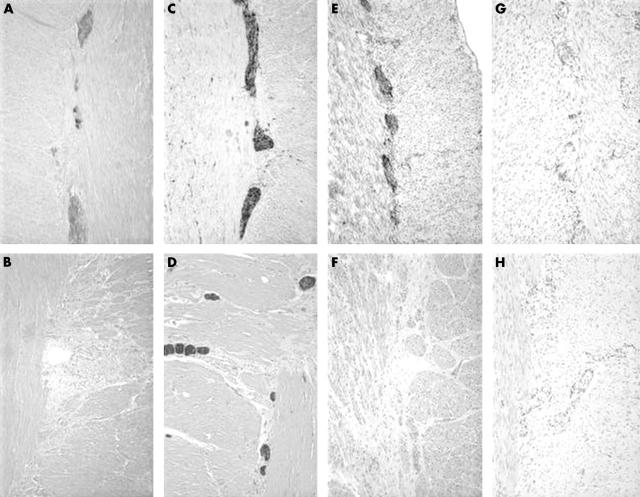
S100 immunostaining demonstrated the proliferation of the nerve fibres. In contrast to the prominent S100 immunostaining, calretinin immunoexpression was negative in the aganglionic segment. No ganglion cells were demonstrated and calretinin immunostaining was also negative in the nerve fibres (table 1; fig 2).
Table 1.
Calretinin, S100, NSE, and c-kit expression in different areas of the colon in patients with HD
| Calretinin staining* | S100 staining in nerve fibres | NSE staining in ganglionic cells | Abnormal c-kit staining | ||
| Ganglionic cells | Nerve fibres | ||||
| Ganglionic segment | 24/24 | 24/24 | 24/24 | 24/24 | 4/24 |
| Aganglionic segment | No ganglion cells | 0/17 | 17/17 | 0/17 | 17/17 |
| Transitional zone | 10/13 | 12/13 | 13/13 | 12/13 | 10/13 |
*Includes submucosal and myenteric plexus.
Hirschsprung’s disease; NSE, neurone specific enolase.
Figure 2.
(A) Calretinin expression in ganglion cells and nerve fibres in the ganglionic segment of Hirschsprung’s disease (HD), compared with (B) a lack of expression in nerve fibres in the aganglionic segment. S100 immunopositivity in nerve fibres in (C) ganglionic and (D) aganglionic segments of HD. Note the proliferation of nerve fibres in (C). (E) Immunopositivity for neurone specific enolase in the ganglion cells of the ganglionic segment, whereas the aganglionic segment is negative (F). (G) Immunopositivity for c-kit in the cells surrounding the myenteric plexus in the ganglionic segment, whereas the aganglionic segment is negative (H). The altered organisation of the c-kit positive cells was considered abnormal also.
Transitional zone of the bowel in patients with HD
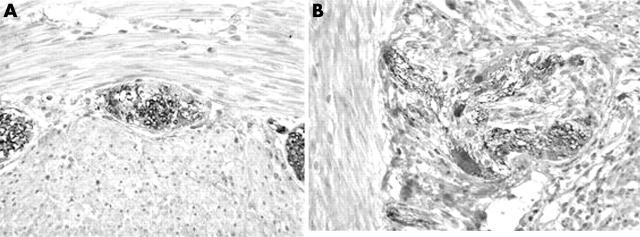
The transitional zone showed a broad spectrum of histomorphological and immunohistochemical patterns. Staining for NSE showed positive ganglion cells in 12 of 13 slides, but many cases showed only sparse and small ganglia cells in the myenteric and submucosal plexus. S100 showed partial or focal nerve fibre proliferation and was positive in all cases. Calretinin immunostaining was at least focally positive in the nerve fibres in 12 of 13 slides, and was positive in the ganglion cells in 10 of 13 slides, both in the submucosal and in the myenteric plexus. Many of the calretinin positive ganglion cells were small (table 1; fig 3). c-kit immunostaining showed abnormal ICC organisation in 10 of 13 cases, including very rare positive cells and cluster formation.
Figure 3.
The transitional zone. (A) Calretinin immunopositivity within the nerve fibres without staining of the ganglion cells. (B) Partial staining of the nerve fibres and focal ganglion cell immunopositivity.
Ganglionic bowel of patients with HD
The patterns of immunostaining for S100 and NSE were similar to those seen in the normal colon specimens. Calretinin immunostaining was similar to the control sections and more than 80% of the ganglia cells and nerve fibres in the submucosal and myenteric plexus were immunopositive. Compared with normal colon, slightly fewer ICCs were immunopositive for c-kit in four of the 24 slides (table 1; fig 2).
DISCUSSION
We found loss of calretinin immunostaining as a result of the absence of ganglion cells in the aganglionic colon of HD.
