Abstract
Aim: Angiogenesis correlates with disease progression in various haematological malignancies. This study investigated the association between microvascular density (MVD) and the Ki-67 proliferation index (Ki-67 PI), bone marrow infiltration, and C reactive protein (CRP) in patients with multiple myeloma.
Methods: Bone marrow MVD was examined in 44 biopsies at diagnosis and 15 in plateau phase by immunostaining the endothelial cells with a monoclonal antibody to CD34. The Ki-67 PI was evaluated by a double immunostaining technique using the monoclonal antibodies MIB-1 and CD38.
Results: MVD, Ki-67 PI, bone marrow infiltration, and CRP were significantly higher in pretreatment patients than in controls and decreased in patients achieving plateau phase. MVD significantly correlated with Ki-67 PI and infiltration, and Ki-67 correlated with infiltration.
Conclusion: In multiple myeloma, apart from being a marker of proliferative activity, Ki-67 is also associated with bone marrow angiogenesis and tumour burden.
Keywords: microvascular density, angiogenesis, proliferation index, Ki-67, multiple myeloma
Angiogenesis has been shown to play an important role in solid tumour invasion and metastasis.1–3 In some types of cancer, the degree of angiogenesis has been shown to have an adverse effect on prognosis.4–6 Although initial studies were performed with solid tumours, several recent studies have shown that angiogenesis also plays an important role in haematological malignancies.7–11 Increased angiogenesis, measured as bone marrow microvessel density (MVD), and increased serum angiogenic factors have been measured in patients with acute and chronic leukaemia, non-Hodgkin lymphomas, myelodysplastic syndromes, and multiple myeloma (MM).12–19 More specifically, for MM, histomorphometric studies have shown that the number of arterioles and arterial capillaries is significantly increased compared with osteoporosis9 and monoclonal gammopathy of undetermined significance.10 It has also been reported that bone marrow angiogenesis is a predictive factor of poor survival in newly diagnosed myeloma.11,17,18
“Although initial studies were performed with solid tumours, several recent studies have shown that angiogenesis also plays an important role in haematological malignancies”
The monoclonal antibody to Ki-67 (MIB1) is a marker strictly associated with cell proliferation because it recognises a nuclear antigen present during the G1, S, G2, and M phases of the cell cycle, but not the G0 phase. It has been used as a marker of proliferative activity in several human tumours, including MM.20–22 However, the determination of Ki-67 in MM is not a routine examination because there is little information regarding its clinical relevance and its association with prognostic factors in MM. The aim of our current study was to investigate the possible relation and usefulness of determining bone marrow MVD and Ki-67 expression in patients with MM at diagnosis and after remission following chemotherapy.
PATIENTS AND METHODS
Patients
Forty four patients with MM—21 men aged 51–84 years (median, 67) and 23 women aged 37–81 years (median, 64)—were enrolled in our study. At the time of diagnosis, 12 patients were in stage I, 15 in stage II, and 17 in stage III of the disease, according to the criteria of Durie and Salmon’s myeloma staging system.23 Bone marrow samples (for the measurement of bone marrow MVD, Ki-67 proliferative index (PI) and plasma cell infiltration) were taken twice for treatment evaluation. The first sample was taken immediately at diagnosis in previously untreated patients, and the second one longer than three months after the end of treatment in the plateau phase.24 Treatment with conventional dose chemotherapy was performed and consisted of melphalan and prednisone or vincristine, adriamycin, and dexamethasone. The plateau phase was defined as a decrease in serum paraprotein to 50% or less of the initial value in association with normal concentrations of serum β2 microglobulin and C reactive protein (CRP). Venous blood drawn directly into sterile tubes from patients before and after treatment and from 15 age and sex matched normal controls was allowed to clot at room temperature for one hour. The tubes where then centrifuged at 1850×g and the separated serum was collected. Serum concentrations of CRP were measured using immunonephelometry (Dade-Behring Marburg GmbH, Marburg, Germany).
