Abstract
Aims: To assess the potential value of chromosome in situ hybridisation (CISH), Ki-67, and telomerase immunocytochemistry in liquid based cervical cytology to help detect carcinoma cells and precursors.
Method: Sixty ThinPrep processed cervical cytology samples were studied: 23 cases within the normal limit, 13 low grade squamous intraepithelial lesions (LSILs), 10 high grade squamous intraepithelial lesions (HSILs), six squamous cell carcinomas, three endocervical adenocarcinomas, two cervical adenosquamous cell carcinomas, and three endometrial adenocarcinomas. CISH was performed with DNA probes specific for the pericentromeric regions of chromosome 11 and 16. Hybridisation signals were visualised with the streptavidin–biotin peroxidase technique. The monoclonal MIB1 and polyclonal TRT-H231 antibodies were used to detect Ki-67 and telomerase immunoreactivity, respectively.
Results: Non-specific background staining was almost absent in CISH slides. Normal squamous and glandular cells showed a diploid chromosomal pattern. A relative gain in chromosomes 11 and 16 (aneusomy) was seen in HSIL and the carcinomas (p<0.0001). In MIB1 stained smears, normal cells and koilocytes showed inconspicuous immunoreactivity, whereas strongly immunoreactive nuclei were found in cancer cells and HSIL (p<0.0001). Not only carcinoma and HSIL cells, but also some normal cells, showed cytoplasmic staining for telomerase.
Conclusions: These preliminary results indicate that ThinPrep processed cervical smears are suitable for CISH and immunocytochemical studies. The neoplastic squamous and glandular cells were easily identified based on nuclear aneusomy and strong Ki-67 immuoreactivity in the context of abnormal nuclear morphology. This is the first study to apply CISH in cervical cytology using an immunoenzymatic approach.
Keywords: liquid based cervical cytology, chromosome in situ hybridisation, Ki-67, telomerase
Cervical cancer is one of the most common forms of cancer in women worldwide. Cytological examination of cervical smears is the most widely applied screening method for cervical cancer and its precursors. However, the success of the Papanicolaou smear test is limited with respect to sensitivity and specificity. False negative rates for cervical premalignant lesions and cervical cancer lie between 15% and 50% and false positive rates of approximately 30% have been reported.1 Such suboptimal performance may be related to the subjectivity of cytological diagnosis. ThinPrep processing, an automated cytopreparatory method, has been reported to produce good quality cervical cytology preparations. Moreover, it allows the use of auxiliary laboratory techniques, which may help to distinguish neoplastic from benign diseases, and thus may further improve the efficiency of cancer detection in cervical cytology.
A wide array of immunohistochemical and molecular markers have been tested to evaluate their specificity in staining dysplastic cells in cervical smears.2–8 Immunohistochemical detection of the MIB1 (Ki-67) antigen is one of the most frequently used methods for studying cell proliferation in cancer.9 We have shown in earlier studies that Ki-67 expression in cervical carcinoma has prognostic relevance.10 Immunocytochemical detection of Ki-67 was also found to be useful in the evaluation of atrophic cervical smears.11,12
“ThinPrep processing, an automated cytopreparatory method, has been reported to produce good quality cervical cytology preparations”
Another possible parameter is the assessment of telomerase activity in cervical samples. We have shown previously, by the polymerase chain reaction based telomeric repeat amplification protocol (TRAP) assay, that telomerase activity is present in all stages of cervical cancer, whereas such activity is weak in the normal cervix, suggesting that it is an early event in cancer.13 Telomerase activity in cancer lesions was found to be quantitatively distinct from that in premalignant lesions, which may mean a much more pronounced activation of telomerase in cancers than in squamous intraepithelial lesions (SILs).14 It has been suggested that the telomerase assay using cervical scrapings might be a useful screening method for cervical lesions, especially when combined with a Papanicolaou smear test.15
The application of fluorescence in situ hybridisation (FISH) as an adjunct to conventional cytology in diagnosing malignant diseases has been reported.16–21 These studies take advantage of the fact that, although normal cells are usually diploid, many of the common malignant tumours harbour numerical chromosomal abnormalities that can be detected by FISH. Most of these chromosome in situ hybridisation (CISH) studies use fluorescence for visualisation. In our opinion, visualisation of such signals using a non-fluorescence immunoenzymatic approach might be a more convenient means of evaluation in pathology laboratories.22,23
The objective of our study was to assess the potential value of CISH, Ki-67, and telomerase immunohistochemistry in liquid based cytology samples for the detection of carcinoma cells and precursors. To the best of our knowledge, this is the first report documenting such an application in ThinPrep residues of cervical cytology samples.
