Abstract
The human placenta is an underexamined organ. The clinical indications for placental examination have no gold standards. There is also inconsistency in the histological reports and the quality is variable. There is great interobserver variability concerning the different entities. Although there are still grey areas in clinicopathological associations, a few mainstream observations have now been clarified. The histopathological examination and diagnosis of the placenta may provide crucial information. It is possible to highlight treatable maternal conditions and identify placental or fetal conditions that can be recurrent or inherited. To achieve optimal benefit from placental reports, it is essential to standardise the method of placenta examination. This article summarises the clinical indications for placenta referral and the most common acknowledged clinicopathological correlations.
Keywords: placenta, pathology, protocol, examination
According to the guidelines of the Royal College of Pathology, samples of diagnostic value removed from the human body should be histologically examined, with only a few exceptions.1 One of the exceptions is the healthy human placenta, but even with valid indications the human placenta is one of the most underexamined specimens.2 There is also evidence that the quality of reports on the investigation of the placenta is very variable.3 According to a recent study, there is a considerable discrepancy rate in the diagnosis of placental disease, and it is common for general surgical pathologists not to recognise placental lesions that may have clinical relevance.4 In this best practice article, we summarise those circumstances in which it is recommended that the placenta should be examined, the minimum criteria of sampling, and the acknowledged clinicopathological correlations.
“It is common for general surgical pathologists not to recognise placental lesions that may have clinical relevance”
Lesions of the placenta often reflect or explain the condition in which the baby was born and some have clinicopathological implications. However, in most cases, there is no clinicopathological relevance to a placental examination, such as in the case of normal pregnancy and delivery.
CLINICAL APPROACH
What do we expect from the pathological examination?
The placenta forms a functional unit between the mother and the fetus. Therefore, any pathological event that concerns the mother or the fetus will influence the normal function of the placenta, occasionally resulting in morphological change. Severe abnormalities of the placenta may lead to adverse fetal outcome. However, placental lesions are not necessarily the cause of unfavourable outcome, and some structural changes may be the consequences of poor fetal condition. The placenta is an easily available specimen and the costs of a routine pathological examination are moderate.
The benefits that can be expected from the examination include revealing the aetiology of stillbirth, preterm delivery, intrauterine growth restriction (IUGR), and neurodevelopmental impairment. It may be possible to decide whether the pathological condition that endangered the well being of the fetus was an acute or a chronic process.5,6
In the case of twin pregnancies, the type of twinning can be identified and pathological aspects of twin pregnancy (for example, twin-to-twin transfusion syndrome) can be studied.
Conditions with the risk of recurrence can be recognised, resulting in adequate treatment and preventive measures during subsequent pregnancies.
Placental examination may have medicolegal aspects—for example, concerning the aetiology of longterm neurodevelopmental sequelae or the approximate timing of an intrauterine death.7,8
Which placentas should be examined?
There are different approaches to the examination of the placenta. It would produce a pointless increase in workload if all placentas, including those from normal pregnancies and normal deliveries resulting in a healthy infant, were examined in a routine pathology laboratory setting.
Because it is the decision of the midwife and/or obstetrician which placentas to send to the pathology department, a clinically oriented approach (fig 1) may be used to define the indications for histopathological examination.
Figure 1.
Indications for pathological referral of the placenta. IU, intrauterine; IUGR, intrauterine growth restriction; NNU, neonatal unit.
Referral is not indicated for:
cholestasis of pregnancy
hepatitis B, human immunodeficiency virus, etc
other maternal disease with normal pregnancy outcome
normal pregnancy
placenta praevia
postpartum haemorrhage.
A RATIONAL SORTING OF THE REFERRED PLACENTAS
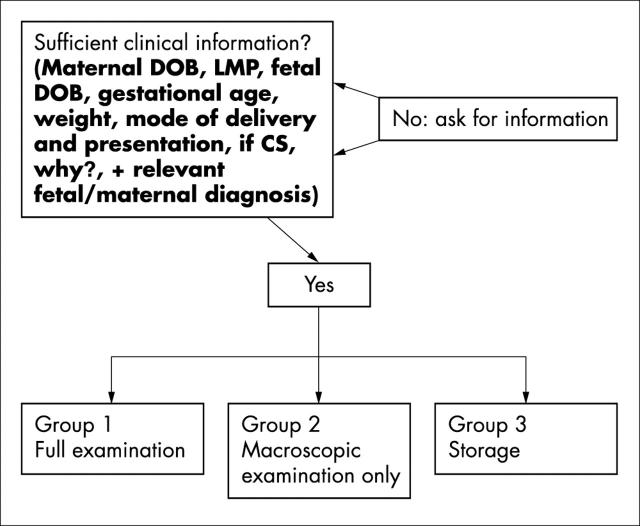
Figure 2 is an algorithm for selecting which of the referred placentas should be subjected to further study. Figure 3 contains a recommendation for sampling the placentas based on the clinical context.
