Abstract
Aims: To determine gastric expression of trefoil family factor 2 (TFF2) and MUC6 in Helicobacter pylori positive and negative subjects, and its association with antralisation at the gastric incisura.
Methods: Gastric biopsies from the antrum, incisura, and body of 76 dyspeptic patients without ulcers were used for the determination of H pylori infection, histological changes, and epithelial TFF2 and MUC6 expression.
Results: In the foveola, the rates of TFF2 and MUC6 immunostaining were greater in H pylori infected (n = 27) than in uninfected patients (n = 49) at the antrum (59.3% v 4.1% for TFF2 and 63.0% v 4.1% for MUC6; both p < 0.001) and incisura (44.4% v 2.0% for TFF2 and 48.1% v 0% for MUC6; both p < 0.001). In the deeper glands, the rates were also greater in H pylori infected than in uninfected patients at the incisura (85.2% v 22.4% for both TFF2 and MUC6; p < 0.001). Antral-type mucosa was present at the incisura in 28 of the 76 patients. TFF2 and MUC6 expression in the foveola and deeper glands was significantly associated with antral-type mucosa, independent of H pylori status.
Conclusions: Helicobacter pylori infection increases the expression of TFF2 and MUC6 in the gastric epithelium. Aberrant TFF2 and MUC6 expression is associated with antralisation of gastric incisura.
Keywords: Helicobacter pylori, trefoil family factor 2, MUC6, antralisation
Mucins are high molecular weight glycoproteins that are the major components of the mucous viscous gel covering the surface of epithelial tissues. To date, 11 distinct human epithelial mucins have been identified.1 Mucins are expressed in a site specific fashion. The gastric mucosa normally expresses mucins MUC1, MUC5AC, and MUC6.1–5 These mucins are believed to play an important role in the protection of the gastric mucosa from extremes of pH, proteases, toxins, mechanical irritation, and pathogenic organisms.1–5
“Whether or not trefoil family factor 2 and mucins are involved in antralisation is unknown”
Trefoil family factor (TFF) peptides are a relatively newly discovered family of peptides comprising small secretory peptides bearing one or more trefoil domain. In humans, three TFF peptides have been identified, namely: TFF1/pS2 (or breast cancer oestrogen inducible peptide), TFF2/hSP (or spasmolytic polypeptide), and TFF3/ITF (or intestinal trefoil factor).6–8 TFFs are mainly synthesised and secreted by mucin secreting epithelial cells lining the gastrointestinal tract and are thus closely associated with mucins.1,9 In normal gastric mucosa, TFF1 co-localises with MUC5AC in the surface/foveolar epithelium, whereas TFF2 is expressed together with MUC6 in the mucous cells of the regenerative zone of the body mucosa and in the antral glands.1,10,11 The role of TFFs in mucosal defence and healing is also becoming increasingly clear, although the mechanism needs to be further elucidated.8,12 More recently, it has been found that the expression pattern of mucins and TFF peptides alters in gastric precancerous lesions and neoplasia, and these altered expression patterns may be used as prognostic indicators, implying involvement of these factors in tumour progression.3,7,8,11–18
Helicobacter pylori is recognised as a major cause of chronic gastritis and peptic ulcer disease, and is a definite pathogen for the development of gastric cancer.19–21 However, the exact mechanisms by which infection with the organism leads to gastric cancer have not been fully elucidated. Previous studies have shown that H pylori infection is associated with changes in the expression of mucins in the gastric epithelium.3–5 However, these studies only investigated gastric biopsies taken from the gastric antrum, so that global changes in the expression of these factors in the stomach could not be revealed. Moreover, the possible changes in gastric epithelial expression of TFF2 in the presence of H pylori infection have rarely been reported.22 In addition, our previous studies have shown that H pylori infection is associated with antralisation—that is, the presence of antral-type mucosa in the gastric incisura, body, and/or fundus or gastric mucosal transformation from transitional or body type to antral type, which is strongly associated with gastric atrophy and intestinal metaplasia.23 Moreover, cell proliferation and apoptosis and their related proteins such as Bcl-2 and Bax alter in the antralised gastric incisura.24 These observations suggest that antralisation is primarily the result of H pylori infection in most cases, and may be an important step (or histological marker) in the process of gastritis progressing to precancerous lesions. However, whether or not TFF2 and mucins are involved in antralisation is unknown. Therefore, the aim of our study was to determine the expression of TFF2 and MUC6 in normal and H pylori infected gastric epithelium at the gastric antrum, incisura, and body, and to explore the association between the aberrant expression of TFF2 and MUC6 and antralisation at the incisura.