Bone marrow samples
Bone marrow samples were collected from patients with MM. Patients underwent transiliac bone marrow biopsy using a Bone Temnotrephine (10 G 11 cm; Allegiance Healthcare Corporation, McGaw Park, Illinois, USA). Biopsies were fixed in 10% formalin, decalcified in 10% EDTA (Titriplex III, M = 372.24 g/mol, catalogue number 64271; Merck, Darmstadt, Germany) for 48 hours, and embedded in Paramat extra (BDH, Poole, Dorset, UK). Initially, haematoxylin and eosin stained, 3 μm thick sections were examined by light microscopy. Additional sections were stained with Giemsa, periodic acid Schiff, Gomori, Masson trichrome, and Perls’ methods. The pattern of infiltration of the bone marrow by MM was highlighted by immunostaining the neoplastic plasma cells with a monoclonal antibody to CD38 (Code M7077; Dako, Glostrup, Denmark) using an alkaline–antialkaline phosphatase (APAAP) method.25 Monoclonality and percentages of κ/λ neoplastic cells in the bone marrow were assessed by in situ hybridisation (SS ISH detection system, catalogue number DA030-SS; Biogenex, San Ramon, California, USA; κ/λ mRNA peptide nucleic acid probes/fluorescein isothiocyanate; catalogue number Y5202; Dako).
Double immunostaining for CD38 and Ki-67 expression by neoplastic plasma cells
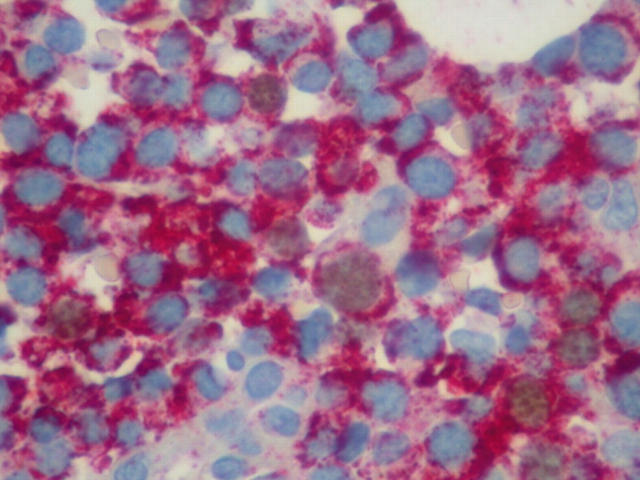
After dewaxing and gradual rehydration in alcohols of decreasing strength down to tap water, 3 μm thick tissue sections were heated at 500 W in 0.01M citrate buffer pH 6.2 for 3.5 minutes, three times; after cooling at room temperature, they were blocked with 3% H2O2 in distilled water for 10 minutes. Next, the sections were incubated with the primary monoclonal antibody antihuman Ki-67/MIB1 antibody (code number M7240; Dako) at a dilution of 1/50 for 90 minutes. At this point, blocking with H2O2 was repeated (three minutes). After incubation with the Dako EnVision reagent/horseradish peroxidase conjugated polymer (kit 5007; Dako) for 25 minutes, the samples were exposed to diaminobenzidine tetrahydrochloride solution, included in the above mentioned kit, for 10 minutes, washed with Tris buffered saline (TBS), and subsequently exposed for 60 minutes to the second primary antibody, the anti-CD38 monoclonal antibody (M-7077; Dako), at a dilution of 1/50 (fig 1). The sections were then incubated with the EnVision reagent/alkaline phosphatase conjugated polymer (kit 1396; Dako) for 20 minutes, followed by incubation for 20 minutes with the Fast Red chromogen plus levamisole (included in kit 1396). The sections were counterstained with Papanikolaou and Harris haematoxylin (KGaA 64271; Merck) for 30 seconds, and coverslipped using glycergel aqueous mounting medium (C-0563; Dako, Carpinteria, California, USA). Throughout the technique TBS was used for rinsing. Positive and negative controls were included in every run.
Figure 1.