Figure 2.
An algorithm to sort the placentas in view of further action. CS, caesarian section; DOB, date of birth; LMP, last menstrual period.
Figure 3.

Indications to examine the placenta, with examples of the minimum blocks. IU, intrauterine; IUGR, intrauterine growth restriction; NNU, neonatal unit; PET, pre-eclamptic toxaemia; PROM, premature rupture of membranes.
NORMAL VARIANTS
As mentioned above, many features can be judged only in the clinicopathological context. This is partly because of the loose correlation between some histological changes and clinical symptoms, and partly because of the large reserve capacity of the placenta.
To record the macroscopic appearance of the placenta we recommend the use of a worksheet as shown in fig 4. This proforma can be useful to describe normal placentas; however, each abnormality should be documented individually.
Figure 4.

Worksheet for macroscopic examination. DiDi, dichorionic diamniotic; DiMo, diamniotic monochorionic; MoMo, monochorionic monoamniotic.
Umbilical cord
The normal length of the umbilical cord at term varies between 40 and 70 cm and cords of less than 32 cm are considered to be short and those more than 100 cm are considered long. The importance of length and coiling should be treated cautiously, because the proportion of the umbilical cord received in pathology laboratories varies and is thus not reliable. Umbilical cords normally show a degree of coiling. The normal coil index is said to be one coil/5 cm.9,10 The normal cord contains three vessels, and this has to be assessed at least 5 cm from the placental insertion.11 False knots may be the site of thrombosis, or rarely bleeding, but most often they have no clinical relevance.
Embryonic remnants of the vitelline duct and urachus are normal findings. Cysts may arise from these vestigial remnants. It may be necessary to differentiate the embryonic remnants of the cord from teratomas and haemangiomas.12
Extraplacental membranes and the fetal surface12–14
The importance of circummarginate and circumvallate placentas is uncertain, although an association with IUGR and acute and chronic maternal haemorrhage has been proposed in circumvallate placentas. Amnion nodosum (granular grey/white nodules, consisting of keratin and vernix) are a sign of oligo/anhydramnios, but squamous metaplasia of the amnion is a normal feature.
A small amount of subchorionic fibrin deposition (Langhans fibrinoid) is not pathological, because it accumulates from eddying of the intervillous flow.
Placenta
A low placental weight is found in “small for gestational age” placentas. Normal values of fetal to placenta weight ratio change during the course of gestation, and vary between 1 at 14 weeks and 7.23 at term. Hydrops or congestion can result in a high placental weight, but the placenta weight can vary to some degree (a table of normal values can be found in Benirschke and Kaufmann13). Deviation from the round or oval shape such as an irregularly shaped, bilobed, or multilobed placenta can be attributed to disturbed implantation or uterine abnormalities, but it can be assessed only in the clinicopathological context. Increased calcification has been mentioned in association with maternal smoking and high socio-economic status, but the feature itself has no clinical relevance.
Minor perivillous fibrin deposition is almost always present in term placentas. This is of no clinical relevance if marginal, or if it does not exceed 10% of the villous tissue. A range of values is found in the literature with regard to the amount of the villous tissue loss required to define whether infarction or perivillous fibrin deposition is “extensive” or relevant—that is, large enough to account for adverse fetal outcome. The reported percentage of minimal villous tissue loss ranges from 10% to 30% in the case of significant placental infarcts and 20% to 30% in perivillous fibrin deposition. In general, there is no clinical relevance if the lesion is single, marginal, and/or involves less than approximately 5% of the villous tissue. Obviously, the functional reserve capacity of the placenta depends not only on the quantity, but also on the quality of the uninvolved tissue and the original size of the placenta. In the case of a small placenta, a smaller amount of parenchymal loss can lead to fetal demise or morbidity.
X cell islands (extravillous cytotrophoblast islands, X-cell proliferation) are considered to be a normal feature.