PATIENTS AND METHODS
Patients and gastric biopsies
Seventy six patients (M/F, 35/41; mean age, 42; SD, 13 years) referred for upper endoscopy at Queen Mary Hospital, Hong Kong because of dyspeptic and reflux symptoms were selected for our study. At endoscopy, four biopsies were obtained from each patient: two from the gastric antrum, one from the body, and one from the incisura. One antral biopsy was used for an in house rapid urease test. The other biopsies were fixed in formalin and embedded in paraffin wax for histological examination and immunohistochemistry. The following groups of patients were excluded from our study: (1) those who had been taking aspirin or non-steroidal anti-inflammatory drugs over the past six months; (2) those who had been taking antibiotics, H2 receptor blockers, bismuth, or proton pump inhibitors in the preceding four weeks; and (3) those who had undergone previous gastric surgery, those with endoscopic evidence of gastroduodenal ulceration, or those with histological evidence of gastric precancerous lesions (atrophy, intestinal metaplasia, and dysplasia), gastric carcinoma, or lymphoma.
All patients gave informed written consent, and this project was approved by the ethics committee of The University of Hong Kong.
Detection of H pylori infection and histological examination
Sections (4 μm thick) of the paraffin wax embedded tissue were stained with haematoxylin and eosin. Histological changes and helicobacter-like organisms were examined by experienced pathologists who were blinded to all clinical and endoscopic information. The in house rapid urease test used in our study has been shown to have a sensitivity of 99% and specificity of 100% for the detection of H pylori.25 Patients were defined as H pylori positive if positive by both the rapid urease test and histology. Patients were defined as H pylori negative if all tests were negative. Patients positive by the rapid urease test only or histology only were not included in our study.
The updated Sydney system was used to assess the severity and activity of gastric inflammation.26 Chronic gastritis was said to be definitely present when the severity of inflammation at any site of the stomach was classified as grade 2 or 3. The mucosa of gastric biopsies taken from different sites was classified as antral (mucous secreting or pyloric) type, body (acid secreting or oxyntic) type, or transitional (junctional) type according to the definitions set out in the updated Sydney system.26 The characteristic feature of antral-type mucosa is the presence of coiled and branching antral glands, which are lined by mucous cells that are interspersed with endocrine cells (chiefly G and D types), and a few parietal cells. The glands in body-type mucosa are straight tubes that constitute acid producing parietal cells, along with scattered mucous cells in their upper portion and mainly chief cells in their lower portion, with scattered argyrophilic endocrine cells. Transitional-type mucosa is a mixture of the architectural features and cell types found in the antral and body types.26
Immunohistochemistry for TFF2 and MUC6 in gastric epithelium
Biopsy specimens taken from the antral, body, and incisura of all patients were subjected to immunohistochemistry for the assessment of TFF2 and MUC6 expression in the gastric epithelium. Briefly, tissues embedded in paraffin wax were cut into sections 4 µm thick, dewaxed, and dehydrated for 10 minutes. The sections were treated in 3% hydrogen peroxide for five minutes, and then incubated with monoclonal antibody against TFF2 (undiluted), MUC6 (diluted 1/100; both from Novocastra Laboratories Ltd, Newcastle upon Tyne, UK) overnight at 4°C, followed by the application of biotinylated link antibody and streptavidin–horseradish peroxidase (LSAB kit; Dako Corporation, Carpinteria, California, USA). Finally, the sections were developed with diaminobenzidine–hydrogen peroxidase substrate, and lightly counterstained with haematoxylin.
The intensity of cytoplasmic immunostaining for TFF2 and MUC6 was scored as follows: 0, negative; 1+, weak; 2+, moderate; and 3+, strong. Cells with moderate or strong immunostaining were classed as positively stained. In addition, the percentage of positively stained cells over the total number of cells counted was calculated. TFF2 and MUC6 expression in the gastric foveola was defined as present when the percentage of positively stained cells over the total number of foveolar cells counted (> 300) was ⩾ 5%. Because it has been found that TFF2 and MUC6 are expressed ubiquitously in the regenerative zone of the gastric mucosa, the expression of TFF2 and MUC6 only in the deeper gastric glands outside the regenerative zone was measured in our study, and was defined as present if the percentage of positively stained cells over the total number of cells counted (> 300) within these glands was ⩾ 5%.