MATERIALS AND METHODS
Clinical samples and preparation of slides
All ThinPrep reagents were obtained from Cytyc, Boxborough, Massachusetts, USA. Sixty ThinPrep processed cervical cytology samples were collected from the department of pathology, Queen Mary Hospital, the University of Hong Kong. Sampling was performed using a cervexbrush (Rovers Medical Devices BV, Oss, the Netherlands), which was then rinsed in the ThinPrep transport solution (PreservCyt). A ThinPrep thin layer cytological smear was prepared from the cell suspension using the ThinPrep 2000 processor.24 If necessary, the specimens were treated with CytoLyt solution to lyse red blood cells and remove mucous. Table 1 lists the cytological diagnoses, which were subsequently confirmed by follow up or biopsy data. The sediment was resuspended in PreservCyt solution, and additional thin layer slides were prepared using the ThinPrep 2000 processor and fixed in 95% alcohol. These unstained ThinPrep slides remained in alcohol until use for immunocytochemistry and CISH. The cytological diagnosis was revealed only after the performance and evaluation of the immunocytochemistry and CISH was completed.
Table 1.
Diagnosis of liquid based cytology samples used in our study
| Cytological diagnosis | Number of cases | Histological diagnosis in follow up biopsy |
| Within normal limit | 23 | |
| LSIL | 13 | CIN I and or condyloma (n = 9) |
| CIN II (n = 1) | ||
| Cervicitis (n = 1) | ||
| HSIL | 10 | CIN III (n = 3) |
| Squamous cell carcinoma (n = 2) | ||
| Squamous cell carcinoma | 6 | Squamous cell carcinoma (n = 6) |
| Endocervical adenocarcinoma | 3 | Endocervical adenocarcinoma (n = 3) |
| Cervical adenosquamous cell carcinoma | 2 | Adenosquamous cell carcinoma (n = 2) |
| Endometrial adenocarcinoma | 3 | Endometrial adenocarcinoma (n = 3) |
CIN, cervical intraepithelial neoplasia; HSIL, high grade intraepithelial lesion; LSIL, low grade intraepithelial lesion.
Chromosome in situ hybridisation
CISH was performed as described previously.22,23,25 DNA probes specific for the pericentromeric regions of chromosome 11 (D11Z1) and chromosome 16 (D16Z3) (American Type Culture Collection, Rockville, Maryland, USA) were labelled with biotin by the nick translation method (Boehringer, Mannheim, Germany). These probes were chosen because, based on our experience, they produced good CISH signals with little non-specific background that would affect the interpretation.
ThinPrep cytology specimens were prepared using 2% aminopropyltriethoxysilane coated slides from the ThinPrep cellular residues of the above samples. The slides were stored in 95% alcohol at room temperature until used for the CISH studies. The slides were incubated first with sodium thiocyanate (Sigma, St Louis, Missouri, USA) and then proteinase K (250–500 μg/ml) and 0.1% Triton X-100 at 50°C. The labelled probes were added to the hybridisation mix (60% formamide, 10% dextran sulfate, and 2× saline sodium citrate) and applied to the tissue sections at probe concentrations of 1 ng/μl of hybridisation mixture. Denaturation was performed at 90°C, followed by overnight hybridisation at 37°C. Immunocytochemistry was performed using avidin, biotinylated mouse anti-avidin, rabbit antimouse peroxidase, and 3,3′-diaminobenzidine (Dakopatts Ltd, Ely, Cambridgeshire, UK); hydrogen peroxidase was used to visualise the peroxidase activity.
Scoring criteria of CISH
The criteria published by Florentine et al were followed.20 The morphology of the nuclei was taken into consideration. Around 10 to 100 cells were evaluated. Artefactual hyperdiploidy—that is, nuclei that might be misinterpreted as hyperdiploid as a result of artificial cellular clumping or multinucleation—was ruled out. A sample was considered malignant if at least four nuclei were hyperdiploid for one or more chromosomes. The sample was considered inconclusive if fewer numbers of these nuclei were seen or if artefactual hyperdiploidy could not be ruled out. A sample was considered benign (negative) if all of the nuclei analysed gave diploid signals.
Immunocytochemistry for Ki-67 and telomerase
From the ThinPrep residue, ThinPrep thin layer cytology was first prepared using the ThinPrep 2000 processor and dried overnight in an oven at 37°C. Immunocytochemical studies were performed using a streptavidin–biotin peroxidase (Dako, Glostrup, Denmark) technique after preheating in a microwave oven.26 Endogenous peroxidase was blocked using 3% H2O2 in distilled water. The slides were immersed in 10mM (pH 6) sodium citrate buffer in a thermoresistant plastic box and were processed in a microwave oven for five minutes at 700 W. The slides were then cooled in phosphate buffered saline before immunostaining. The monoclonal antibody MIB1 (Zymed Laboratories, South San Francisco, California, USA) and the polyclonal antibody TRT-H231 (Santa Cruz Biotechnology, Santa Cruz, California, USA), neat and at a 1/50 dilution, respectively, were applied. Appropriate negative and positive controls were included. Diaminobenzidine hydrogen peroxide was used as chromogen. A light haematoxylin counterstain was used.