The origin of septal cysts is unknown. They are reported to occur more frequently in oedematous placentas, but are of no clinical relevance.13
Examination of twin placentas
Twin placentas should be labelled after the delivery to identify which cord belongs to which fetus. The examination of placentas from multiple gestations should establish the chorionicity of the sample and whether there are signs of twin-to-twin transfusion syndrome. Separated twin placentas have to be examined in the same way as those of singletons. Fused placentas can be monochorionic or dichorionic. The dividing membrane should be studied to identify the chorionicity. The dividing membrane in monochorionic pregnancy is thin and translucent (with no chorionic layer), whereas that of a dichorionic placenta is thicker, because it contains two chorionic layers between the amniotic sacs. The dividing membrane can be sampled as a membrane roll or in “T section” form. A properly oriented T section is the best sample to prove chorionicity. Fetal vessel anastomoses and inter-twin blood transfusion occur normally in monochorionic placentas. Imbalance in the blood flow may lead to acute or chronic twin-to-twin transfusion. Acute transfusion occurs either during labour or after the death of either of the twins, and frequently results in severe neurological damage or the death of the co-twin. Chronic twin-to-twin transfusion manifests as discordant fetal growth, oligohydramnios in the donor, and polyhydramnios in the recipient fetus, and is often associated with poor fetal outcome.15 Arterio–arterial anastomoses (AAA) may have a protective role against chronic twin-to-twin transfusion syndrome, but may be the route of acute blood loss after compromise or the death of one twin. Veno–venous anastomoses (VVA) are associated with poor outcome.16 The anatomical background of chronic twin-to-twin transfusion syndrome seems to be a unidirectional arteriovenosus shunt between the donor and the recipient twin. Injection studies can be performed in fresh specimens to clarify the type of the anastomosis.17 In fixed placentas, arteries may be identified by the fact that they are always superficial to the veins. Arterio–venous anastomosis (AVA) may be identified by the presence of an impaired vessel from one twin feeding an area drained by the co-twin. In monochorionic diamniotic placentas, it may be useful to record the sites of insertion and distance between the cord insertions, the relative size of the placental territories serving each twin, the number and minimum diameter of superficial anastomoses (AAA/VVA), and the number and direction of deep anastomoses (AVA).
“A properly oriented T section is the best sample to prove chorionicity”
It is recommended that fused dichorionic placentas should be separated. Evidence of a vanished twin might be found in singleton or twin placentas. This varies in appearance from an amorphous, fibrotic plaque to a well formed fetus papyraceous. Histological and x ray examinations are helpful to identify calcification.18
RECOGNISED CLINICOPATHOLOGICAL CORRELATIONS
Table 1 summarises the clinical relevance of placental abnormalities.
Table 1.
The clinical relevance of placental abnormalities
| Disorder | Clinicopathological correlation |
| Umbilical cord | |
| Short cord (less than 40 cm) | High fetal and neonatal mortality rates and increased frequency of neurological abnormality |
| Long cord (longer than 70 cm)19 | Maternal factors: systemic diseases, delivery complications, increased maternal age Fetal factors: non-reassuring fetal status, respiratory distress, vertex presentation, cord entanglement, male sex, increased birth weight Gross placental features: increased placental weight, overcoiled cord, true knots, congestion, cord prolapse causing fetal distress |
| Marginal cord insertion | IUGR, still birth, neonatal death, premature birth, low birth weight |
| Velamentous insertion | Fetal haemorrhage, fetal death, low birth weight, premature birth, maternal smoking, advanced maternal age |
| Overcoiling or undercoiling of the cord9,10 | Fetal demise fetal intolerance to labour, IUGR, chorioamnionitis |
| True knot | If tight, associated with perinatal mortality of 10% and umbilical vessel thrombosis |
| Single umbilical artery20,21 | Single umbilical artery is associated with fetal malformation chromosome aberration in 25–50%, with IUGR and increased perinatal mortality in normally formed infants |
| Thrombosis of umbilical cord vessels12,13,22,23 | Thromboembolic spread to placental or fetal vessels. The consequences of cord vessel thrombosis for the fetus may be wide. Severe sequelae such as fetal death, cerebral palsy and IUGR have been described, but delivery of a healthy, live neonate may also occur |
| Umbilical cord vessel vasculitis and funisitis12 | Umbilical cord vessel vasculitis and funisitis are associated with cord vessel thrombosis, preterm delivery, amniotic infection, vasospasm of cord vessels |
| Necrotising funisitis12,13,24,25 | It is often associated with premature rupture of the membranes, preterm labour, IUGR, intrauterine death. Usually seen with acute chorioamnionitis. Candida, streptococci, herpes, and syphilis are reported to play a role in the pathogenesis of necrotising funisitis. Mostly associated with chorioamnionitis NOS |
| Membranes | |
| Acute chorioamnionitis (including “subchorial intervillositis”)26–34 | Strong association with premature rupture of membranes and preterm delivery. Fetal intrauterine infection may occur. Maternal fever and tachycardia are described, but may be asymptotic. Recently, chorioamnionitis has been implicated as a risk factor for periventricular leucomalacia and cerebral palsy |