Statistical analysis
Data were expressed as the percentage of positive cases over the total number of cases. Statistical analysis was performed using SPSS system (version 10.0; SPSS Inc, Chicago, Illinois, USA). The association between the expression of TFF2 and MUC6 and H pylori infection at different gastric sites was assessed using the χ2 test, with Yates’s correction if required, or Fisher’s exact test; odds ratios (OR), 95% confidence intervals (CI), and two tailed p values were calculated. The α level of significance was set at p < 0.05.
RESULTS
Expression of TFF2 and MUC6 in normal gastric epithelium at different gastric sites
In the normal stomach, staining for TFF2 and MUC6 in the gastric foveola was very weak or not discernable. In the gastric antrum, there was uniform staining of TFF2 and MUC6 in the regenerative zone and the deeper mucous glands (fig 1). In gastric body mucosa, TFF2 and MUC6 were expressed together only in the regenerative zone, where mucous neck cells were present (fig 2). In gastric incisura mucosa, the expression of TFF2 and MUC6 was limited to the regenerative zone in most cases (fig 3; table 1).
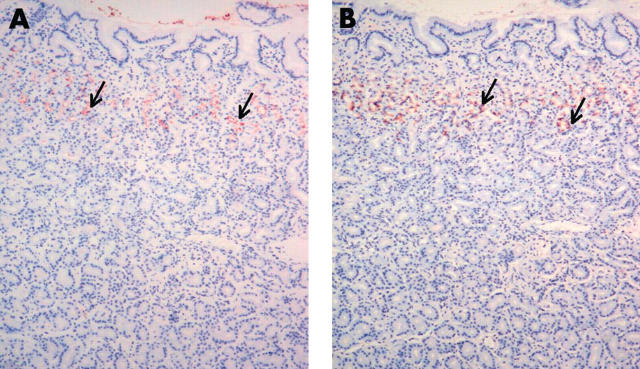
Figure 1.
The expression of trefoil family factor 2 (TFF2) and MUC6 in normal antral mucosa. There is uniform staining of (A) TFF2 and (B) MUC6 in the regenerative zone and the deeper mucous glands (arrows). Original magnification, ×100.
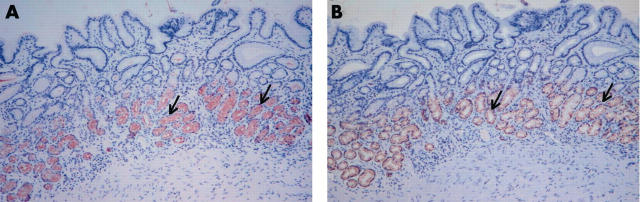
Figure 2.
The expression of trefoil family factor 2 (TFF2) and MUC6 in normal body mucosa. The expression of (A) TFF2 and (B) MUC6 is limited to the mucous neck cells in the regenerative zone (arrows). Original magnification, ×100.
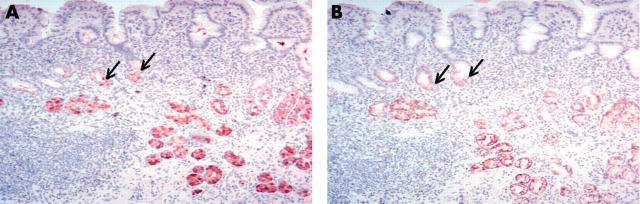
Figure 3.
The expression of trefoil family factor 2 (TFF2) and MUC6 in normal incisura mucosa. The mucosa exhibits transitional or body-type histology, and the expression of (A) TFF2 and (B) MUC6 is limited to the mucous neck cells (arrows) in most cases. Original magnification, ×100.
Table 1.
