Abstract
Aims: To investigate the practicality and sensitivity of supervised automated microscopy (AM) for the detection of micrometastasis in sentinel lymph nodes (SLNs) from patients with breast carcinoma.
Methods: In total, 440 SLN slides (immunohistochemically stained for cytokeratin) from 86 patients were obtained from two hospitals. Samples were selected on the basis of: (1) a pathology report mentioning micrometastases or isolated tumour cells (ITCs) and (2) reported as negative nodes (N0).
Results: From a test set of 29 slides (12 SLN positive patients, including positive and negative nodes), 18 slides were scored positive by supervised AM and 11 were negative. Routine examination revealed 17 positive slides and 12 negative. Subsequently, automated reanalysis of 187 slides (34 patients; institute I) and 216 slides (40 patients; institute II) from reported node negative (N0) patients showed that two and seven slides (from two and five patients, respectively) contained ITCs, respectively, all confirmed by the pathologists, corresponding to 5.9% and 12.5% missed patients. In four of the seven missed cases from institute II, AM also detected clusters of four to 30 cells, but all with a size ⩽ 0.2 mm.
Conclusions: Supervised AM is a more sensitive method for detecting immunohistochemically stained micrometastasis and ITCs in SLNs than routine pathology. However, the clinical relevance of detecting cytokeratin positive cells in SLNs of patients with breast cancer is still an unresolved issue and is at the moment being validated in larger clinical trials.
Keywords: micrometastasis, breast carcinoma, sentinel lymph nodes, immunohistochemistry, automated microscopy
Axillary lymph node status is one of the most powerful prognostic factors in breast cancer, despite the ongoing search for molecular markers that predict the behaviour of the primary tumour. Classic lymph node staging requires the removal of most of the axillary nodes, a procedure with the potential for considerable postoperative complications. As an alternative, sentinel lymph node (SLN) biopsy has been proposed, based on the postulate that anatomically tumour cells have to pass through one or a few lymph nodes (the “sentinel lymph nodes”) before spreading into other nodes of the lymphatic system. This theory is strongly supported by data showing that SLNs can predict axillary status in 95% of cases.1–4
Strategies for investigating SLN were designed in a consensus meeting of the College of American Pathologists primarily aiming at detecting metastases of size 2 mm or larger, because only the presence of metastases of this size had been shown to correlate well with survival.5 Furthermore, it was recommended that multiple sectioning of the whole SLN should be carried out at 2 mm intervals to increase detection sensitivity. Despite the increased workload, many centres apply multiple sectioning at even smaller intervals, because it has been shown that the sensitivity for detecting occult cells and micrometastases < 0.2 mm increases up to a certain level with the number of sections investigated.6,7 Although they may not have prognostic impact, such micrometastases or even isolated tumour cells (ITCs) are accompanied by second echelon metastases in a considerable proportion of patients, and are therefore clinically relevant because they (according to current standards) indicate the need for further axillary dissection.6,8,9 In addition, immunohistochemical (IHC) staining of breast cells using cytokeratin specific antibodies is being used to improve the ability to recognise smaller metastases and ITCs in particular. As an example, the application of IHC in combination with the analysis of multiple sections results in the detection of up to 35% more positive nodes compared with conventional histopathology.10–15 Finally, techniques such as reverse transcription polymerase chain reaction are increasingly used to investigate whether breast tissue specific mRNA molecules are present in SLN biopsies.
“Until recently, automated image analysis was either too slow, or not capable of hands off analysis of relatively large numbers of specimens, so that it was not cost effective”
Thus, whereas the term “positive lymph node” according to recommendations of the College of American Pathologists has been reserved for “the presence of a metastasis of size > 2 mm”, results of alternative methods and introduction of the term “micrometastasis” allow nodes to be classified as positive “as soon as one single tumour cell is found”. To bring the terminology and classification of SLN status up to date, the definition of micrometastasis was revisited and incorporated into the AJCC staging system, effective from 1 January 2003.16,17 Basically, micrometastases have been further defined with a lower limit and are designated pN1mi for metastases > 0.2 mm but not > 2 mm. Metastases that are not larger than 0.2 mm have been designated as ITCs and are classified pN0 with modifiers to indicate pN0(i+): ITCs present but no clusters larger than 0.2 mm. The pN0(i−) category has also been defined for lymph nodes assessed above routine single level screening but found negative. pN0(mol− and +) are identical for molecular methods.17–19 In this article we have used the AJCC staging system criteria.