Scoring for Ki-67 and telomerase immunoreactivity
The whole slide was screened. At least 10 epithelial groups with the highest immunoreactivity were evaluated and marked in each smear. Cells with abnormal cytological features were included for assessment. The expression of Ki-67 and telomerase in the marked cell groups in each smear was recorded as negative, inconclusive, or positive based on the percentage of positive nuclei (< 10%, 10–50%, and > 50%, respectively).11
RESULTS
Chromosome in situ hybridisation
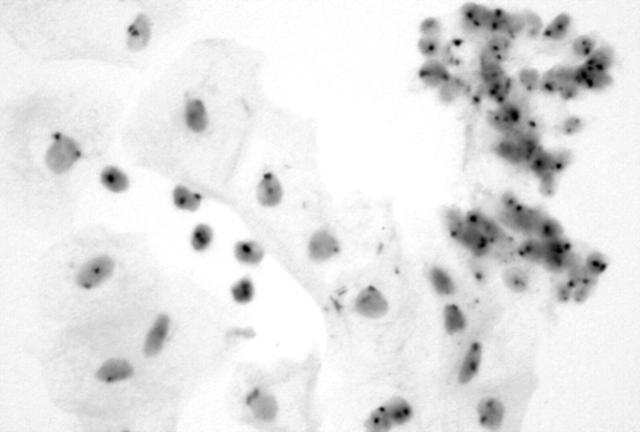
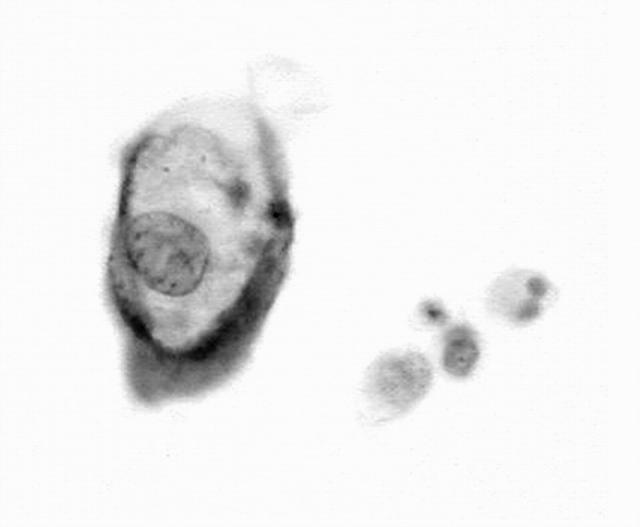
Non-specific background staining was almost absent. Table 2 summarises the results of the CISH signal analysis. Normal squamous and glandular cells (fig 1) and koilocytes (fig 2) showed a diploid chromosomal pattern. A relative gain in chromosomes 11 and 16 (aneusomy) was seen in high grade SIL (HSIL; fig 3) and in the carcinomas. Smears within the normal limit and low grade SIL (LSIL) were significantly more likely to display disomy for chromosome 11 (p < 0.0001) and chromosome 16 (p < 0.0001) than were HSIL or cancer cases.
Table 2.
Cytological diagnosis and results of the CISH studies for chromosomes 11 and 16 in the cervical cytology samples
| Cytological diagnosis | No of Cases | Chromosome 11 | Chromosome 16 | |||||
| Cases with pure diploid signals | Inconclusive findings | Cases with >4 nuclei with ⩾3 ISH signals | Cases with pure diploid signals | Inconclusive findings | Cases with >4 nuclei with ⩾3 ISH signals | |||
| Negative | 23 | 23 | 0 | 0 | 23 | 0 | 0 | |
| LSIL | 13 | 13 | 0 | 0 | 13 | 0 | 0 | |
| HSIL | 10 | 0 | 1 | 9 | 0 | 2 | 8 | |
| SCC | 6 | 0 | 0 | 6 | 0 | 0 | 6 | |
| Cx AdenoCA | 3 | 0 | 1 | 2 | 0 | 1 | 2 | |
| Cx AdenoSqCA | 2 | 0 | 0 | 2 | 0 | 0 | 2 | |
| Em AdenoCA | 3 | 0 | 0 | 3 | 0 | 1 | 2 | |
| p<0.0001 | p<0.0001 | |||||||
CISH, chromosome in situ hybridisation; Cx AdenoCA, endocervical adenocarcinoma; Cx AdenoSqCA, cervical adenosquamous cell carcinoma; Em AdenoCA, endometrial adenocarcinoma; HSIL, high grade intraepithelial lesion; ISH, in situ hybridisation; LSIL, low grade intraepithelial lesion; negative, cases within the normal limit; SCC, squamous cell carcinoma.
Figure 1.
Normal squamous and endocervical cells showing two copies of the in situ hybridisation signals for chromosome 11.
Figure 2.
Low grade squamous intraepithelial lesion (koilocytes) showing two copies of the in situ hybridisation signals for chromosome 11.
Figure 3.
High grade squamous intraepithelial lesion (cervical intraepithelial neoplasia III) showing multiple copies of the in situ hybridisation signals for chromosome 11.