Several dark (brown) Ki-67 stained nuclei of neoplastic plasma cells can be seen. Double immunostaining: EnVision system, diaminobenzidine/fast red; original magnification, ×600
Immunostaining and counting of microvessels
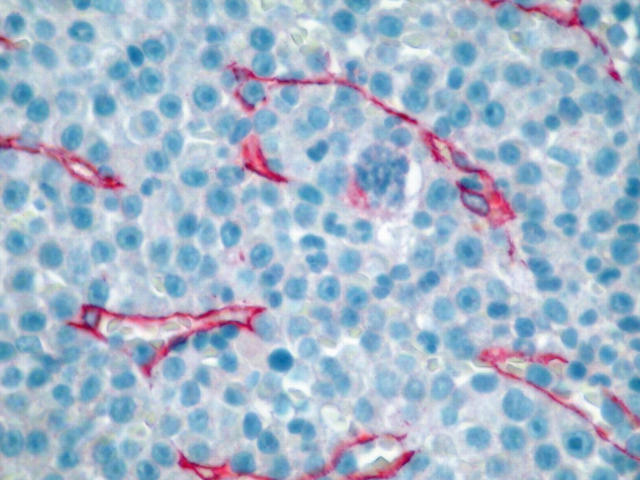
Blood vessels were highlighted by immunostaining endothelial cells with a monoclonal antibody to CD3410 (catalogue number 0786; Immunotech, Marseille, France) and the same APAAP method used above. Known positive controls and negative controls were included in every run of the immunostaining. MVD was assessed by two independent observers (ENS, KD) using a standard 16 Zeiss microscope equipped with plan objectives and Kpl/W10x/18 eyepieces. The two investigators assessed the MVD without previous knowledge of the patients’ data or outcome. The mean of the two independent counts was considered to be the final measurement for each counting field and hot spot. Both the observers obeyed the standard criteria for the recognition and identification of elements of the microvasculature. The overall interobserver variation was close to 10%. Because of the heterogeneity encountered in the MVD of the specimens, three areas of neoplastic infiltration from each specimen containing the highest number of microvessels (capillaries and venules) representing the most intense microvasculature (hot spots) were examined. Initially, the sections were scanned at low magnification (×100) and the hot spots located before counting. Microvessels in close proximity to the trabeculae and those in sclerotic areas were not considered when counting. After the hot spots were identified, individual microvessels were counted at ×400 magnification. For systematic examination of the specimens, a squared and numbered (with 100 indexed squares of equal dimension) eyepiece graticule (micrometer, grid), 1 × 1 cm in dimension (NE35 mm; Graticules Ltd, Kent, UK), was introduced into one of the eyepieces and calibrated against a calibration 1 mm stage micrometer for transmitted light (Zeiss, 5+100/100 mm) with 100 divisions of 1/100 mm each, mounted on a 76 × 76 mm slide. The measurements were performed on the part of the field corresponding to the projection of the eyepiece graticule, covering a 0.0625 mm2 surface area. During microvessel counting, the stage of the microscope was moved in such a way that the whole area of each of the hot spots was examined. An effort was made to ensure that each counting square was full of tissue. Any red staining cells morphologically compatible with endothelial cells and any cluster of endothelial cells with or without a (rudimentary or well formed) lumen were considered to be microvessels and were counted. Finally, the mean microvessel count of the three hot spots was calculated and expressed as vessels/0.0625 mm2 (fig 2).
Figure 2.
Increased angiogenesis in a case of multiple myeloma with heavy infiltration of bone marrow by neoplastic plasma cells. Immunostaining for CD34 using an APAAP method; original magnification, ×400.
Fifteen bone marrow specimens within normal limits from individuals undergoing biopsies for various reasons (mainly reactive bone marrow specimens) were included as study controls for the calculation of MVD.
In a supplemental evaluation, the percentage of Ki-67 positive plasma cells was examined in hot spot versus non-hot spot areas. To achieve this measurement, additional sections were stained in patients with sufficient histological specimens. The sections were double stained as previously described for Ki-67 and CD34. The areas of increased microvasculature were identified and plasma cells in the vicinity were identified based on morphological criteria. The percentage of Ki-67 positive plasma cells was counted in the hot spot areas and expressed as the mean for three hot spots in the same patient. The same procedure was followed in three areas with very sparse or no microvasculature (termed non-hot spot areas) and the mean number of Ki-67 positive plasma cells was calculated. This comparative measurement was performed in 26 patients before treatment. In the rest of the cases, a lack of histological material did not allow this measurement to be carried out.
STATISTICAL ANALYSIS
Data analysis was carried out using SYSTAT 8.0 statistical software (SPSS Inc, Chicago, Illinois, USA). Results were expressed as mean (SD), unless otherwise indicated. ANOVA was used to test the differences in the studied parameters between the various stages of the disease. The Wilcoxon test was used to compare pretreatment, post-treatment, and control parameter values. The Spearman’s rank correlation coefficient was used to determine the correlation between pretreatment values of the various studied parameters. A p value< 0.05 was consider to be significant.