One of the key questions will be whether manual methods provide sufficient sensitivity and whether examination of multiple sections can be performed in a cost effective way. The rate of missed metastases (micrometastases or macrometastases) tested by routine microscopy re-evaluation ranges from 2% to 9%.20 Metastases may also be missed when IHC staining is applied, and this has been ascribed to human failure and fatigue; problems that can be circumvented to a large extent when unbiased image analysis and automation is applied. Recent studies have indicated that image analysis is more sensitive than manual microscopy.21,22 A preliminary study, in which IHC negative SLN biopsies were reanalysed by automated microscopy, revealed the presence of single tumour cells and groups up to 30 μm in size.22 However, until recently, automated image analysis was either too slow, or not capable of hands off analysis of relatively large numbers of specimens, so that it was not cost effective; these shortcomings have prevented its introduction into routine pathology practice so far.
In this article, we report the results of the use of “hands off” microscopy and supervised image analysis to reanalyse sections from SLNs that were IHC stained as part of routine common practice in two clinical centres in the Netherlands.
MATERIAL AND METHODS
Patients
The patients consisted of a group of consecutive patients diagnosed with breast cancer in the year 2001, in which the SLN biopsy was performed as a standard procedure. Clinical procedures and details on tissue processing of SLN have been described elsewhere.6,7
Basically, immunostaining for cytokeratin was performed using CAM 5.2 (Becton Dickinson, Franklin Lakes, New Jersey, USA) or AE1/AE3 (Dako, ITK Diagnostics, Uithoorn, the Netherlands) (institute I and II, respectively; see later), and 3,3′-diaminobenzidine as endpoint product; all sections were counterstained with haematoxylin. Four to five levels were cut at 250 μm intervals from each block. The SLN tumour load was classified according to Greene et al.16,17 In the original manual pathology examination, a node was called positive when at least one IHC positive tumour cell was found. The CAM 5.2 antibody also stains dendritic cells. Although these cells will sometimes be selected by automated microscopy, they can very easily be identified by the reviewing pathologist.
Test set (selected positive and negative nodes from SLN positive patients)
Conditions and software algorithms to detect IHC positive cells had been developed before and are described elsewhere.19 To test their performance on IHC stained SLN slides (haematoxylin counterstaining), a test set consisting of 32 slides from 12 patients (archives of the department of pathology of the VU Medical Centre of Amsterdam, The Netherlands) was used. This test set contained positive samples selected on the basis of a pathology report of micrometastasis, in addition to similarly stained slides from negative nodes. The investigators performing the supervised AM were blinded to the composition of the set.
Study of reported negative SLNs from two centres
Reported negative slides were obtained from the VU Medical Centre of Amsterdam (34 patients, 192 slides; institute I) and from the St Antonius Hospital in Nieuwegein, the Netherlands (40 patients, 216 slides; institute II). These slides had been IHC stained for cytokeratin, as part of common clinical practice in both institutions, and had been reported as tumour negative samples.
Supervised automated microscopy
Hardware: for automated microscopy, an Ariol SL-50 image analysis system (Applied Imaging Corp, Santa Clara, California, USA) was used. The operation and pathologist review of this system is centralised to a single or networked station.
In brief, the system consists of a microscope (Olympus BX-61) providing bright field capabilities, a trinocular head, and ×1.25, ×10, ×40, and ×50 objectives. The microscope is equipped with an automated scanning stage, an automated filter wheel containing filters for red, green, and blue light, and automated focusing and a B/W CCD camera with light integration capability (COHU 4910, Cohu, Poway, California, USA). A personal computer (Dell Precision 530) is used to control all microscope functions, perform image acquisition and processing, and perform user interface functions. Key to the hands off microscopy approach is a slide feeder system capable of handling 50 slides in a fully automated way (for example, overnight).
Analysis procedure
All slides were bar coded and loaded into the slide feeder system. The focus position was determined automatically, and the slide was scanned using low magnification (×1.25; NA 0.04 objective) to locate the section(s) on the slide and store the coordinates as regions of interest (time needed, one minute). Subsequently, all regions of interest were analysed using a ×10 objective (NA 0.30) to identify IHC positive cells automatically (time needed: image acquisition, four minutes; analysis, 10–15 minutes depending on the size of the tissue section). The automatically detected IHC positive cells and their characteristics were stored in a database. The steps described above (slide loading, focus finding, changing objective lenses, and analysis) were performed fully hands off (typically overnight) in batches of 50 glass slides (the capacity of the slide loader).