Ki-67 immunoreacitvity
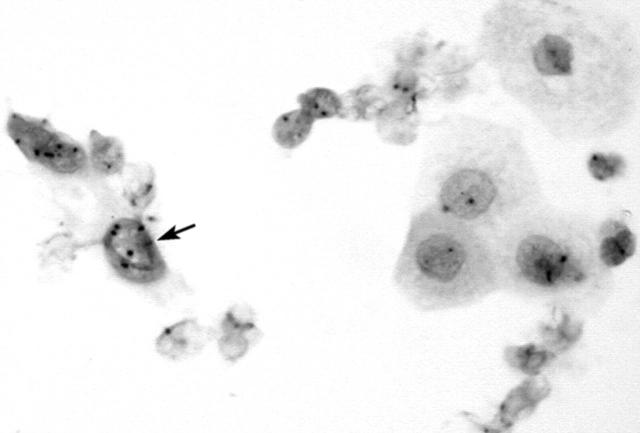
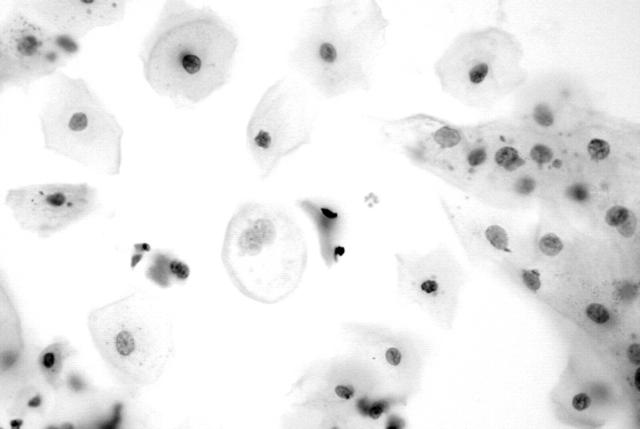
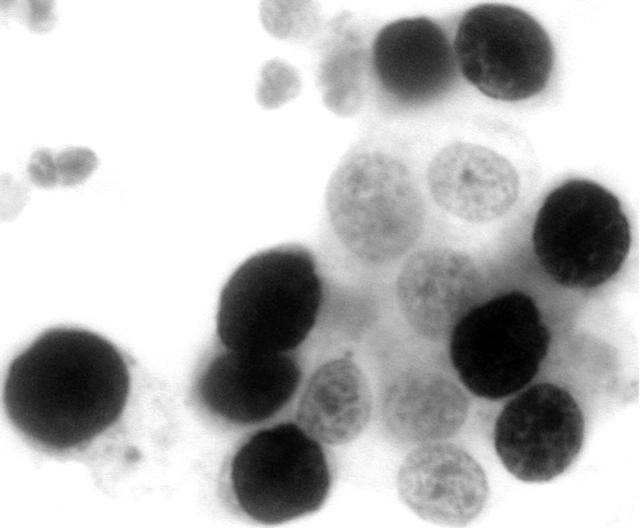
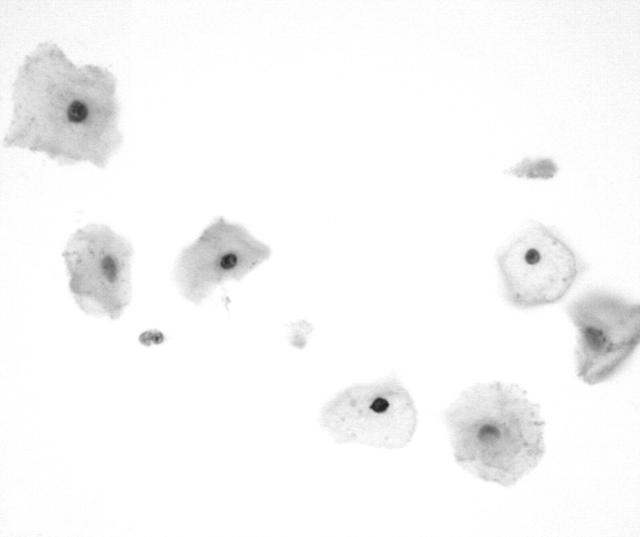
Immunoreactivity for the Ki-67 antigen was exclusively confined to the nuclei, with no cytoplasmic staining detected. Table 3 summarises the results of the Ki-67 staining analysis. Normal squamous cells, koilocytes, and endocervical cells showed a lack of immunoreactivity (fig 4). Only occasional endometrial cells (fig 5) and mildly dyskaryotic cells in LSIL showed nuclear staining. Numerous strongly immunoreactive nuclei were found in all types of carcinoma cells (figs 6 and 7) available on the smear. HSIL cells (fig 8) were also Ki-67 immunopositive. There was a significant difference in Ki-67 immunoreactivity between the group containing normal or LSIL cases and the group containing HSIL or cancer cases (p < 0.0001).
Table 3.
Cytological diagnosis and results of Ki-67 immunocytochemistry
| Cytological diagnosis | No of cases | Ki-67 | ||
| Negative | Inconclusive | Positive | ||
| Negative | 23 | 23 | 0 | 0 |
| LSIL | 13 | 13 | 0 | 0 |
| HSIL | 10 | 0 | 2 | 8 |
| SCC | 6 | 0 | 1 | 5 |
| Cx AdenoCA | 3 | 0 | 0 | 3 |
| Cx AdenoSqCA | 2 | 0 | 0 | 2 |
| Em AdenoCA | 3 | 0 | 1 | 2 |
| p<0.0001 | ||||
Cx AdenoCA, endocervical adenocarcinoma; Cx AdenoSqCA, cervical adenosquamous cell carcinoma; Em AdenoCA, endometrial adenocarcinoma; HSIL, high grade intraepithelial lesion; LSIL, low grade intraepithelial lesion; negative, cases within the normal limit; SCC, squamous cell carcinoma.