| Chronic chorioamnionitis12,13,35 | Association with premature rupture of membranes, preterm delivery, and prolonged rupture of membranes has been observed. It has been described in herpes virus infection |
| Amnion epithelial vacuolisation12,13,36 | Cell degeneration and necrosis of amniotic epithelial cells can be seen in normal and abnormal pregnancies and the evaluation of these alterations might be fairly uncertain because of artefact effects. Small, lipid containing vacuoles in the cytoplasm are the feature, strongly associated with gastroschisis |
| Pigmented macrophages, meconium staining12,13,32,37 | The presence of meconium staining is not necessarily associated with adverse fetal outcome. Meconium staining indicates the danger of meconium aspiration and with other histological signs of fetal distress may underline the diagnosis. Vasospasm of cord vessels and fetal chorionic vessels is reported as a consequence of meconium exposure |
| Deciduitis, acute deciduitis, chronic decidual necrosis13 | Acute deciduitis in the decidua capsularis is often associated with ascending infiltrations of the placental membranes, and may be unimportant in isolation. Severe, necrotising, acute deciduitis can be found in placentas with retroplacental haematoma. Chronic deciduitis with scattered infiltration may represent a physiological condition of maternal lymphocyte response |
| Placenta | |
| Low placental weight, below 10th centile for gestational age38–40 | IUGR, pre-eclampsia, increased intervillous fibrin deposition, villitis of unknown origin, and trisomy |
| High placental weight 38,39 | Maternal diabetes mellitus, maternal or fetal anaemia, fetal hydrops; may also be seen in congenital syphilis, Beckwith-Wiedemann syndrome, congenital nephrotic syndrome |
| Thin placenta (placenta annulare or placenta membranacea)38,41,42 | Average thickness less than 2 cm, placenta with large membranous area. Risk of maternal bleeding, placenta praevia, placenta accreta. Often premature delivery occurs. Possibly more frequent in IUGR |
| Placental haemorrhage | |
| Retroplacental haematoma12,13 | Large retroplacental haematomas can cause extensive infarction involving a sufficient proportion of the villous tissue to cause fetal hypoxia or lead to perinatal death. Early separation is called abruptio placentae, and can be lethal if extensive. The AFP concentration may be raised if an old haematoma |
| Subchorionic haematoma (massive subchorial thrombosis, Breus’s mole)12,13 | This is a normal finding when patchy, focal, or diffuse. However, subchorionic thromboses of large size have been reported in association with abortion, premature delivery, and live-born infants also |
| Placenta praevia | Associated with high risk of third trimester bleeding |
| Placenta accreta, increta, and percreta | Potentially life threatening clinical conditions, causing uterine rupture and massive postpartum haemorrhage, or leading to caesarean section if prenatally diagnosed. It is often an indication of postpartum hysterectomy because of excessive bleeding. To make the pathological diagnosis of a placenta accreta, examination of the entire uterus is necessary |
| Placental chorionic villi and intervillous space abnormalities | |
| Syncytial knots12,13 | Increased numbers of syncytial knots occur in: pre-eclampsia, hypertension, diabetes mellitus, maternal anaemia, pregnancy at high altitude, thick section (artefact). A correlation between increased syncytial knotting and fetal hypoxia has not been reported. An excessive increase of syncytial knotting may result from reduced fetal perfusion and placental hypoxia or can be the sign of accelerated maturation if the duration of pregnancy was less than 40 weeks |
| Infarct (acute or old)13 | No clinical relevance if it is single, marginal, and/or involves less than about 5% of the villous tissue |
| Extensive placental infarction12,13,31,43 | Involving more than 10% of villous tissue: fetal hypoxia, IUGR, stillbirth, pregnancy induced hypertension, abruptio placentae, neurological abnormalities |
| Nucleated RBC13 | Raised numbers may occur in many causes of chronic hypoxia, IUGR, stillbirth, acute fetal blood loss, maternal diabetes, and erythroblastosis fetalis |
| Villous basal membrane thickening12 | Pre-eclampsia, essential hypertension, diabetes mellitus |
| VSM deficiency12,13 | An increase of VSM is described in pregnancies at high altitude, pre-eclampsia, maternal heart failure, and maternal anaemia. VSM deficiency was reported in pre-eclampsia, materno–fetal rhesus incompatibility, maternal diabetes, low birth weight and stillbirths |
| Villous stromal fibrosis and sclerosis12,13 | Extensive stromal fibrosis occurs in terminal villous deficiency, in IUGR, and in avascular villi as a result of stem vessel thrombosis, and in CMV infection |
| Villous oedema12,13,31 | Placentas from pregnancies with hydrops fetalis may show a combination of immaturity and oedema. Villous oedema occurs in infections (syphilis, CMV, toxoplasma), in cases of fetal hydrops, and in hydatidiform moles. It is correlated with neurological impairment and cerebral palsy. May be normal if focal |
| Dysmaturity/immaturity12,44 | A failure of villous maturation was found to be associated with fetal hypoxia, IUGR, maternal diabetes, and materno–fetal rhesus incompatibility. Failure of maturation can lead to intrauterine death |