Supervision consisted of reviewing the database with detected candidate cells. The operator preselected only morphologically recognisable IHC positive cells, excluding dirt, and candidate objects were thereafter presented to the pathologist for confirmation as tumour cells by reviewing the images and, if necessary, by relocating these objects under the microscope.
RESULTS
Supervised AM compared with conventional pathology
Test set
In total, 32 IHC stained slides were available (positive and negative nodes from 12 node positive patients); three slides were rejected because of irrelevant material (fat). Using routine manual microscopy, 17 slides were scored positive and 12 negative. Automated analysis resulted in 19 positive and 10 negative slides. Visual evaluation of the discrepancies by the pathologist revised one machine positive slide (with non-specific staining of cells) as negative. However, one machine positive slide (with one single cell and three clusters) was confirmed as having been missed by the pathologist in routine analysis. Fortunately, other slides of the same node had been found positive by manual examination, so this mistake had no impact on the patient. On average, 562 objects were analysed by the operator for each slide, resulting in 49 candidate tumour cells (from 29 analysed lymph nodes) to be confirmed by the pathologist.
Institute I
In total, 192 slides (from 34 patients) were available; two slides were rejected because of irrelevant material (fat), three slides could not be analysed automatically because of strong non-specific staining of sinus lining cells by the monoclonal antibody. Automated analysis resulted in 11 candidate positive slides. In review, the pathologist scored two slides as positive. Slides scored as negative by the pathologist contained positively stained macrophages or debris, not showing the morphological characteristics of tumour cells. From the two cases that were missed by manual examination, one slide contained five IHC positive single cells. The other slide contained one cell that was scored as “uncertain”. The positively classified patient had a breast carcinoma of the left side. The SLN in the left axilla was then negative but the SLN in the right axilla was examined because of a new primary breast tumour on the right side. Based on the negatively reported SLN, this patient had been spared further axillary lymph node dissection. On average, 849 objects were analysed by the operator for each slide, resulting in 11 slides with a total of 25 (range, 1–10/slide) candidate tumour cells to be confirmed by the pathologist.
Institute II
In total, 216 slides (from 40 patients) were analysed. Automated analysis resulted in 10 positive slides, which were reviewed by the pathologist. In seven of the 10 slides the pathologist confirmed the findings of automated microscopy; that is, the presence of clusters of cells (n = 4) or single cells (n = 3). The single cells were detected in three sequential sections, separated by 250 μm, from one patient. In the other four positive cases, distinct clusters were recognised, with sizes varying from 0.04 to 0.14 mm in largest dimension (four to 30 cells). On average, 61 objects were analysed by the operator for each slide, resulting in 10 slides with a total of 15 (range, 1–3/slide) candidate tumour cells to be confirmed by the pathologist. All seven patients reported negative by routine manual microscopy but found positive by supervised AM had been spared axillary lymph node dissection. Since the time of the original SLN investigation (2001), none of them had shown signs of recurrent disease. Table 1 shows a summary of the results of the automated lymph node analysis. Altogether, two slides (including one uncertain case) were missed by institute I, and seven by institute II, corresponding to 1.1% (0.5% excluding the uncertain case) and 3.2%, respectively, at the slide level. At the clinically more relevant patient level, for institute I, two of 34 patients (5.9%) (one of 34 patients (2.9%) when excluding the uncertain case), and for institute II, five of 40 patients (12.5%) had been missed in the original manual analysis. Figure 1 shows images of IHC positive cells detected by supervised AM.
Table 1.
Results of supervised AM of IHC stained SLN biopsy specimens originally reported as N0 by manual examination
| Institute I | Institute II | |
| Patients | 34 | 40 |
| Slides | 187 | 216 |
| Scored positive by AM (no of slides) | 2 | 7 |
| Scored positive by AM (no of patients) | 2 | 5 |
| Slides positive by supervised AM (all confirmed) | 1.1% (0.5%)* | 3.2% |
| Patients positive by supervised AM (all confirmed) | 5.9% (2.9%)* | 12.5% |
*One classification was uncertain; including this result among the positives gives rise to the first percentage but not including it gives rise to the second number in parenthesis (recommended according the UICC classification for haematoxylin and eosin stained sections).
AM, automated analysis; IHC, immunohistochemistry; SLN, sentinel lymph node.
Figure 1.