Figure 4.
Koilocyte and normal squamous cells showing a lack of reactivity for MIB-1 (Ki-67).
Figure 5.
Normal endometrial cells showing a lack of reactivity for MIB-1 (Ki-67).
Figure 6.
Endometrial carcinoma (grade I) showing reactivity for MIB-1 (Ki-67).
Figure 7.
Endometrial carcinoma (grade III) showing extensive reactivity for MIB-1 (Ki-67).
Figure 8.
High grade intraepithelial lesion (cervical intraepithelial neoplasia III) showing reactivity for MIB-1 (Ki-67).
Telomerase immunoreactivity
Telomerase immunoreactivity was found in both the nuclei and the cytoplasm. Background staining was present in some cases. Table 4 summarises the results of the telomerase staining analysis. The carcinoma cells and cells in HSIL (fig 9) showed positive staining. Some koilocytes were also immunoreactive (fig 10). A negative or weakly positive reaction was occasionally seen in some squamous cells (fig 11), metaplastic cells, normal endocervical cells, and endometrial cells. However, the staining was slightly stronger in cancer cells and HSIL cells. There was no significant difference in telomerase immunoreactivity between the group containing normal or LSIL cases and the group containing HSIL or cancer cases (p = 0.112).
Table 4.
Cytological diagnosis and results of telomerase immunocytochemistry
| Cytological diagnosis | No of cases | Telomerase– (%) | Telomerase inconclusive | Telomerase+ (%) |
| Negative | 23 | 10 | 10 | 3 |
| LSIL | 13 | 2 | 4 | 7 |
| HSIL | 10 | 3 | 1 | 6 |
| SCC | 6 | 1 | 1 | 4 |
| Cx AdenoCA | 3 | 1 | 1 | 1 |
| Cx AdenoSqCA | 2 | 0 | 1 | 1 |
| Em AdenoCA | 3 | 0 | 1 | 2 |
| p = 0.112 |
Cx AdenoCA, endocervical adenocarcinoma; Cx AdenoSqCA, cervical adenosquamous cell carcinoma; Em AdenoCA, endometrial adenocarcinoma; HSIL, high grade intraepithelial lesion; LSIL, low grade intraepithelial lesion; negative, cases within the normal limit; SCC, squamous cell carcinoma.
Figure 9.
High grade intraepithelial lesion (cervical intraepithelial neoplasia III) showing immunoreactivity for telomerase.
Figure 10.
Low grade intraepithelial lesion (koilocytes) showing immunoreactivity for telomerase.
Figure 11.
Normal squamous cells showing lack of immunoreactivity for telomerase.
DISCUSSION
The use of Papanicolaou smears to screen for cervical carcinoma is widely recognised as an effective measure. However, erroneous and equivocal diagnoses still exist.1,27 Various approaches are being explored to improve the sensitivity and specificity of the test. In particular, human papillomavirus testing has permitted the sensitive identification of women who will progress to HSIL or even carcinoma.27
In addition, molecular and biological markers have been tested for the detection of malignant cells in cervical smears.4–6 Because it is known that complex numerical chromosomal abnormalities evolve during anaplastic transformation,22,23 in situ hybridisation can be performed in cytology specimens,16,20,21 including cervical cytology samples,19 to evaluate the chromosome composition.
“Our approach using immunoenzymatic visualisation of in situ hybridisation signals makes evaluation much easier and the signals are more permanent”
The liquid based cytological preparation technique has been developed to collect exfoliated cervical cells in liquid buffer for transportation to the cytology laboratory for preparation as thin layer slides. It has received Food and Drug Administration approval for clinical use. ThinPrep cytology has shown an equivalent or increased rate of detection of SIL and carcinoma in comparison with conventional Papanicolaou smears.24 Because the production of a ThinPrep slide uses only a fraction of the cells collected in PreservCyt buffer, the residual cells remaining in the vial are available for ancillary studies. It is hoped that this could facilitate reliable detection and minimise equivocal diagnoses based on a single clinical collection, without the need for repeated patient appointments. Combined cytopathological diagnosis and ancillary tests from PreservCyt specimens is an interesting option.
Most of the earlier studies analysing CISH on cytology preparations use fluorescence approaches.16–21,28 Although hyperdiploid signals could be detected, quantitative assessment of in situ hybridisation (ISH) signals may be difficult in such specimens, and may not be a convenient measure in the routine cytology laboratory. Our approach using immunoenzymatic visualisation of ISH signals makes evaluation much easier and the signals are more permanent. We found that the detection of cells with chromosomal aneusomy is a reliable marker for malignancy, including HSIL and different types of cancer.
The number of abnormal cells in each slide varied in different cases. In some cases, only a small number of dysplastic or malignant cells could be identified for assessment of the ISH signals. The interpretation is more reliable when more abnormal cells are available for assessment. However, the observation of a distinctly raised copy number of targeted chromosomes indicates the high sensitivity of the CISH test, which can highlight even small numbers of abnormal cells.