Expression of trefoil family factor 2 (TFF2) and MUC6 in Helicobacter pylori positive and negative patients at different gastric sites in relation to the mucosal types
| Percentage of cases with positive TFF2/MUC6 staining |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antrum |
Incisura |
Body |
|||||||
| N | Foveola | Gland | N | Foveola | Gland | N | Foveola | Gland | |
| H pylori negative | 49 | 4.1/4.1 | 100/100 | 49 | 2.0/0 | 22.4/22.4 | 49 | 4.1/2.0 | 2.0/2.0 |
| Antral type | 45 | 4.4/4.4 | 100/100 | 8 | 0/0 | 75.0†/75.0† | 0 | NA | NA |
| Transitional type | 4 | 0 | 100/100 | 28 | 3.6/0 | 17.9/17.9 | 3 | 0/0 | 0/0 |
| Body type | 0 | NA | NA | 13 | 0/0 | 0/0 | 46 | 4.3/2.2 | 2.2/2.2 |
| H pylori positive | 27 | 59.3*/63.0* | 100/100 | 27 | 44.4*/48.1* | 85.2*/85.2* | 27 | 14.8/14.8 | 14.8/11.1 |
| Antral type | 27 | 59.3/63.0 | 100/100 | 20 | 55.0/60.0 | 100†/100† | 1 | 100/100 | 100/100 |
| Transitional type | 0 | NA | NA | 6 | 16.7/16.7 | 50.0/50.0 | 3 | 33.3/33.3 | 66.7/66.7 |
| Body type | 0 | NA | NA | 1 | 0/0 | 0/0 | 23 | 8.7/8.7 | 4.3/0 |
*p<0.001, compared with H pylori negative cases; †p<0.001, compared among antral type, transitional type, and body type, χ2 test, df = 2.
df, degrees of freedom; NA, not available.
Expression of TFF2 and MUC6 in H pylori infected gastric epithelium at different gastric sites
Of the 76 patients, 27 were positive for H pylori infection. Chronic gastritis (grade 2 or 3) was present in 25 H pylori positive patients and four H pylori negative patients.
In general, H pylori infection was associated with increased expression of TFF2 and MUC6 in both the gastric foveola and the deeper gastric glands at the antrum (fig 4), body (fig 5), and incisura (fig 6). Foveolar staining for TFF2 and MUC6 appeared to be limited to the bottom of the pits.
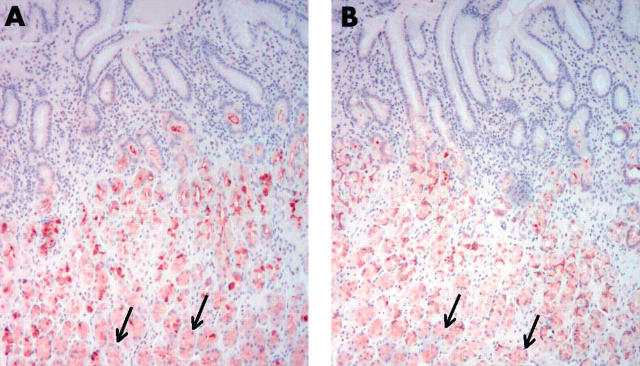
Figure 4.
The expression of trefoil family factor 2 (TFF2) and MUC6 in antral mucosa with Helicobacter pylori infection. In addition to the regenerative zone and the deeper mucous glands, the expression of (A) TFF2 and (B) MUC6 is also seen in the foveola (arrows). Original magnification, ×100.
Figure 5.
The expression of trefoil family factor 2 (TFF2) and MUC6 in body mucosa with Helicobacter pylori infection. The expression of (A) TFF2 and (B) MUC6 is seen in the regenerative zone and in the deeper glands (arrows). Original magnification, ×100.
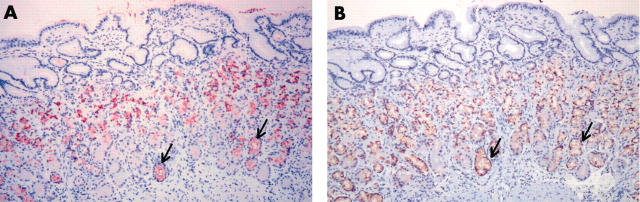
Figure 6.
The expression of trefoil family factor 2 (TFF2) and MUC6 in the incisura mucosa with Helicobacter pylori infection. The mucosa exhibits antral-type histology, and the expression of (A) TFF2 and (B) MUC6 is seen in the deeper glands (arrows), in addition to the regenerative zone. Original magnification, ×100.