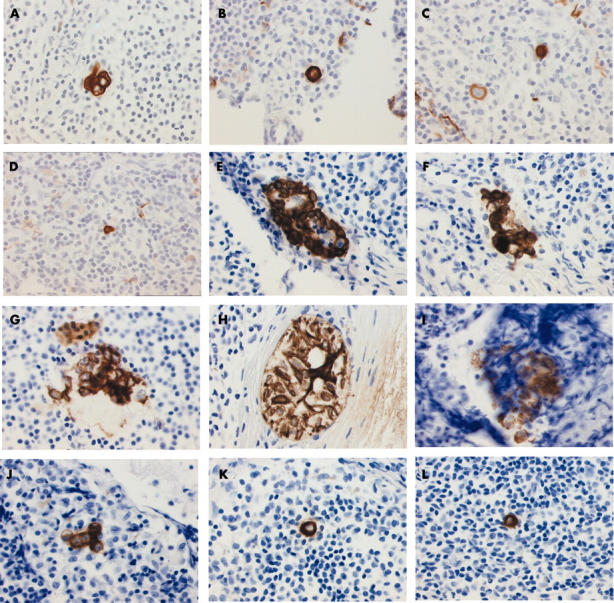
Examples of cytokeratin immunostained cells and cell clusters detected in sections manually classified as negative. Images were taken using a Sony Power HAD 3 CCD colour video camera. Cells shown were found in (A) test set, in (B–D) the series from institute I, and (E–L) the series from institute II.
Time needed for automated analysis
Batches of 50 slides, each with typically two sections, were analysed overnight, without affecting hands on time. The number of detected objects varied from 849 for institute I, to only 61 for institute II, and depended strongly on the level of fine tuning of the IHC staining conditions with respect to staining artefacts (dye precipitates, non-specific binding). Note also that the two institutes used different cytokeratin specific antibodies—CAM 5.2 and AE1/AE3 for institutes I and II, respectively. Because each memory page simultaneously displays 80 cells, detected objects were easily reviewed in less than a minute; relocating a particular object using the microscope hardware obviously took more time. Only a few (one to five) objects were relocated for each section using the microscope. Note that a clearly positive section takes less time to analyse than a negative one, because finding one micrometastasis or single tumour cell finalises the classification. In this particular study, the average verification of the detected events in 50 slides (100 sections) took from one to about four hours, depending of the number of positive slides; that is, slightly more than one minute of hands on time for each section. Note that the hands on time primarily relates to the time needed for the cytotechnologist to select cells from the image memories; the time spend by the pathologist on the cytopathological confirmation of these cells is very limited (table 2).
Table 2.
Comparison between manual and automated screening of sections of breast cancer SLN
| Manual | Automated (supervised) | |
| Staining | H&E or IHC | IHC |
| Sensitivity to detect micrometastases | Low to moderate | High |
| Hands on time/section | 30–60 sec | 1 min* |
| Costs of hardware | 20 k$ | 100 k$ |
| Digital documentation | No | Yes |
| Fun factor | Low | High |
| Fatigue factor | High | Low |
| Reproducibility | Good | Very good |
*Most of the hands on time relates to the preselection of cells from the digitally stored images; the time spend by the pathologist for cytopathological confirmation is very small.
H&E, haematoxylin and eosin; IHC, immunohistochemistry; SLN, sentinel lymph node.
DISCUSSION
Supervised AM of 403 IHC stained SLN sections from 74 patients with stage N0 breast cancer showed that the missed positive rate of routine manual microscopy ranges from 2.9% to 12.5% (depending on the hospital). It should be stressed again, that both centres for manual diagnosis aimed at reporting single cells and small groups of cells; therefore, the classification “node negative” of the material used in our study not only excluded the presence of metastasis > 2 mm, but also the presence of micrometastases or single tumour cells. Institute I, the academic hospital with vast SLN experience, performed somewhat better than institute II, a large regional hospital.6–8 This may reflect a correlation between experience and performance in this area. Therefore, it is likely that the reanalysis of cases taken from even less experienced, smaller centres will reveal even higher numbers of cases in which automated analysis results in finding tumour cells or small groups of cells.