Several studies have shown that Ki-67 immunocytochemistry can be used to distinguish proliferating and non-proliferating cells in both histological and cytological specimens.29–32 These studies showed that the number and distribution of Ki-67 positive cells correlated well with the grade of cervical intraepithelial neoplasia (CIN). The Ki-67 labelling index is low in normal cases and gradually increases with CIN.33–35 Ki-67 immunoreactivity has been found to be a useful and reliable proliferation marker in smears from postmenopausal women.11,34
In our study, Ki-67 immunoreactivity was mostly positive in HSIL and carcinoma cells and negative in normal cells and LSIL, so that this marker could be used to distinguish between these two groups. The findings suggest that Ki-67 could be a convenient and reliable marker to assist in the cytological diagnosis in ThinPrep samples.
Telomerase is an enzyme that replenishes short stretches of repeat nucleotides lost from the telomeric ends of chromosomes with each round of replication. Studies in both tumour cell lines and human tumour specimens have shown that, in contrast to normal somatic cells, most malignant cells are characterised by increased telomerase activity.36–39 Telomerase activity has been reported in association with CIN II/III and cervical cancer, with a difference being seen between premalignant and invasive cervical cancerous lesions.13,38,40,41
The determination of telomerase activity has been suggested as a test for early cancer detection. Assessment of telomerase activity in cervical scrapings has been reported to be potentially useful for screening of cervical cancer even in cases with a negative Papanicolaou smear and for triage of women with borderline smears,42–47 and has thus been explored as a molecular marker for cervical cancer screening.
We intended to investigate the usefulness of telomerase immunocytochemistry in ThinPrep cell residues because this could be a convenient way of assessing telomerase activity. However, unfortunately, this was hampered by significant background staining. Moreover, telomerase staining was unhelpful in the distinction between normal or LSIL samples and any of the lesions examined. There may be several reasons for this. The specificity of the antibody may be a problem. Alternatively, these results also agree with our recent findings that the detection of telomerase activity in cervical scrapings by TRAP had no significant association with HSIL.46
“We found that the detection of cells with chromosomal aneusomy is a reliable marker for malignancy, including high grade squamous cell intraepithelial lesions and different types of cancer”
Our preliminary results indicate that ThinPrep processed cervical smears are suitable for CISH and immunocytochemical studies. The abnormal squamous and glandular cells could easily be identified based on nuclear aneusomy and strong Ki-67 immunoreactivity in the context of abnormal nuclear morphology. CISH, Ki-67, and telomerase immunocytochemistry in liquid based cervical cytology can assist in the detection of carcinoma cells and precursors. To the best of our knowledge, this is the first study illustrating the application of CISH in cervical cytology using an immunoenzymatic approach, which may be a more convenient approach in the service pathology laboratory. However, our study was limited by the number of cases included for each category and more cases should be studied to confirm this application.
Take home messages.
ThinPrep processed cervical smears appear to be suitable for chromosome in situ hybridisation using an immunoenzymatic approach and immunocytochemical studies
Neoplastic squamous and glandular cells were easily identified based on nuclear aneusomy and strong Ki-67 immuoreactivity in the context of abnormal nuclear morphology
Immunohistochemistry for telomerase was not useful in distinguishing between neoplastic and non-neoplastic cells
Larger studies need to be undertaken to verify the results of these preliminary investigations
Acknowledgments
This study was supported by University of Hong Kong Conference and Research Grant. Part of the study has been presented to the American Association for Cancer Research 92nd Annual Meeting. Some of the ThinPrep reagents were discounted by Cytyc Inc (Boxborough, Massachusetts, USA).