RESULTS
Table 1 shows the mean (SD) values for each of the variables measured in the pretreatment group of patients. The proportion of Ki-67 positive plasma cells was significantly higher in stage III of the disease than in stages I and II (p < 0.01). There were also significant differences in the proportion of Ki-67 positive plasma cells among the untreated patients and those in plateau phase after chemotherapy (p < 0.01). Of note, the double staining of normal control bone marrows revealed no Ki-67 positive plasma cells. The single Ki-67 staining performed on these bone marrows (which depicts the proliferative activity in the bone marrow progenitors) was 2.42 (SD, 2.4). The small numbers of plasma cells (⩽ 2%) seen on the initial standard staining were resting, non-proliferating cells.
Table 1.
Mean (SD) values of the measured parameters in the groups of patients with different disease stage before treatment
| Stage I (N = 12) | Stage II (N = 15) | Stage III (N = 17) | |
| Ki-67 (%) | 3.67 (1.97)** | 8.07 (10.73)** | 15.2 (8.0) |
| Infiltration (%) | 25.8 (14.4)** | 36.5 (20.1)** | 53.2 (22.3) |
| CRP (mg/l) | 6.42 (3.15)* | 10.27 (6.57) | 13.12 (8.70) |
| MVD (/0.0625 mm2) | 6.18 (1.72)* | 8.53 (6.23) | 11.4 (5.9) |
*p<0.05 v stage III; **p<0.01 v stage III.
CRP, C reactive protein; MVD, microvascular density.
MVD was significantly higher in stage III than in stage I of MM disease (p < 0.05; table 1). Significant differences were also found regarding the MVD value in the pretreatment and post-treatment groups (p < 0.01; table 2), and between untreated patients with MM and controls (p < 0.0001; table 3). Significant differences were also found regarding MVD values between post-treatment and control groups (p < 0.05).
Table 2.
Mean (SD) values for Ki-67/CD38 positivity and infiltration
| Pre-T (N = 15) | Post-T (N = 15) | Pre-T v post-T | |
| Ki-67+CD38+ (%) | 14.6 (13.0) | 2.74 (4.17) | p<0.01 |
| Infiltration (%) | 44.3 (24.8) | 7.27 (7.96) | p<0.01 |
Pre-T, pretreatment; post-T, postreatment.
Table 3.
Mean (SD) values for CRP and MVD before and after treatment and in controls
| Pre-T (N = 15) | Post-T (N = 15) | Controls (N = 15) | Pre-T v post-T | Pre-T v controls | Post-T v controls | |
| CRP (mg/l) | 10.20 (7.34) | 4.93 (2.18) | 3.8 (0.9) | p<0.05 | p<0.0001 | NS |
| MVD (vessels/0.0625 mm2) | 10.14 (6.29) | 4.107 (2.285) | 2.77 (0.88) | p<0.01 | p<0.0001 | p<0.05 |
CRP, C reactive protein; MVD, microvascular density; NS, not significant.
Plasma cell infiltration and CRP values increased significantly with increasing stage of disease in untreated patients with MM (p < 0.01 and p < 0.05, respectively). Comparisons between pretreatment and post-treatment values indicated that bone marrow infiltration and CRP concentrations decreased significantly (p < 0.01 and p < 0.05, respectively).
In the pretreatment group of patients, a correlation between bone marrow MVD and plasma cell infiltration was found (r = 0.325; p < 0.05). We also found a significant relation between Ki-67 PI, bone marrow MVD (r = 0.474; p < 0.01), and plasma cell infiltration (r = 0.630; p < 0.0001). Bone marrow MVD and Ki-67 PI did not correlate with CRP.
With regard to the comparative analysis of plasma cell Ki-67 positivity in hot spot versus non-hot spot areas, the results from Mann Whitney and Wilcoxon testing showed that the percentage of Ki-67 positive plasma cells was significantly higher in areas of increased microvasculature than in areas with decreased vasculature. Specifically, the mean (SD) of Ki-67 positive plasma cells was found to be 15.0 (13.3 %) in hot spot areas and 4.7 (4.8%) in non-hot spot areas (p < 0.001).