In H pylori negative patients, the positive rate for TFF2 expression in the foveola at the antrum, incisura and body was 4.1%, 2.0%, and 4.1%, respectively. However, in H pylori infected patients, the rates increased to 59.3%, 44.4%, and 14.8%, respectively. TFF2 expression in the foveola was seen more often in patients with H pylori infection than in those without H pylori infection at the gastric antrum (OR, 34.2; 95% CI, 6.83 to 171.0; χ2 = 29.30; p < 0.001) and incisura (OR, 38.4; 95% CI, 4.61 to 320.2; χ2 = 19.19; p < 0.001; table 1). In H pylori positive patients, TFF2 expression in the deeper glands was present in 100%, 85.2%, and 14.8% of cases at the antrum, incisura, and body, respectively, whereas the rates were 100%, 22.4%, and 2.0%, respectively, in those without H pylori infection. The positive rate for TFF2 expression in the deeper glands at the incisura was significantly higher in H pylori infected patients than in uninfected patients (OR, 19.9; 95% CI, 5.66 to 69.8; χ2 = 27.71; p < 00.01; table 1).
MUC6 expression was similar to TFF2 expression (table 1). In the foveola, MUC6 expression was significantly higher in H pylori positive patients than in H pylori negative patients at the antrum (63.0% v 4.1%; OR, 40.0; 95% CI, 7.94 to 201.1; χ2 = 32.19; p < 0.001) and incisura (48.1% v 0%; χ2 = 25.17; p < 0.001). In the deeper glands, MUC6 expression was present more frequently in H pylori positive patients than in H pylori negative patients at the incisura (85.2% v 22.4%; OR, 19.9; 95% CI, 5.66 to 69.8; χ2 = 27.71; p < 0.001; table 1).
Association between the expression of TFF2 and MUC6 and antralisation of gastric incisura
Antral-type mucosa was present at the incisura in 28 of 76 of patients—20 of the 27 H pylori positive and eight of the 49 H pylori negative patients (table 1). Seventy two patients had antral-type mucosa and four patients had transitional-type mucosa in the antrum. At the gastric body, antral-type mucosa was seen in only one H pylori positive patient and the transitional type in six (three H pylori positive and three H pylori negative) patients (table 1).
At the gastric incisura, the expression of TFF2 and MUC6 was significantly associated with the presence of antral-type mucosa. The rates of TFF2 and MUC6 expression in the foveola were 39.3%, 5.9%, and 0%, respectively, in antral-type, transitional-type, and body-type mucosa (degrees of freedom = 2; χ2 = 43.74; p < 0.001). In the deeper glands, the rates of TFF2 and MUC6 expression were 92.9%, 23.5%, and 0%, respectively, in antral-type, transitional-type, and body-type mucosa (degrees of freedom = 2; χ2 = 15.62; p < 0.001). When patients with and those without H pylori infection were analysed separately, there was an association between the presence of antral-type mucosa and the expression of TFF2 and MUC6 in the deeper glands, but not in the foveola (table 1).
DISCUSSION
Our present study showed that H pylori infection was associated with significant changes in the expression of TFF2 and MUC6 at the gastric antrum, incisura, and body. In the normal gastric antrum, there was no expression of TFF2 and MUC6 in the foveolar epithelium. Both TFF2 and MUC6 were expressed in the regenerative zone and the deeper portion of the antral glands. These findings are in agreement with previous studies using antral biopsies.3–5 Moreover, we found that TFF2 and MUC6 were expressed only in the regenerative zone of the body mucosa, and, in most cases, of the incisura mucosa. However, in the presence of H pylori infection, the distribution of TFF2 and MUC6 expression was altered: these molecules were frequently expressed in the foveola of the antrum and incisura. This finding supports the previous observation by Byrd et al, who only used antral biopsies, that MUC6 expression was limited to the mucous glands of H pylori negative patients, but that 72% of H pylori positive patients expressed MUC6 in the surface mucous cells.3 Moreover, our present study showed that both TFF2 and MUC6 were also expressed in the deeper glands of the body, especially the incisura mucosa, in the presence of H pylori infection.
TFF2 is upregulated in diverse pathological conditions, and its expression profile broadens to include the regenerative epithelia of the entire gastrointestinal tract.27 For example, expression of TFF2 and other trefoil peptides is increased at sites of gastric ulceration, duodenal ulceration, and Crohn’s disease.28–30 Thus, increased expression of TFF2 may be a potentially important mucosal defence mechanism. Previous studies have shown that there is an association between TFFs and mucins.1,9–11 TFFs interact with mucous, and stabilise the mucous gel by binding the long mucous molecules together.17 It has been suggested that TFF2 may function with MUC6 to strengthen the mucous barrier.17 Moreover, Hoosein et al showed that a highly purified preparation of porcine TFF2 was able to stimulate [3H] thymidine incorporation in HCT116 colon carcinoma cells, suggesting a role in cell proliferation.31 Recently, Farrell et al also showed that TFF2 deficient mice have a 35% reduction in the fundic proliferation rate compared with wild-type mice.18 Those results suggest a physiological role for TFF2 in promoting mucosal healing through the stimulation of cell proliferation.