| Advanced maturation/maturitas praecox12,13 | Accelerated maturation can be seen in prematurely delivered placentas in pre-eclampsia. It is considered to be an ischaemic feature |
| Mesenchymal dysplasia45,46 | Associated with Beckwith-Wiedeman syndrome |
| Perivillous fibrin32, extensive perivillous fibrin deposition | When more than 20–30% of the villous tissue and functional placenta is involved it is associated with IUGR and fetal death. In these cases often 70–80% of the villous population is enveloped by fibrin. The maternal serum AFP values is raised, sometimes extremely so |
| Maternal floor infarct12,13,39,47, Gitter infarct | Massive basal plate perivillous fibrin deposition is termed “maternal floor infarct” and is associated with high mortality and IUGR. Massive perivillous fibrin deposition in a netlike pattern is the “Gitter infarct”. Neither of these is an infarct. Massive perivillous fibrin can recur (18%) and is associated with IUGR and fetal death |
| Villitis | |
| Acute villitis12,13 | Clinical consequences depend on the type of the pathogenic agent. Acute villitis is usually associated with severe maternal infection, preterm delivery and might lead to intrauterine infection and IUD |
| Chronic villitis: basal, parenchymal, granulomatous, and VUE12,13,32 | Chronic villitis: more often of unknown aetiology (VUE) than known. Fetal infections causing chronic villitis: CMV, toxoplasma, connatal syphilis. Chronic villitis is associated with: IUGR and/or stillbirth |
| VUE: IUGR, preterm birth, is often recurrent! | |
| Chronic histiocytic intervillositis, (chronic perivillositis)48–50 | Associated with raised maternal serum AFP, recurrent abortion, IUGR, preterm delivery. Malaria infection should be excluded |
| Abnormalities of the fetal vessels | |
| Fetal chorionic vessel thrombosis and avascular villi; stem vessel thrombosis (single/or multiple); recanalisation of chorionic vessels8,31,32,51,52 | Extensive avascular villi as a result of fetal vessel thrombosis was reported in association with stillbirth, IUGR, maternal and fetal coagulopathy, and fetal thromboembolic disease leading to cerebral palsy |
| Intimal fibrin cushion37,53 | Neonatal asphyxia, association with disseminated capillary thrombi of fetal vessels. The severity of the fetal consequences depends more on the accompanying vascular lesions, mainly on fetal vessel thrombosis |
| HEV and haemorrhagic villitis12,13,54–56 | HEV was reported to be a postmortem artefact and was doubted as being a specific disease entity. HEV was found to be associated with meconium staining and postmaturity. Earlier reports revealed an association with stillbirth, IUGR, neurological disability, maternal hypertension. Can occur in live births, associated with perinatal complications, fetal distress, IUGR. Interlesional relations exist between thrombotic, chronic inflammatory, and chronic vaso-occlusive lesions |
| Chorangiosis13,57,58 | Perinatal death, congenital malformation, and cerebral palsy were found to be associated with chorangiosis as a response to low grade tissue hypoxia. Although others have supported this observation, it is still unclear how chronic hypoxia results in increased vascularisation. The importance of this alteration needs further investigation |
| Abnormalities of the maternal vessels13 | |
| Failure of physiological adaptation of maternal vessels, uteroplacental vessel fibrinoid necrosis,12 acute atherosis, uteroplacental vessel thrombosis59 | Uteroplacental or decidual arteriopathy is closely related to pregnancy induced hypertension, maternal essential hypertension, and pre-eclampsia, and results in fetal complications such as IUGR, SGA, and stillbirth. It is associated with APA, SLE, and thrombophilia |
| Haemorrhages of the placenta | |
| Intervillous haemorrhage and intervillous thrombus | Most often intervillous haemorrhage is related to a maternal vessel lesion and is of maternal origin. Its consequence can be fetal compromise or death depending on the functional placenta parenchyma loss and the rest of the unaffected placenta |
| Kline’s haemorrhage12,13 | In some cases, intervillous haemorrhage and thrombus is a sign of fetal bleeding into the maternal circulation, as described by Kline. Only a few of these alterations lead to a large amount of fetal blood loss and stillbirth or severe anaemia followed by ischaemic lesions of parenchymal organs |
| Twin placenta, chorionicity | It is important to know the type of twinning because the twin-to-twin transfusion syndrome is associated with diamniotic monochorionic placentas |
| Angiomas | |
| Angioma of the placenta (chorangioma)12,13,58 | Large lesions often lead to cardiac failure, hydrops, and death of the fetus. Transplacental bleeding and fetomaternal transfusion have been also described, leading to anaemia. Chorangioma was reported to be associated with pre-eclampsia, multiple gestation, premature delivery, fetal thrombocytopenia, and fetal angioma (Kasabach-Merritt syndrome) |
| Angioma in the cord60,61 | The lesion can be associated with raised AFP, fetal DIC, and fetal hydrops; fetal death has been described too |
AFP, α fetoprotein; APA, anti-phospholipid syndrome; CMV, cytomegalovirus; DIC, disseminated intravascular coagulopathy; HEV, haemorrhagic endovasculitis; IUD, intrauterine death; IUGR, intrauterine growth restriction; NOS, not otherwise specified; RBC, red blood cell; SGA, small for gestational age; SLE, systemic lupus erythematosus; VSM, vasculo–syncytial membrane; VUE, villitis of unknown aetiology.