DISCUSSION
In myeloma disease, prognostic factors are useful for distinguishing stable or slowly progressive disease from the more aggressive forms.26,27 Of the various factors reported, Ki-67 expression and angiogenesis have been shown to be of prognostic importance.6,11,18,20,28 The results of our study link plasma cell proliferation to angiogenic activity in MM because the Ki-67 index correlated closely to MVD. This association has also been investigated in another study,18 although a different methodology was used: immunostaining for von Willebrand factor to estimate MVD and the plasma cell labelling index as a plasma cell proliferation marker. These authors also found that proliferative activity (expressed by the plasma cell labelling index) correlated with MVD; however, in contrast to our study, they did not find an association between MVD and plasma cell infiltration. The method of CD34 staining used in our study has been advocated as a more accurate method than factor VIII or CD31 staining for the estimation of microvessels in the bone marrow.29 Furthermore, ours is the first study that directly supports a relation between the proliferative activity of malignant cells and the development of microvessels, although in a limited number of samples. Thus, in the vicinity of the increased microvasculature, the percentage of proliferating plasma cells is significantly higher than in areas of decreased vasculature, suggesting that angiogenesis is one of the major determinants of tumour growth in MM. The importance of bone marrow angiogenesis for the proliferation of neoplastic plasma cells has been investigated in several studies by separate groups.17–19 There is evidence to support this on a biological basis because it has been shown that the exposure of stromal and microvascular endothelial cells to vascular endothelial growth factor induces an increase in interleukin 6, which is a strong growth factor in myeloma cells.10
The observed decrease in Ki-67 and MVD after chemotherapy is probably a result of the direct cytotoxic effect of the drugs on both plasma cells and endothelial cells, leading to a reduction in the release of angiogenic factors from the myeloma cells.29 In vitro and in vivo studies have been performed that support the cytotoxic effect of certain chemotherapeutic agents on endothelial cell function and proliferation of microvessels in solid tumours and MM.30–32
“Ours is the first study that directly supports a relation between the proliferative activity of malignant cells and the development of microvessels, although in a limited number of samples”
However, it should be noted that although the proliferative activity of plasma cells was almost normalised after effective treatment, MVD was decreased, but still remained higher than normal. This has also been noted by others,33 and suggests that a pathological angiogenic process still remains, which may contribute to myeloma relapse and renders clinical studies using a combination of antineoplastic and antiangiogenic agents extremely useful.
With regard to the role of Ki-67 expression in MM, in an earlier study Ki-67 values did not differ significantly between patients with MM at diagnosis and those in plateau phase, but were found to be significantly higher in patients with relapsing MM.34,35 On the contrary, our data are in accordance with another study36 demonstrating a significant decrease in Ki-67 in patients who entered plateau phase compared with prechemotherapy values. Double staining of Ki-67 and CD38 for assessing MM proliferative activity is not routinely used in myeloma bone biopsies. However, we have shown that it can be applied efficiently in myeloma. In older studies, there was a degree of scepticism regarding Ki-67 staining in MM because it could not differentiate MM from reactive plasmacytosis.37 Double staining of CD38 and Ki-67 may be useful in this situation and has already been applied to bone marrow samples for flow cytometry.38
The other parameters measured in our study were in accordance with the literature showing that plasma cell infiltration and serum concentrations of CRP were significantly higher in patients with MM than in controls and increased with advancing stage of disease.39 However Ki-67 and MVD were related to plasma cell infiltration but not to CRP. CRP production in MM seems to depend on the cytokine milieu, especially interleukin 6, and not directly on the number of tumour cells or microvessels.
Take home messages.
Microvessel density (MVD) and Ki-67 index are significantly higher in pretreatment patients than in controls and decreased in patients achieving plateau phase
MVD significantly correlated with the Ki-67 index and plasma cell infiltration, and Ki-67 correlated with plasma cell infiltration
The assessment of bone marrow MVD and Ki-67 expression in bone marrow plasma cells are important indicators of disease activity
However, MVD remained higher than normal after chemotherapy and the role of this in future MM relapse and the value of adjacent antiangiogenic drugs in standard treatment need to be evaluated further
In conclusion, our results provide evidence that patients with active MM have raised MVD and Ki-67 PI values, whereas those in plateau phase experience a reduction of these factors. Thus, the assessment of bone marrow MVD and Ki-67 expression in bone marrow plasma cells may be considered important indicators of disease activity. However, although the Ki-67 proliferative activity was almost normalised after treatment, MVD remained higher than normal. The role of this in future MM relapse and the value of adjacent antiangiogenic drugs40 in standard treatment need to be further evaluated.