The pathological diagnosis of HD is established by demonstrating the lack of ganglion cells in the colonic neural plexus. The traditional approach is a full thickness segment,14 but alternatives such as “suction” or mucosal rectal biopsies are also frequently used.4,15 Suction biopsies are more difficult to interpret than full thickness biopsies because they only show the superficial portion of the submucosal neural plexus, and do not contain the more abundantly ganglionated and more easily evaluated myenteric plexus.8 The loss of ganglion cells is usually associated with the proliferation of nerve fibres. Acetylcholinesterase stains, mainly in frozen tissues, demonstrate the increased network of coarse, thickened, and irregular cholinergic nerve fibres within the muscularis mucosa in affected segments.3 The nerve fibres may also be highlighted using immunohistochemical stains such as PGP 9.5 or antibodies to S100, NSE, neurofilament protein, or the microtubule associated protein MAP5. NSE immunostains produce intense staining of ganglia, facilitating the recognition of small immature ganglion cells. S100 immunostaining highlights ganglion cells as prominent negatively stained cells surrounded by positive Schwann cells. However, despite the fact that these immunomarkers can facilitate the diagnosis in some cases, many specimens are still difficult to evaluate and may require careful and repeat deeper sections. In addition, the fact that the loss of calretinin expression in aganglionic segments was evident both in the submucosal and myenteric plexus, might help in the interpretation of smaller or submucosal biopsies.
The enteric nervous system, which controls the gastrointestinal tract, is composed of different types of neurones, which subserve various functions.7 In the normal colon, these neurones include motor neurones to the circular and longitudinal muscle layers, sensory neurones, ascending and descending interneurones, and secretomotor neurones.9 Immunohistochemical markers of many types have been used in an attempt to differentiate the functional subclasses of neurones in the enteric nervous system. These include antibodies directed against the following: (1) various peptides, such as the C-terminal region of substance P, tachykinins, vasoactive intestinal peptide, enkephalin, and neuropeptide Y; (2) enzymes, such as tyrosine hydroxylase, choline acetyltransferase, and nitric oxide synthase; and (3) the calcium binding proteins calretinin and calbindin.6,7 The calcium binding proteins are involved in the physiological buffering of excess cytosolic calcium ions, calcium transport, and protection against calcium ion overload.16–18 In their absence, there is an accumulation of excess calcium ions inside the cytoplasm, causing hyperexcitability, which often leads to neurodegeneration.16,18 Nerve cell bodies in both submucosa and myenteric ganglia of guinea pig19,20 and in the human gastrointestinal tract were found to show immunopositivity for calretinin and calbindin.21 In a previous study, immunoreactivity to calretinin was present in 23% of neurones projecting anally and 3% projecting orally within the myenteric plexus.9 Myenteric neurones with calretinin immunoreactivity were demonstrated with projections to the circular muscle in the myenteric ganglia in both the mucosa and submucosa. In addition, the interneurones with calretinin immunoreactivity were found to have anal polarity.9
“The fact that the loss of calretinin expression in aganglionic segments was evident both in the submucosal and myenteric plexus, might help in the interpretation of smaller or submucosal biopsies”
In our study, there was an absence of immunostaining for calretinin in the aganglionic colon of HD. No ganglion cells were noted. In addition, immunoexpression of calretinin by nerve fibres, which was present in the ganglionic colon of HD and in normal colon, was absent. This contrasts with the prominent nerve fibre proliferation demonstrated by S100 immunostaining. Therefore, the lack of calretinin immunostaining in the nerve fibres also represents the absence of related ganglion cells.
Take home messages.
Ganglion cells and nerve fibres express calretinin in ganglionic areas of Hirschsprung’s disease (HD) and in normal colon, whereas in patients with HD aganglionic segments and their associated nerve fibres show a lack of expression
The absence of calretinin expression may serve as a diagnostic aid in identifying aganglionic segments in HD
ICCs are distributed throughout the gastrointestinal tract and are thought to be pacemaker cells, which control neural and muscular intestinal activity.22
A recent study has demonstrated an altered distribution of ICCs in HD. Moreover, abnormalities in ICC organisation were found not only in the aganglionic segments, but in the entire resected bowel, suggesting that the persistent dysmobility problems after surgery in HD may be the result of the altered distribution and impaired function of ICCs throughout the colon.8 Special attention was given to the transitional zone, which was characterised by small and sparse ganglia accompanied by hypertrophic nerve bundles. The ICCs were evident as single cells or cell clusters closely related to the small myenteric ganglia, without the formation of the typical network of the normal bowel. In our study, in addition to the abnormalities of the organisation of the ICCs already described, some of the ganglion cells were immunonegative for calretinin in the transitional zone, whereas the nerve fibres were mostly positive. Calretinin immunopositive nerve fibres may represent connections to proximal calretinin positive ganglion cells. However, the abnormalities in the organisation of the ICCs in combination with the alterations in calretinin immunoreactivity in these regions may be some of the developmental abnormalities that occur between the ganglionic colon and the obvious aganglionic colon.