“Our present study showed that both trefoil family factor 2 and MUC6 were also expressed in the deeper glands of the body, especially the incisura mucosa, in the presence of Helicobacter pylori infection”
Helicobacter pylori infection is associated with antralisation (or so called pseudopyloric metaplasia) at the gastric incisura, body, and fundus.23,24,32 It has been established that H pylori infection induces the apoptosis of gastric epithelial cells, and subsequently stimulates cell proliferation in the gastric mucosa.33 Indeed, Wong et al found that resected specimens taken from the gastric body or fundus, most infected with H pylori, showed the presence of antral-type glands entirely surrounded by acid secreting mucosa.34 Moreover, they reported that there was a pronounced increase of mucous neck cells in fundus glands adjacent to areas of pseudopyloric metaplasia, and that the secretory phenotype of pseudopyloric metaplasia in the fundus (typically, expression of TFF2) resembled that of the mucous neck cells.34 These findings indicate that pseudopyloric metaplasia occurs in the body glands as a result of hyperplasia of mucous neck cells, and represents a mucosal response to damage associated with H pylori infection.23,34,35 Furthermore, Schmidt et al reported that the spasmolytic polypeptide (TFF2) expressing metaplastic (SPEM) lineage was closely associated with fundic H pylori infection.36 Thus, we propose that the hyperplastic mucous neck cells move both upwards and particularly downwards in the oxyntic tubule, replacing the specialised parietal and chief cells, to create a mucous cell lineage. This process can occur focally, occupying a single oxyntic tubule, groups of tubules, or on a fairly massive scale with many tubules involved.37 Eventually a mucous gland, which resembles a pyloric gland, is formed, and thus antralisation of the proximal gastric mucosa, particularly the incisura, follows. It is most likely that the weakened antralised mucosa in the proximal stomach is prone to further damage by H pylori, resulting in ulceration even in the presence of subnormal acid production.32,38
More importantly, the expression pattern of mucins and TFFs alters in gastric precancerous lesions and neoplasia, and thus the altered expression pattern of these factors may be involved in the development of gastric cancer.2,3,6–8,11–18,39 It has been shown that mice lacking TFF1 develop antral adenoma or carcinoma, and TFF1 expression is lost in 50% of human gastric cancers, suggesting that TFF1 may be a tumour suppressor gene.8,17 More recently, studies have shown that SPEM is associated with the development of gastric cancer, suggesting that SPEM is a potential precursor lesion of gastric cancer.40,41 In our present study, we found that alteration of TFF2 and MUC6 expression was associated with antralisation of gastric incisura, indicating that antralisation is histogenetically identical to SPEM. Because there was a close topographical association between the expression of TFF2 and MUC6 in the deeper glands and antralisation of gastric incisura was present in both H pylori positive and negative patients, we suggest that the expression of TFF2 and MUC6 in the deeper glands may be a useful biological marker for antralisation. Furthermore, based on the findings of previous and present studies, we hypothesise that the increased expression of TFF2 and MUC6 in H pylori infection is a host response to damage induced by the infection. These factors are associated with the regulation of cell proliferation; thus, an increase in these factors, along with other growth factors, will be followed by increased cell proliferation.17,30 However, H pylori induced mucosal damage (including epithelial apoptosis) that is accompanied by an overmatched cell proliferation may result in hyperplasia of the gastric mucosa and transformation of the gastric mucosa into antral-type mucosa (antralisation), which is believed to increase the risk of the development of gastric neoplasia.23,24,32,33 Further studies are required to verify this hypothesis.
In conclusion, H pylori infection increases the expression of TFF2 and MUC6 in the gastric epithelium, which may be a protective response of the host to the mucosal damage induced by H pylori infection. Aberrant expression of TFF2 and MUC6 is associated with antralisation of the gastric incisura, and thus may be involved in and used as a biological marker for the process.