Abbreviations
APAAP, alkaline–antialkaline phosphatase
CRP, C reactive protein
MM, multiple myeloma
MVD, microvascular density
PI, proliferation index
TBS, Tris buffered saline
REFERENCES
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27–31. [DOI] [PubMed] [Google Scholar]
- 2.Acenero MJ, Conzales JF, Gallego GM, et al. Vascular enumeration as a significant prognosticator for invasive breast carcinoma. J Clin Oncol 1998;16:1684–8. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. New perspectives in clinical oncology from angiogenesis research. Eur J Cancer 1996;14:2534–9. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary R, Bromfey M, Clarke NW, et al. Prognostic relevance of microvessel density in cancer of the urinary bladder. Anticancer Res 1999;19:3479–84. [PubMed] [Google Scholar]
- 5.Gallego GM, Acenero MJ, Sanz Ortega J, et al. Vascular enumeration as a prognosticator for colorectal carcinoma. Eur J Cancer 2000;36:55–60. [DOI] [PubMed] [Google Scholar]
- 6.Fox SB. Tumor angiogenesis and prognosis. Histopathology 1997;30:294–301. [DOI] [PubMed] [Google Scholar]
- 7.Moehler TM, Neben K, Ho AD, et al. Angiogenesis in hematologic malignancies. Ann Hematol 2001;80:695–705. [DOI] [PubMed] [Google Scholar]
- 8.Alexandrakis MG, Passam FH, Boula A, et al. Relationship between circulating serum soluble interleukin-6 receptor and the angiogenic cytokines basic fibroblast growth factor and vascular endothelial growth factor in multiple myeloma. Ann Hematol 2003;82:19–23. [DOI] [PubMed] [Google Scholar]
- 9.Laroche M, Brousset P, Ludot I, et al. Increased vascularization in myeloma. Eur J Haematol 2001;66:89–93. [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Mesa RA, Fonseca R, et al. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clin Cancer Res 2002;8:2210–16. [PubMed] [Google Scholar]
- 11.Sezer O, Niemoller K, Eucher J, et al. Bone marrow microvessel density is a prognostic factor for survival in patients with multiple myeloma. Ann Hematol 2000;79:574–7. [DOI] [PubMed] [Google Scholar]
- 12.Aguayo A, Kantarjian H, Manshouri T, et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndrome. Blood 2000;150:2240–5. [PubMed] [Google Scholar]
- 13.Perez-Atayde AR, Sallan SE, Tedrow U, et al. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol 1997;150:815–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Padro T, Ruiz S, Bieker R, et al. Increased angiogenesis in the bone marrow of patients with acute leukemia. Blood 2000;95:2637–44. [PubMed] [Google Scholar]
- 15.Bruneri G, Bertolini F, Soligo D, et al. Angiogenesis in myelodysplastic syndromes. Br J Cancer 1999;81:1398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vacca A, Ribatti D, Ruco L, et al. Angiogenesis extent and macrophage density increase simultaneously with pathological progression in B-cell non Hodgkin’s lymphomas. Br J Cancer 1999;79:965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sezer O, Niemoller K, Jacob C, et al. Relationship between bone marrow angiogenesis and plasma cell infiltration and serum B2-microglobulin levels in patients with multiple myeloma. Ann Hematol 2001;80:598–601. [DOI] [PubMed] [Google Scholar]
- 18.Rajkumar SV, Leong T, Roche PC, et al. Prognostic value of bone marrow angiogenesis in multiple myeloma. Clin Cancer Res 2000;6:3111–16. [PubMed] [Google Scholar]
- 19.Vacca A, Ribatti D, Roncali L, et al. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol 1994;87:503–8. [DOI] [PubMed] [Google Scholar]
- 20.Thaler J, Fechner F, Herold M, et al. Interleukin-6 in multiple myeloma: correlation with disease activity and Ki-67 proliferation index. Leuk Lymphoma 1994;12:265–71. [DOI] [PubMed] [Google Scholar]
- 21.Schluter C, Duchrow M, Wohlenberg C, et al. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining protein. J Cell Biol 1993;123:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanavaros P, Stefanaki K, Vlachonikolis J, et al. Immunohistochemical expression of the p53, p21/waf-1, Rb, p16 and Ki-67 proteins in multiple myeloma. Anticancer Res 2000;20:4619–26. [PubMed] [Google Scholar]