In summary, there was a lack of immunostaining for calretinin in aganglionic segments in patients with HD and in the nerve fibres in these areas, whereas both ganglion cells and nerve fibres showed calretinin expression in ganglionic areas of HD and in normal colon.
Abbreviations
HD, Hirschsprung’s disease
ICC, interstitial cell of Cajal
NSE, neurone specific enolase
REFERENCES
- 1.Bodian M , Stephens FD, Ward BCH. Hirschsprung’s disease and idiopathic megacolon. Lancet 1949;1:6–11. [DOI] [PubMed] [Google Scholar]
- 2.Larsson LT. Hirshprung’s disease—immunohistochemical findings. Histol Histopathol 1994;9:615–29. [PubMed] [Google Scholar]
- 3.Qualman SJ, Murray R. Aganglionosis and related disorders. Hum Pathol 1994;25:1141–9. [DOI] [PubMed] [Google Scholar]
- 4.Yunis EJ, Dobbins EW, Sherman FE. Rectal suction biopsy in the diagnosis of Hirschsprung’s disease in infants. Arch Pathol Lab Med 1976;100:329. [PubMed] [Google Scholar]
- 5.Maia DM. The reliability of frozen-section diagnosis in the pathologic evaluation of Hirschsprung’s disease. Am J Surg Pathol 2000;24:1675–7. [DOI] [PubMed] [Google Scholar]
- 6.Marty TL, Seo T, Matlak ME. Gastrointestinal function after surgical correction of Hirschsprung’s disease: long term follow-up in 135 patients. J Pediatr Surg 1995;30:655–8. [DOI] [PubMed] [Google Scholar]
- 7.Furness JB, Costa M. The enteric nervous system. Edinburgh: Churchill-Livingstone, 1987.
- 8.Rolle U , Piotrowska AP, Nemeth L, et al. Altered distribution of interstitial cells of Cajal in Hirschsprung disease. Arch Pathol Lab Med 2002;126:928–33. [DOI] [PubMed] [Google Scholar]
- 9.Wattchow DA, Porter AJ, Brookes SJ, et al. The polarity of neurochemically defined myenteric neurons in the human colon. Gastroenterology 1997;113:497–506. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen L , Robinson TL, Lee JCF, et al. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med 1998;4:848–50. [DOI] [PubMed] [Google Scholar]
- 11.Torihashi S , Horisawa M, Watanabe Y. c-kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst 1999;75:38–50. [DOI] [PubMed] [Google Scholar]
- 12.Guedson JL, Ternynck T, Avrameas S. The use of avidin–biotin interaction in immunoenzymatic technique. J Histochem Cytochem 1997;27:1131–9. [DOI] [PubMed] [Google Scholar]
- 13.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 1996;84:345–57. [DOI] [PubMed] [Google Scholar]
- 14.Swenson O , Fisher JH, MacMahon HE. Rectal biopsy as an aid in the diagnosis of Hirschsprung’s disease. N Engl J Med 1955;253:632–5. [DOI] [PubMed] [Google Scholar]
- 15.Dobbins WO III, Bill AH Jr. Diagnosis of Hirschsprung’s disease excluded by rectal suction biopsy. N Engl J Med 1965;272:990–3. [DOI] [PubMed] [Google Scholar]
- 16.Heizmann CW. Paraalbumin, an intracellular calcium binding protein: distribution, properties and possible roles in mammalian cells. Experimentia 1984;40:910–21. [DOI] [PubMed] [Google Scholar]
- 17.Jande SS, Maler L, Lawson DEM. Immunocytochemical mapping of vitamin D-dependent calcium binding proteins in brain. Nature 1981;294:765–7. [DOI] [PubMed] [Google Scholar]
- 18.Braun K . Calcium-binding proteins in avian and mammalian central nervous system: localization, development and possible functions. Prog Histochem Cytochem 1990;21:1–64. [DOI] [PubMed] [Google Scholar]
- 19.McConalogue K , Furness JB. Calretinin immunoreactivity of motor neurons in the guinea-pig distal colon and tinia coli. Cell Tissue Res 1996;284:367–72. [DOI] [PubMed] [Google Scholar]
- 20.Messenger JP, Bornstein JC, Furness JB. Electrophysiological and morphological classification of myenteric neurons in the proximal colon of guinea-pig. Neuroscience 1994;60:227–44. [DOI] [PubMed] [Google Scholar]
- 21.Walters JR, Bishop AE, Facer P, et al. Calretinin and calbindin-D28k immunoreactivity in the human gastrointestinal tract. Gastroenterology 1993;104:1381–9. [DOI] [PubMed] [Google Scholar]
- 22.Tuneberg L . Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol 1982;71:1–130. [PubMed] [Google Scholar]