CONCLUSION
We recommend that relevant placentas are discussed regularly at perinatal mortality or morbidity meetings. This could reveal new clinicopathological correlations, would increase appreciation of the profession, and would serve team building and communication between the different medical teams. We have presented an algorithm of indications for placental examination and discussed the methods of histopathological examination. Common placental lesions with their clinicopathological correlation are reviewed. Our intent is to outline the acknowledged entities with their clinical consequences. Often, the clinicopathological correlation appears to be strong, significant, and well documented. In other instances, lesions may have a tendency to occur with clinical conditions and in the rest of the cases there is only an anecdotal association. A major problem with the literature related to the placenta is that most of it has been produced based solely on abnormal placentas, so that for many features it is not clear what is pathologically abnormal and what is a normal variant. Basic studies are necessary to analyse normal placentas statistically and to identify the normal variants of histological lesions during the course of pregnancy.
It is also apparent that because function depends on the reserve capacity of the placenta, several findings can be judged only in the clinical context: the importance of a particular lesion depends on its localisation and on the extent of the lesion (the proportion of the placenta involved and the size and the condition of the uninvolved placenta). Some features can be within normal limits in term placentas, whereas earlier in pregnancy they may be pathological. In addition, the assessment of the lesions is even more complex because several pathological conditions can coexist in the same placenta.
Acknowledgments
The authors are grateful to TY Khong, Associate Professor, Department of Obstetrics and Gynaecology, University of Adelaide, Australia for his advice during the preparation of this manuscript.
Abbreviations
AAA, arterio–arterial anastomosis
AVA, arterio–venous anastomosis
IUGR, intrauterine growth restriction
VVA, veno–venous anastomosis
REFERENCES
- 1. Histopathology of limited or no clinical value. Report of a working group of the Royal College of Pathologists. Guideline of the Royal College of Pathologists, London: Royal College of Pathologists, August, 2002.
- 2.Spencer MK, Khong TY. Conformity to guidelines for pathologic examination of the placenta. Arch Pathol Lab Med 2003;127:205–7. [DOI] [PubMed] [Google Scholar]
- 3.Khong TY, Gordijn SJ. Quality of placental pathology reports. Pediatr Dev Pathol 2003;6:54–8. [DOI] [PubMed] [Google Scholar]
- 4.Sun CC, Revell VO, Belli AJ, et al. Discrepancy in pathologic diagnosis of placental lesions. Arch Pathol Lab Med 2002;126:706–9. [DOI] [PubMed] [Google Scholar]
- 5.Langston C, Kaplan C, Macpherson T, et al. Practice guideline for examination of the placenta: developed by the placental pathology practice guideline development task force of the College of American Pathologists. Arch Pathol Lab Med 1997;121:449–76. [PubMed] [Google Scholar]
- 6.Salafia CM, Vintzileos AM. Why all placentas should be examined by a pathologist in 1990. Am J Obstet Gynecol 1990;163:1282–93. [DOI] [PubMed] [Google Scholar]
- 7.Altshuler G. Placenta within the medicolegal imperative. Arch Pathol Lab Med 1991;115:688–95. [PubMed] [Google Scholar]
- 8.Kraus FT. Cerebral palsy and thrombi in placental vessels of the fetus: insights from litigation. Hum Pathol 1997;28:246–8. [DOI] [PubMed] [Google Scholar]
- 9.Machin GA, Ackerman J, Gilbert-Barness E. Abnormal umbilical cord coiling is associated with adverse perinatal outcomes. Paediatr Dev Pathol 2000;3:462–71. [DOI] [PubMed] [Google Scholar]
- 10.van Diik CC, Franx A, De Laat MV, et al. The umbilical coiling index in normal pregnancy. J Matern Fetal Neonatal Med 2002;11:280–3. [DOI] [PubMed] [Google Scholar]
- 11.Fujikura T. Fused umbilical arteries near placental cord insertion. Am J Obstet Gynecol 2003;188:765–7. [DOI] [PubMed] [Google Scholar]
- 12.Fox H. Pathology of the placenta. London: WB Saunders Company Ltd, 1997.
- 13.Benirschke K, Kaufmann P. Pathology of the human placenta. 4th ed. New York: Springer-Verlag, 2000.