Abbreviations
CIN, cervical intraepithelial neoplasia
CISH, chromosome in situ hybridisation
FISH, fluorescence in situ hybridisation
LSIL, low grade squamous intraepithelial lesion
HSIL, high grade squamous intraepithelial lesion
ISH, in situ hybridisation
SIL, squamous intraepithelial lesion
TRAP, telomeric repeat amplification protocol
REFERENCES
- 1.Jacobs MV, Snijders PJF, Voorhorst FJ, et al. Reliable high risk HPV DNA testing by polymerase chain reaction: an intermethod and intramethod comparison. J Clin Pathol 1999;52:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rimm DL. Molecular biology in cytopathology—current applications and future directions. Cancer Cytopathol 2000;90:1–9. [DOI] [PubMed] [Google Scholar]
- 3.Weaver EJ, Kovatich AJ, Bibbo M. Cyclin E expression and early cervical neoplasia in ThinPrep specimens—a feasibility study. Acta Cytol 2000;44:301–4. [DOI] [PubMed] [Google Scholar]
- 4.Keating JT, Ince T, Crum CP. Surrogate biomarkers of HPV infection in cervical neoplasia screening and diagnosis. Adv Anat Pathol 2001;8:83–92. [DOI] [PubMed] [Google Scholar]
- 5.Negri G , Egarter-Vigl E, Kasal A, et al. p16INK4a is a useful marker for the diagnosis of adenocarcinoma of the cervix uteri and its precursors: an immunohistochemical study with immunocytochemical correlations. Am J Surg Pathol 2003;27:187–93. [DOI] [PubMed] [Google Scholar]
- 6.Gong Y , Sun X, Michael CW, et al. Immunocytochemistry of serous effusion specimens: a comparison of ThinPrep vs cell block. Diagn Cytopathol 2003;28:1–5. [DOI] [PubMed] [Google Scholar]
- 7.Han AC, Filstein MR, Hunt JV, et al. N-cadherin distinguishes pleural mesotheliomas from lung adenocarcinomas: a ThinPrep immunocytochemical study. Cancer 1999;87:83–6. [DOI] [PubMed] [Google Scholar]
- 8.Leung SW, Bedard YC. Immunocytochemical staining on ThinPrep processed smears. Mod Pathol 1996;9:304–6. [PubMed] [Google Scholar]
- 9.Cheung ANY. The use of markers of cell proliferation and cell loss in gynaecological cancers. Curr Opin Obstet Gynecol 1997;9:32–6. [PubMed] [Google Scholar]
- 10.Liu SS, Tsang BK, Cheung AN, et al. Anti-apoptotic proteins, apoptotic and proliferative parameters and their prognostic significance in cervical carcinoma. Eur J Cancer 2001;37:1104–10. [DOI] [PubMed] [Google Scholar]
- 11.Ejersbo D , Jensen HA, Holund B. Efficacy of Ki-67 antigen staining in Papanicolaou (Pap) smears in post-menopausal women with atypia—an audit. Cytopathology 1999;10:369–74. [DOI] [PubMed] [Google Scholar]
- 12.Bulten J , Van Der Laak JAWM, Gemmink JH, et al. MIB1, a promising marker for the classification of cervical intraepithelial neoplasia. J Pathol 1996;178:268–73. [DOI] [PubMed] [Google Scholar]
- 13.Zhang DK, Ngan HY, Cheng RY, et al. Clinical significance of telomerase activation and telomeric restriction fragment (TRF) in cervical cancer. Eur J Cancer 1999;35:154–60. [DOI] [PubMed] [Google Scholar]
- 14.Kyo S , Takakura M, Tanaka M, et al. Telomerase activity in cervical cancer is quantitatively distinct from that in its precursor lesions. Int J Cancer 1998;79:66–70. [DOI] [PubMed] [Google Scholar]
- 15.Kyo S , Takakura M, Ishikawa H, et al. Application of telomerase assay for the screening of cervical lesions. Cancer Res 1997;57:1863–7. [PubMed] [Google Scholar]
- 16.Chen Z , Wang DD, Peier A, et al. FISH in the evaluation of pleural and ascitic fluids. Cancer Genet Cytogenet 1995;84:116–19. [DOI] [PubMed] [Google Scholar]
- 17.Cajulis RS, Frias-Hidvegi D, Yu GH, et al. Detection of numerical chromosomal abnormalities by fluorescence in situ hybridisation of interphase cell nuclei with chromosome-specific probes on archival cytologic samples. Diagn Cytopathol 1996;14:178–81. [DOI] [PubMed] [Google Scholar]
- 18.Johnson TM, Kuffel DG, Dewald GW. Detection of hyperdiploid malignant cells in pleural effusions with chromosome-specific probes and fluorescence in situ hybridization. Mayo Clin Proc 1996;71:643–8. [DOI] [PubMed] [Google Scholar]
- 19.Hariu H , Matsuta M. Cervical cytology by means of fluorescence in situ hybridization with a set of chromosome-specific DNA probes. J Obstet Gynaecol Res 1996;22:163–70. [DOI] [PubMed] [Google Scholar]
- 20.Florentine BD, Sanchez B, Raza A, et al. Detection of hyperdiploid malignant cells in body cavity effusions by fluoresence in situ hybridization on ThinPrep slides. Cancer 1997;81:299–308. [DOI] [PubMed] [Google Scholar]
- 21.Inoue T , Nasu Y, Tsushima T, et al. Chromosomal numerical aberrations of exfoliated cells in the urine detected by fluorescence in situ hybridization: clinical implication for the detection of bladder cancer. Urol Res 2000;28:57–61. [DOI] [PubMed] [Google Scholar]
- 22.Cheung AN, Tin VP, Ngan HY, et al. Interphase cytogenetic study of endometrial stromal sarcoma by chromosome in situ hybridization. Mod Pathol 1996;9:910–18. [PubMed] [Google Scholar]