Take home messages
Helicobacter pylori infection increases the expression of TFF2 and MUC6 in the gastric epithelium, and this may be a protective response of the host to the mucosal damage induced by H pylori infection
Aberrant TFF2 and MUC6 expression is associated with antralisation of gastric incisura
This increased expression of TFF2 and MUC6 might be a useful biological marker for antralisation
Acknowledgments
This work was supported by competitive earmarked research grants from the Research Grants Council of Hong Kong Special Administrative Region, China (HKU7318/01M and HKU7493/03M) to HHX Xia. This work was presented, in part, at Digestive Disease Week of the American Gastroenterological Association, Orlando, FL, May 17–22 2003 (Gastroenterology 2003;124:A464) and at Australian Gastroenterology Week 2003, Cairns, QLD, October 7–10 2003 (J Gastroenterol Hepatol 2003;18(suppl):B108).
Abbreviations
CI, confidence interval
OR, odds ratio
SPEM, spasmolytic polypeptide expressing metaplastia
TFF, trefoil family factor
The first two authors contributed equally to this work
REFERENCES
- 1.Longman RJ, Douthwaite J, Sylvester PA, et al. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut 2000;47:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HS, Lee HK, Kim HS, et al. MUC1, MUC2, MUC5AC, and MUC6 expressions in gastric carcinomas: their roles as prognostic indicators. Cancer 2001;92:1427–34. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JC, Yan P, Sternberg L, et al. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology 1997;113:455–64. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Bresalier RS. Alterations in gastric mucin synthesis by Helicobacter pylori. World J Gastroenterol 2000;6:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgenstern S, Koren R, Fraser G, et al. Gastric corpus mucin expression after partial gastrectomy, in relation to colonization with Helicobacter pylori. J Clin Gastroenterol 2001;32:218–21. [DOI] [PubMed] [Google Scholar]
- 6.Wong WM, Poulsom R, Wright NA. Trefoil peptides. Gut 2001;44:890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulsom R, Wright NA. Trefoil peptides: a newly recognized family of epithelial mucin-associated molecules. Am J Physiol 1993;265:G205–13. [DOI] [PubMed] [Google Scholar]
- 8.Williams GR, Wright NA. Trefoil factor family domain peptides. Virchows Arch 1997;431:299–304. [DOI] [PubMed] [Google Scholar]
- 9.Wright NA. Interaction of TFF with mucins: clues to their mechanism of action? Gut 2001;48:293–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanby AM, Poulsom R, Singh S, et al. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology 1993;105:1110–16. [DOI] [PubMed] [Google Scholar]
- 11.Nogueira AMMF, Machado JC, Carneiro F, et al. Patterns of expression of trefoil peptides and mucins in gastric polyps with and without malignant transformation. J Pathol 1999;187:541–8. [DOI] [PubMed] [Google Scholar]
- 12.Playford RJ, Marchbank T, Chinery R, et al. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology 1995;108:108–16. [DOI] [PubMed] [Google Scholar]
- 13.Ho SB, Shekels LL, Toribara NW, et al. Mucin expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res 1995;55:2681–90. [PubMed] [Google Scholar]
- 14.Reis CA, David L, Correa P, et al. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, MUC6) expression. Cancer Res 1999;59:1003–7. [PubMed] [Google Scholar]
- 15.Felicity EBM, Bruce RW. Trefoil proteins: their role in normal and malignant cells. J Pathol 1997;183:4–7. [DOI] [PubMed] [Google Scholar]
- 16.Machado JC, Nogueira AM, Carneiro F, et al. Gastric carcinoma exhibits distinct types of cell differentiation: an immunohistochemical study of trefoil peptides (TFF1 and TFF2) and mucins (MUC1, MUC2, MUC5AC, and MUC6). J Pathol 2000;190:437–43. [DOI] [PubMed] [Google Scholar]
- 17.Wright NA, Hoffmann W, Otto WR, et al. Rolling in the clover: trefoil factor family (TFF)-domain peptides, cell migration and cancer. FEBS Lett 1997;408:121–3. [DOI] [PubMed] [Google Scholar]
- 18.Farrell JJ, Taupin D, Koh TJ, et al. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest 2002;109:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Agency for Research on Cancer. Schistosomes, liver flukes and Helicobacter pylori. In: IARC monographs on the evaluation of carcinogenic risks to humans. IARC monographs, Vol. 61. Lyon, 1994:177–241. [PMC free article] [PubMed]