It is also evident that the number of positive nodes (using one single confirmed tumour cell as criterion) will increase upon analysis of more sections.6,7 In our present study, an average of four levels were analysed for each SLN, with an interval of 250 μm.23 The sectioning and staining of all resected lymph nodes is too labour intensive for use in a clinical setting. However, for the analysis of SLNs, which most of the time involves only one to three lymph nodes, step sectioning is highly recommended by the Association of Directors of Anatomic and Surgical Pathology and is practically feasible.24 In fact, automated analysis of multiple sections from a few SLNs is easily accomplished using hands off analysis overnight. The limiting step becomes the time needed to inspect the database with images. In our study, about one minute was needed for each section. The pathologist involved in our study stated that reviewing a few preselected images is intellectually more satisfying than manually scanning a complete slide at varying magnifications. Our results suggest that supervised AM is a powerful technique to analyse IHC stained SLN biopsies, particularly when multiple sectioning is applied. A recent review of the European Working Group for Breast Screening Pathology states that currently published data do not allow the relevance of micrometastases or ITCs to be established. However, it is suggested that approximately 18% of the cases may be associated with further nodal (non-SLN) metastases; that is, approximately 2% of all patients staged by SLN biopsy.25 It should be realised that this conclusion is based on published data obtained by manual examination of a few IHC stained sections for each node; a conclusion that is expected to change when supervised AM of multiple sections as used here is applied. Furthermore, another important question is whether the presence of micrometastases or single tumour cells correlates with prognosis and survival. In a recent multi-institutional study of 736 patients, the presence of immunohistochemically detected occult cells in axillary lymph node metastases, in postmenopausal women, was found to be an independent predictor of overall survival.9 In contrast, a large study of the John Wayne Cancer Centre showed a decreased five year disease free survival only for patients with nodal metastasis > 2.0 mm, and concluded that IHC based detection of micrometastases does not adversely affect prognosis.24 However, the median follow up of this study was only 38 months, which is generally thought to be too short to be conclusive in breast cancer. Furthermore, although micrometastases may not have prognostic impact, in a large proportion of patients micrometastases or even ITCs are accompanied by second echelon axillary lymph node metastases, and are therefore clinically relevant because they indicate the need for further axillary dissection to achieve local disease control.6,8–10
Take home messages.
In a cohort of adult medical admissions with suspected bacteraemia, neutrophilia and lymphopenia were both associated with bacteraemia, although lymphopenia was the better predictor
Both neutrophilia and lymphopenia were more predictive of bacteraemia than the total white blood cell count
“Institute I, the academic hospital with vast sentinel lymph node experience, performed somewhat better than institute II, a large regional hospital”
Currently, three trials are ongoing to determine the clinical importance of single tumour cells and small micrometastases, namely: the ACOSOG-Z0011 trial and the NSABP-B32 study in the USA and the 2301 IBCSG trial in Europe. It is hoped that these trials will help to predict those patients who will profit from an axillary lymph node dissection. However, a final answer is not expected soon. Thus, a serious dilemma may occur in the near future: should one refrain from using new technology unless its clinical use is confirmed? Or should new technology, which can be applied cost effectively in a routine setting, be provided to clinicians?
From a tumour biology point of view, it is unlikely that a chosen limit of 2 mm for metastasis remains decisive for treatment and patient managements. Furthermore, with the current view that micrometastases indicate further axillary dissection (or radiotherapy) to achieve local disease control, it is clear that not missing smaller metastases or even single cells on the limited (although more extensive than normal) sample investigated SLN biopsies of patients with breast cancer will remain imperative. AM may well facilitate the sensitive and efficient detection of these metastatic cells in routine pathology.
Acknowledgments
This study was financially supported by the “Maurits and Anna de Kock Foundation” the Netherlands.