- 23.Durie BGM, Salmon SE. A clinical staging system for multiple myeloma; correlation of measured myeloma cell mass with presenting clinical features, response to treatment and survival. Cancer 1975;36:842–54. [DOI] [PubMed] [Google Scholar]
- 24.Papadaki H, Kyriakou D, Foudoulakis A, et al. Serum levels of soluble IL-6 receptor in multiple myeloma as indicator of disease activity. Acta Haematol 1997;97:191–5. [DOI] [PubMed] [Google Scholar]
- 25.Cordell JL, Falini B, Erber WN, et al. Immunoenzymatic labelling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APPAP complexes). J Histochem Cytochem 1984;32:219–29. [DOI] [PubMed] [Google Scholar]
- 26.Miguel-Garcia A, Matutes E, Tarin F, et al. Circulating Ki67 positive lymphocytes in multiple myeloma and benign monoclonal gammopathy. J Clin Pathol 1995;48:835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.San Miguel JF, Sanchez J, Gonzalez M. Prognostic factors and classification in multiple myeloma. Br J Cancer 1989;59:113–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodson WH, Moore DH, Ljung BM, et al. The prognostic value of proliferation indices: a study with in vivo bromodeoxyuridine and Ki-67. Breast Cancer Res Treat 2000;59:113–23. [DOI] [PubMed] [Google Scholar]
- 29.Sezer O, Niemoller K, Kaufmann O, et al. Decrease of bone marrow angiogenesis in myeloma patients achieving a remission after chemotherapy. Eur J Haematol 2001;66:238–44. [DOI] [PubMed] [Google Scholar]
- 30.Hotchkiss KA, Ashton AW, Mahmood R, et al. Inhibition of endothelial cell function in vitro and angiogenesis in vivo by docetaxel (Taxotere): association with impaired repositioning of the microtubule organizing center. Mol Cancer Ther 2002;1:1191–200. [PubMed] [Google Scholar]
- 31.Oh HS, Choi JH, Park CK, et al. Comparison of microvessel density before and after peripheral blood stem cell transplantation in multiple myeloma patients and its clinical implications: multicenter trial. Int J Hematol 2002;76:465–70. [DOI] [PubMed] [Google Scholar]
- 32.O’Leary JJ, Shapiro RL, Ren CJ, et al. Antiangiogenic effects of camptothecin analogues 9-amino-20(S)-camptothecin, topotecan, and CPT-11 studied in the mouse cornea model. Clin Cancer Res 1999;5:181–7. [PubMed] [Google Scholar]
- 33.Kumar S, Fonseca R, Dispenzieri A, et al. Bone marrow angiogenesis in multiple myeloma: effect of therapy. Br J Haematol 2002;119:665–71. [DOI] [PubMed] [Google Scholar]
- 34.Lokhorst HM, Boom SE, Terpstra W, et al. Determination of the growth fraction in monoclonal gammopathy with the monoclonal antibody Ki-67. Br J Haematol 1988;69:477–81. [DOI] [PubMed] [Google Scholar]
- 35.Drach J, Gattringer H, Classl H, et al. The biological and clinical significance of the Ki-67 growth fraction in multiple myeloma. Hematol Oncol 1992;10:125–34. [DOI] [PubMed] [Google Scholar]
- 36.Girino M, Riccardi A, Luoni R, et al. Monoclonal antibody Ki-67 as a marker of proliferative activity in monoclonal gammopathies. Acta Haematol 1991;5:26–30. [DOI] [PubMed] [Google Scholar]
- 37.Pellegrini W, Facchetti F, Marocolo D, et al. Assessment of cell proliferation in normal and pathological bone marrow biopsies: a study using double sequential immunophenotyping on paraffin sections. Histopathology 1995;27:397–405. [DOI] [PubMed] [Google Scholar]
- 38.Rawstron A, Barrans S, Blythe D, et al. Distribution of myeloma plasma cells in peripheral blood and bone marrow correlates with CD56 expression. Br J Hematol 1999;104:138–43. [DOI] [PubMed] [Google Scholar]
- 39.Alexandrakis MG, Passam FH, Ganotakis ES, et al. The clinical and prognostic significance of erythrocyte sedimentation rate (ESR) serum interleukin-6 (IL-6) and acute phase protein levels in multiple myeloma. Clin Lab Haem 2003;25:41–6. [DOI] [PubMed] [Google Scholar]
- 40.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 1999;341:1565–71. [DOI] [PubMed] [Google Scholar]