- 14.Fox H, Elston CW. Pathology of the placenta. Major Probl Pathol 1978;7:1–491. [PubMed] [Google Scholar]
- 15.Denbow ML, Fisk NM. The consequences of monochorionic placentation. Baillieres Clin Obstet Gynaecol 1998;12:37–51. [DOI] [PubMed] [Google Scholar]
- 16.Denbow ML, Cox P, Taylor M, et al. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am J Obstet Gynecol 2000;182:417–26. [DOI] [PubMed] [Google Scholar]
- 17.De Paepe ME, Burke S, Luks FI, et al. Demonstration of placental vascular anatomy in monochorionic twin gestations. Paediatr Dev Pathol 2002;5:37–44. [DOI] [PubMed] [Google Scholar]
- 18.Jauniaux E, Elkazen N, Leroy F, et al. Clinical and morphologic aspects of the vanishing twin phenomenon. Obstet Gynecol 1988;72:577–81. [PubMed] [Google Scholar]
- 19.Baergen RN, Malicki D, Behling C, et al. Morbidity, mortality, and placental pathology in excessively long umbilical cords: retrospective study. Paediatr Dev Pathol 2001;4:144–53. [DOI] [PubMed] [Google Scholar]
- 20.Chow JS, Benson CB, Doubilet PM. Frequency and nature of structural anomalies in fetuses with single umbilical arteries. J Ultrasound Med 1998;17:765–8. [DOI] [PubMed] [Google Scholar]
- 21.Rinehart BK, Terrone DA, Taylor CW, et al. Single umbilical artery is associated with an increased incidence of structural and chromosomal anomalies and growth restriction. Am J Perinatol 2000;17:229–32. [DOI] [PubMed] [Google Scholar]
- 22.Heifetz SA. Thrombosis of the umbilical cord: analysis of 52 cases and literature review. Pediatr Pathol 1988;8:37–54. [DOI] [PubMed] [Google Scholar]
- 23.Wolfman WL, Purohit DM, Self SE. Umbilical vein thrombosis at 32 weeks’ gestation with delivery of a living infant. Am J Obstet Gynecol 1983;146:468–70. [DOI] [PubMed] [Google Scholar]
- 24.Craver RD, Baldwin VJ. Necrotizing funisitis. Obstet Gynecol 1992;79:64–70. [PubMed] [Google Scholar]
- 25.Jacques SM, Qureshi F. Necrotizing funisitis: a study of 45 cases. Hum Pathol 1992;23:1278–83. [DOI] [PubMed] [Google Scholar]
- 26.Gibbs RS, Romero R, Hillier SL, et al. A review of premature birth and subclinical infection. Am J Obstet Gynecol 1992;166:1515–28. [DOI] [PubMed] [Google Scholar]
- 27.Naeye RL. Functionally important disorders of the placenta, umbilical cord, and fetal membranes. Hum Pathol 1987;18:680–91. [DOI] [PubMed] [Google Scholar]
- 28.Naeye RL. Acute chorioamnionitis and the disorders that produce placental insufficiency. Monogr Pathol 1991:286–307. [PubMed]
- 29.Redline RW, Faye-Petersen O, Heller D, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Paediatr Dev Pathol 2003;6:435–48. [DOI] [PubMed] [Google Scholar]
- 30.Resch B, Vollaard E, Maurer U, et al. Risk factors and determinants of neurodevelopmental outcome in cystic periventricular leucomalacia. Eur J Pediatr 2000;159:663–70. [DOI] [PubMed] [Google Scholar]
- 31.Redline RW, Wilson-Costello D, Borawski E, et al. Placental lesions associated with neurologic impairment and cerebral palsy in very low-birth-weight infants. Arch Pathol Lab Med 1998;122:1091–8. [PubMed] [Google Scholar]
- 32.Redline RW, O’Riordan MA. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch Pathol Lab Med 2000;124:1785–91. [DOI] [PubMed] [Google Scholar]
- 33.Wu YW, Colford JM Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 2000;284:1417–24. [DOI] [PubMed] [Google Scholar]
- 34.Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev 2002;8:25–9. [DOI] [PubMed] [Google Scholar]
- 35.Gersell DJ, Phillips NJ, Beckerman K. Chronic chorioamnionitis: a clinicopathologic study of 17 cases. Int J Gynecol Pathol 1991;10:217–29. [PubMed] [Google Scholar]
- 36.Grafe MR, Benirschke K. Ultrastructural study of the amniotic epithelium in a case of gastroschisis. Pediatr Pathol 1990;10:95–101. [DOI] [PubMed] [Google Scholar]
- 37.Altshuler G. Some placental considerations related to neurodevelopmental and other disorders. J Child Neurol 1993;8:78–94. [DOI] [PubMed] [Google Scholar]
- 38.Naeye RL. Do placental weights have clinical significance? Hum Pathol 1987;18:387–91. [DOI] [PubMed] [Google Scholar]
- 39.Naeye RL. Disorders of the placenta, fetus and neonate: diagnosis and clinical significance. St Louis: Mosby Year Book,, 1992.