Abbreviations
AM, automated microscopy
IHC, immunohistochemistry
ITC, isolated tumour cell
SLN, sentinel lymph node
REFERENCES
- 1.Tjan-Heijnen VC, Bult P, de Widt-Evert LM, et al. Micro-metastases in axillary lymph nodes: an increasing classification and treatment dilemma in breast cancer due to the introduction of the sentinel node procedure. Breast Cancer Res Treat 2001;70:81–8. [DOI] [PubMed] [Google Scholar]
- 2.Fraile M, Rull M, Julian FJ, et al. Sentinel node biopsy as a practical alternative to axillary lymph node dissection in breast cancer patients: an approach to its validity. Ann Oncol 2000;11:701–5. [DOI] [PubMed] [Google Scholar]
- 3.Sandrucci S, Casalegno PS, Percivale P, et al. Sentinel lymph node mapping and biopsy for breast cancer: a review of the literature relative to 4791 procedures. Tumori 1999;85:425–34. [DOI] [PubMed] [Google Scholar]
- 4.Widt-Levert LM de, Tjan-Heijnen VCG, Bult P, et al. Stage migration in breast cancer: surgical decisions concerning isolated tumour cells and micro-metastases in sentinel lymph node. Eur J Surg Oncol 2003;29:216–20. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic factors in breast cancer: College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:966–78. [DOI] [PubMed] [Google Scholar]
- 6.Torrenga H, Rahusen FD, Meijer S, et al. Sentinel node investigation in breast cancer: detailed analysis of the yield from step sectioning and immunohistochemistry. J Clin Pathol 2001;54:550–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cserni G. Complete step sectioning of axillary sentinel lymph nodes in patients with breast cancer. Analysis of two different step sectioning and immunohistochemistry protocols in 246 patients. J Clin Pathol 2002;55:926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahusen FD, Van Diest PJ, Meijer S. Re: Chu, et al. “Do all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection?”. Ann Surg 2000;231:615–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahusen FD, Torrenga H, van Diest PJ, et al. Predictive factors for metastatic involvement of nonsentinel nodes in patients with breast cancer. Arch Surg 2001;136:1059–63. [DOI] [PubMed] [Google Scholar]
- 10.Cote RJ, Peterson HF, Chaiwun B, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. Lancet 1999;345:896–900. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano AE, Haigh PI, Brennan MB, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol 2000;18:2553–9. [DOI] [PubMed] [Google Scholar]
- 12.Kelley WS, Komorowski RA, Dayer AM. Axillary sentinel lymph node examination in breast carcinoma. Arch Pathol Lab Med 1999;123:533–5. [DOI] [PubMed] [Google Scholar]
- 13.Altinyollar H, Kapucuoglu N, Berberoglu U. Lymphatic mapping and sentinel lymphadenectomy in early stage breast carcinoma. J Exp Cancer Res 2000;19:141–4. [PubMed] [Google Scholar]
- 14.Liu LH, Siziopikou KP, Gabram S, et al. Evaluation of axillary sentinel lymph node biopsy by immunohistochemistry and multilevel sectioning in patients with breast carcinoma. Arch Pathol Lab Med 2000;124:1670–3. [DOI] [PubMed] [Google Scholar]
- 15.Dowlatshahi K, Fan M, Anderson JM, et al. Occult metastases in sentinel nodes of 200 patients with operable breast cancer. Ann Surg Oncol 2001;8:675–81. [DOI] [PubMed] [Google Scholar]
- 16.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6th ed. New York: Springer, 2002.
- 17.Singletary SE, Greene FL, Sobin LH. Classification of isolated tumor cells. Clarification of the 6th edition of the American Joint Committee on Cancer staging manual. Cancer 2003;98:2740–1. [DOI] [PubMed] [Google Scholar]
- 18.Weaver DL. Sentinel lymph nodes and breast carcinoma. Which micrometastases are clinically significant? Am J Surg Oncol 2003;27:842–5. [DOI] [PubMed] [Google Scholar]
- 19.Leers MPG, Schoeffelen RHMG, Hoop JGM, et al. Multiparameter flow cytometry as a tool for the detection of micrometastatic tumour cells in the sentinel lymph node procedure of patients with breast cancer. J Clin Pathol 2002;55:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver DL, Krag DN, Ashikaga T, et al. Pathologic analysis of sentinel and nonsentinel lymph nodes in breast cancer; a multicenter study. Cancer 2000;88:1099–107. [PubMed] [Google Scholar]
- 21.Mesker WE, Doekhie FS, Vrolijk H, et al. Automated analysis of multiple sections for the detection of occult cells in lymph nodes. Clin Cancer Res 2003;9:4826–34. [PubMed] [Google Scholar]
- 22.Weaver DL, Krag DN, Manna EA, et al. Comparison of occult micrometastasis detection in breast cancer sentinel lymph nodes using manual cytokeratin immunohistochemistry and image analysis. Mod Pathol 2002;15:55A–6A. [Google Scholar]
- 23.Van Diest PJ. Histopathologic workup of sentinel lymph nodes: how much is enough [editorial]? J Clin Pathol 1999;52:871–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ADASP recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Virchows Arch 2001;439:601–3. [DOI] [PubMed] [Google Scholar]
- 25.Cserni G, Amendoeira I, Apostolikas N, et al. Pathological work-up of sentinel lymph nodes in breast cancer. Review of current data to be considered for the formulation of guidelines. Eur J Cancer 2003;39:1654–67. [DOI] [PubMed] [Google Scholar]