- 20.Wong BCY, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region in China: a randomized controlled trial. JAMA 2004;291:187–94. [DOI] [PubMed] [Google Scholar]
- 21.Xia HHX, Wong BCY, Lam SK. Helicobacter pylori infection and gastric cancer. Asian J Surg 2001;24:217–21. [Google Scholar]
- 22.Hu GY, Yu BP, Dong WG, et al. Expression of TFF2 and Helicobacter pylori infection in carcinogenesis of gastric mucosa. World J Gastroenterol 2003;9:910–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia HHX, Kalantar J, Talley NJ, et al. Antral-type mucosa in the gastric incisura (antralization)—a link between Helicobacter pylori infection and intestinal metaplasia? Am J Gastroenterol 2000;95:114–21. [DOI] [PubMed] [Google Scholar]
- 24.Xia HHX, Zhang G-S, Talley NJ, et al. Topographic association of gastric epithelial expression of Ki-67, Bax and Bcl-2 expression with antralization in the gastric incisura, body and fundus. Am J Gastroenterol 2002;97:3123–31. [DOI] [PubMed] [Google Scholar]
- 25.Wong BCY, Wong WM, Wang WH, et al. An evaluation of invasive and non-invasive tests for the diagnosis of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther 2001;15:505–11. [DOI] [PubMed] [Google Scholar]
- 26.Dixon MF, Genta RM, Yardley JH. et al and the participants in the International Workshop on the Histopathology of Gastritis, Houston 1994. Classification and grading of gastritis. The updated Sydney System. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 27.Rio MC, Chenard MP, Wolf C, et al. Induction of pS2 and hSP genes as markers of mucosal ulceration of the digestive tract. Gastroenterology 1991;100:375–9. [DOI] [PubMed] [Google Scholar]
- 28.Wright NA, Poulsom R, Stamp G, et al. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology 1993;104:12–20. [DOI] [PubMed] [Google Scholar]
- 29.Alison MR, Chinery R, Poulsom R, et al. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol 1995;175:405–14. [DOI] [PubMed] [Google Scholar]
- 30.Otto WR, Rao J, Cox HM, et al. Effects of pancreatic spasmolytic polypeptide (PSP) on epithelial cell function. Eur J Biochem 1996;235:64–72. [DOI] [PubMed] [Google Scholar]
- 31.Hoosein NM, Thim L, Jorgensen KH, et al. Growth stimulatory effect of pancreatic spasmolytic polypeptide on cultured colon and breast tumor cells. FEBS Lett 1989;247:303–6. [DOI] [PubMed] [Google Scholar]
- 32.Xia HHX, Lam SK, Wong WM, et al. Antralization at the edge of the gastric ulcers at the gastric incisura, body and fundus: does Helicobacter pylori infection play a role? World J Gastroenterol 2003;9:1265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia HHX, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am J Gastroenterol 2001;96:16–26. [DOI] [PubMed] [Google Scholar]
- 34.Wong WM, Garcia SB, Poulson R, et al. Aberrant spasmolytic peptide (TFF2)-expressing cell lineage (SPEM), pseudopyloric metaplasia (PPM) and the response to gastric mucosal damage [abstract]. Gastroenterology 1999;116:G2306. [Google Scholar]
- 35.Hanby AM, Poulsom R, Playford RJ, et al. The mucous neck cell in the human gastric corpus: a distinctive, functional cell lineage. J Pathol 1999;187:331–7. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 1999;79:639–46. [PubMed] [Google Scholar]
- 37.Wright NA. Mechanisms involved in gastric atrophy. In: Hunt R, Tytgat G, eds. Helicobacter pylori. Basic mechanisms to clinical cure 2000. Dordrecht: Kluwer, 2000:239–47.
- 38.Correa P, Miller MJS. Carcinogenesis, apoptosis and cell proliferation. Br Med Bull 1998;54:151–62. [DOI] [PubMed] [Google Scholar]
- 39.Lefebvre O, Chenard MP, Masson R, et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science 1996;274:259–62. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi H, Goldenring JR, Kaminishi M, et al. Identification of spasmolytic polypeptide expressing metaplasia (SPEM) in remnant gastric cancer and surveillance postgastrectomy biopsies. Dig Dis Sci 2002;47:573–8. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi H, Goldenring JR, Kaminishi M, et al. Association of spasmolytic polypeptide-expressing metaplasia with carcinogen administration and oxyntic atrophy in rats. Lab Invest 2002;82:1045–52. [DOI] [PubMed] [Google Scholar]