- 40.Heinonen S, Taipale P, Saarikoski S. Weights of placentae from small-for-gestational age infants revisited. Placenta 2001;22:399–404. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed A, Gilbert-Barness E. Placenta membranacea: a developmental anomaly with diverse clinical presentation. Paediatr Dev Pathol 2003;6:201–3. [DOI] [PubMed] [Google Scholar]
- 42.Redline RW, Patterson P. Patterns of placental injury. Correlations with gestational age, placental weight, and clinical diagnoses. Arch Pathol Lab Med 1994;118:698–701. [PubMed] [Google Scholar]
- 43.Naeye RL. Placental infarction leading to fetal or neonatal death. A prospective study. Obstet Gynecol 1977;50:583–8. [PubMed] [Google Scholar]
- 44.Stalmach T, Hebisch G, Meier K, et al. Rescue by birth: defective placental maturation and late fetal mortality. Obstet Gynecol 2001;97:505–9. [DOI] [PubMed] [Google Scholar]
- 45.Jauniaux E, Nicolaides KH, Hustin J. Perinatal features associated with placental mesenchymal dysplasia. Placenta 1997;18:701–6. [DOI] [PubMed] [Google Scholar]
- 46.Lokan J, Chan YF. Placental mesenchymal dysplasia. Pathology 2002;34:375–8. [DOI] [PubMed] [Google Scholar]
- 47.Bane AL, Gillan JE. Massive perivillous fibrinoid causing recurrent placental failure. Br J Obstet Gynaecol 2003;110:292–5. [PubMed] [Google Scholar]
- 48.Boyd TK, Redline RW. Chronic histiocytic intervillositis: a placental lesion associated with recurrent reproductive loss. Hum Pathol 2000;31:1389–96. [PubMed] [Google Scholar]
- 49.Doss BJ, Greene MF, HIll J, et al. Massive chronic intervillositis associated with recurrent abortions. Hum Pathol 1995;26:1245–51. [DOI] [PubMed] [Google Scholar]
- 50.Ordi J, Ismail MR, Ventura PJ, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol 1998;22:1006–11. [DOI] [PubMed] [Google Scholar]
- 51.Rayne SC, Kraus FT. Placental thrombi and other vascular lesions. Classification, morphology, and clinical correlations. Pathol Res Pract 1993;189:2–17. [DOI] [PubMed] [Google Scholar]
- 52.Redline RW, Pappin A. Fetal thrombotic vasculopathy: the clinical significance of extensive avascular villi. Hum Pathol 1995;26:80–5. [DOI] [PubMed] [Google Scholar]
- 53.DeSa DJ. Rupture of fetal vessels on placental surface. Arch Dis Child 1971;46:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sander CH. Hemorrhagic endovasculitis and hemorrhagic villitis of the placenta. Arch Pathol Lab Med 1980;104:371–3. [PubMed] [Google Scholar]
- 55.Sander CM, Gilliland D, Akers C, et al. Livebirths with placental hemorrhagic endovasculitis: interlesional relationships and perinatal outcomes. Arch Pathol Lab Med 2002;126:157–64. [DOI] [PubMed] [Google Scholar]
- 56.Shen-Schwarz S, Macpherson TA, Mueller-Heubach E. The clinical significance of hemorrhagic endovasculitis of the placenta. Am J Obstet Gynecol 1988;159:48–51. [DOI] [PubMed] [Google Scholar]
- 57.Altshuler G. Chorangiosis. An important placental sign of neonatal morbidity and mortality. Arch Pathol Lab Med 1984;108:71–4. [PubMed] [Google Scholar]
- 58.Ogino S, Redline RW. Villous capillary lesions of the placenta: distinctions between chorangioma, chorangiomatosis, and chorangiosis. Hum Pathol 2000;31:945–54. [DOI] [PubMed] [Google Scholar]
- 59.Magid MS, Kaplan C, Sammaritano LR, et al. Placental pathology in systemic lupus erythematosus: a prospective study. Am J Obstet Gynecol 1998;179:226–34. [DOI] [PubMed] [Google Scholar]
- 60.Kamitomo M, Sueyoshi K, Matsukita S, et al. Hemangioma of the umbilical cord: stenotic change of the umbilical vessels. Fetal Diagn Ther 1999;14:328–31. [DOI] [PubMed] [Google Scholar]
- 61.Sondergaard G. Hemangioma of the umbilical cord. Acta Obstet Gynecol Scand 2003;73:434–6. [DOI] [PubMed] [Google Scholar]