- 23.Shen DH, Khoo US, Xue WC, et al. Ovarian mature cystic teratoma with malignant transformation. An interphase cytogenetic study. Int J Gynecol Pathol 1998;17:351–7. [DOI] [PubMed] [Google Scholar]
- 24.Cheung ANY, Szeto EF, Leung BSY, et al. Liquid based cytology and conventional cervical smears: a comparison study in an Asian screening population. Cancer Cytopathol 2003;99:331–5. [DOI] [PubMed] [Google Scholar]
- 25.Xue WC, Guan XY, Ngan HYS, et al. Malignant placental site trophoblastic tumor: a cytogenetic study using comparative genomic hybridization and chromosome in situ hybridization. Cancer 2002;94:2288–94. [DOI] [PubMed] [Google Scholar]
- 26.Cheung ANY, Ngan HYS, Collins RJ, et al. Assessment of cell proliferation in hydatidiform mole using monoclonal antibody MIB1 to Ki-67 antigen. J Clin Pathol 1994;47:601–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherman ME, Schiffman MH, Lorincz AT, et al. Cervical specimens collected in liquid buffer are suitable for both cytologic screening and ancillary human papillomavirus testing. Cancer 1997;81:89–97. [PubMed] [Google Scholar]
- 28.Segers P , Haesen S, Castelain P, et al. Study of numerical aberrations of chromosome 1 by fluorescent in situ hybridization and DNA content by densitometric analysis on (pre)-malignant cervical lesions. Histochem J 1995;27:24–34. [DOI] [PubMed] [Google Scholar]
- 29.Heatley MK. What is the value of proliferation markers in the normal and neoplastic cervix? Histol Histopathol 1998;13:249–54. [DOI] [PubMed] [Google Scholar]
- 30.Boon ME, van Dunné FMF, Vardaxis NJ. Recognition of atypical reserve cell hyperplasia in cervical smears and its diagnostic significance. Mod Pathol 1995;8:786–94. [PubMed] [Google Scholar]
- 31.van Hoeven KH, Kovatish AJ, Oliver RE. Immunocytochemical detection of squamous intraepithelial lesions in cervical smears. Mod Pathol 1996;9:407–12. [PubMed] [Google Scholar]
- 32.Sahebali S , Depuydt CE, Segers K, et al. Ki-67 immunocytochemistry in liquid based cervical cytology: useful as an adjunctive tool? J Clin Pathol 2003;56:681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Saleh W , Delvenne P, Greimers R, et al. Assessment of Ki-67 antigen immunostaining in squamous intraepithelial lesions of the uterine cervix. Correlation with the histologic grade and human papillomavirus type. Am J Clin Pathol 1995;104:154–60. [DOI] [PubMed] [Google Scholar]
- 34.Bulten J , de Wilde PC, Schijf C, et al. Decreased expression of Ki-67 in atrophic cervical epithelium of post-menopausal women. J Pathol 2000;190:545–53. [DOI] [PubMed] [Google Scholar]
- 35.McCluggage WG, Buhidma M, Tang L, et al. Monoclonal antibody MIB1 in the assessment of cervical squamous intraepithelial lesions. Int J Gynecol Pathol 1996;15:131–6. [DOI] [PubMed] [Google Scholar]
- 36.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994;266:2011–15. [DOI] [PubMed] [Google Scholar]
- 37.Feng J , Funk WD, Wang SS, et al. The RNA component of human telomerase. Science 1995;269:1236–41. [DOI] [PubMed] [Google Scholar]
- 38.Kyo S , Kanaya T, Ishikawa H, et al. Telomerase activity in gynecological tumors. Clin Cancer Res 1996;2:2023–8. [PubMed] [Google Scholar]
- 39.Cheung AN, Zhang DK, Liu Y, et al. Telomerase activity in gestational trophoblastic disease. J Clin Pathol 1999;52:588–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutirangura A , Sriuranpong V, Termrunggraunglert W, et al. Telomerase activity and human papillomavirus in malignant, premalignant and benign cervical lesions. Br J Cancer 1998;78:933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisman GB, De Jong S, Meersma GJ, et al. Telomerase in (pre)neoplastic cervical disease. Hum Pathol 2000;31:1304–12. [DOI] [PubMed] [Google Scholar]
- 42.Zheng PS, Iwasaka T, Zhang ZM, et al. Telomerase activity in Papanicolaou smear-negative exfoliated cervical cells and its association with lesions and oncogenic human papillomaviruses. Gynecol Oncol 2000;77:394–8. [DOI] [PubMed] [Google Scholar]
- 43.Reddy VG, Khanna N, Jain SK, et al. Telomerase—a molecular marker for cervical cancer screening. Int J Gynecol Cancer 2001;11:100–6. [DOI] [PubMed] [Google Scholar]
- 44.Jarboe EA, Liaw KL, Thompson LC, et al. Analysis of telomerase as a diagnostic biomarker of cervical dysplasia and carcinoma. Oncogene 2002;21:664–73. [DOI] [PubMed] [Google Scholar]
- 45.Sen S , Reddy VG, Guleria R, et al. Telomerase—a potential molecular marker of lung and cervical cancer. Clin Chem Lab Med 2002;40:994–1001. [DOI] [PubMed] [Google Scholar]
- 46.Ngan HY, Cheung AN, Liu SS, et al. Telomerase assay and HPV 16/18 typing as adjunct to conventional cytological cervical cancer screening. Tumour Biol 2002;23:87–92. [DOI] [PubMed] [Google Scholar]
- 47.Reesink-Peters N , Helder MN, Wisman GB, et al. Detection of telomerase, its components, and human papillomavirus in cervical scrapings as a tool for triage in women with cervical dysplasia. J Clin Pathol 2003;56:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]