Abstract
Background: Squamous differentiation/squamous metaplasia is often associated with endometrial adenocarcinoma and benign lesions, such as endometrial hyperplasia and chronic endometritis. Morules have distinct histological characteristics, and are referred to as squamous metaplasia or squamoid metaplasia.
Aim: To focus on the histological characteristics of morules and clarify the difference between morules and squamous differentiation.
Materials/Methods: Twenty endometrioid carcinomas with morules or squamous differentiation, five adenosquamous carcinomas, and eight non-carcinomatous endometrial lesions with morules were investigated. Numerous antibodies for epithelial membrane antigen (EMA), involucrin, cytokeratins, neuropeptides, and oncofetal antigens were used for immunohistochemistry. In situ hybridisation and polymerase chain reaction were used to detect human papillomavirus (HPV).
Results: The morules observed were uniform cell clusters, with no squamous differentiation. They were immunonegative for epithelial antigens including involucrin, EMA, and cytokeratins, but were positive for neurone specific enolase. A few morules were immunopositive for acetylcholine esterase, and one case was positive for somatostatin; neither oncofetal nor proliferative cell markers, including blood group A, B, and AB, or other neuropeptides were demonstrated in the morules. HPV DNA was not found in either the morules in the carcinomas or in the benign lesions. However, true squamous differentiation tissue in four endometrioid carcinomas and two adenosquamous carcinomas was HPV positive using in situ hybridisation.
Conclusion: Morules are histologically distinct from squamous metaplasia/squamous differentiation tissue. Morules are thought to be neuroectodermal-like cell clusters, and are not infected with HPV. In contrast, some of the true squamous differentiation tissue was associated with HPV infection.
Keywords: morules, squamous differentiation, endometrial carcinoma, immunohistochemistry, human papillomavirus
A well known change in endometrial adenocarcinoma is the so called squamous metaplasia/phenotype change from an adenocarcinoma to a squamous cell component.1–5 Metaplastic epithelium is usually well differentiated and is associated with a relatively good prognosis.2 Formerly, the term squamous metaplasia was used in adenocarcinoma and also in benign lesions of the endometrium, such as endometrial hyperplasia and chronic endometritis, but recently squamous differentiation has become common.4–6 It primarily involves the glands, often filling the lumen. The squamous cells are usually well differentiated. In cases where the squamous cell component is benign and the adenomatous element is malignant, the lesion is defined as an adenoacanthoma. When both elements are malignant, it is named adenosquamous carcinoma or adenocarcinoma with squamous differentiation. The term adenocarcinoma with squamous differentiation is used in association with either the benign or malignant squamous elements.5 Furthermore, recently the term squamous metaplasia has been used for benign endometrial glandular lesions,6 whereas squamous differentiation has been used for carcinomas and benign lesions.4,5
The source of the squamous cells is probably the reserve cells, which are thought to lie between the epithelium of the glands and the stroma, and it seems that there is a direct transition from gland epithelium or stroma to squamous cells.3,7 Up until now, morules have been thought of as peculiar squamous cell foci with a prominent oval or spindle shaped cell appearance, and have been categorised as squamous metaplasia or squamous differentiation.1,2,4,5,8,9 The nuclei of the cells are bland, uniform, and lack prominent nucleoli. Mitotic figures are not usually seen, and the cells are thought to be benign. In addition to adenocarcinoma cases, morules are associated with several benign lesions—for example, those involving senile endometrium, chronic endometritis, radiation, submucosal myoma, endometrial hyperplasia, and lesions resulting from the use of an intrauterine contraceptive device.1
“We would like to confirm the notion that morules in the endometrium are not composed of true squamous cells, and are distinct from both squamous metaplasia in benign endometrial lesions and squamous differentiation in endometrial adenocarcinomas”
Furthermore, morules have been reported in fetal carcinoma of the lung,10–14 adenocarcinoma of the thyroid,15 and carcinoma/adenoma of the colon16–18 in rare instances when these lesions showed neuroectodermal characteristics. Expression of oncofetal antigens has also been reported in adenocarcinoma of the thyroid.15 In addition, an association between β catenin gene mutation and morules has been reported.19,20 β Catenin can act as a transcriptional activator of the Wnt signalling pathway. Nakatani et al reported that abnormal regulation of the Wnt signalling pathway might be a common denominator in the development of a tumour with morules.20 Therefore, we have analysed mutation of the β catenin gene in our present study.
In addition, we would like to confirm the notion that morules in the endometrium are not composed of true squamous cells, and are distinct from both squamous metaplasia in benign endometrial lesions and squamous differentiation in endometrial adenocarcinomas. To this end, we describe a detailed immunohistochemical investigation and molecular analysis of endometrial morules. Furthermore, human papillomavirus (HPV) has been detected in squamous cell carcinoma of the uterine cervix. In Okinawa, a subtropical island in southern Japan, we have reported many cases of HPV positive squamous cell carcinoma of the uterine cervix, lung, oesophagus, and head and neck. We have also reported that HPV is one of the causative factors of squamous differentiation/phenotype change from adenocarcinoma to squamous cell carcinoma.21,22 In this context, we investigated HPV infection in morules and squamous differentiation tissue in endometrial samples, by means of the polymerase chain reaction (PCR) and in situ hybridisation. In previous studies, 13% (by detection of the HPV E6 region) and 28% (by detection of the HPV L1 region) of endometrioid carcinomas in mainland Japan were reported to be positive for HPV,23 and 9.1% of endometrial carcinomas in Hong Kong Chinese women were positive for HPV.24 However, it was unclear whether the HPV positive cases in these reports were associated with squamous differentiation or with morules. In the UK, O’Leary25 reported that HPV-6 was found in squamous cell components in 19 of 41 cases of endometrial adenoacathoma. In contrast, Czerwenka et al reported that there was no association between HPV infection and endometrial carcinoma with squamous differentiation.26
MATERIALS AND METHODS
Samples
Tissue samples were obtained from the department of gynaecology and obstetrics, Ryukyu University Hospital, Japan. Samples comprised biopsied materials and tumours surgically removed in 2002. The age of the patients ranged from 42 to 79 years. The samples were as follows: 20 cases of endometrial adenocarcinoma (endometrioid carcinoma) with morules or squamous differentiation; five cases of adenosquamous carcinoma, which was composed of at least 20% each of the squamous cell carcinoma component and the adenocarcinoma component22; and eight non-carcinomatous endometrial biopsy samples with morules (six complex type atypical endometrial hyperplasia, one intrauterine contraceptive device associated endometrium, and one benign submucosal leiomyoma).
Morphological examination
Samples fixed in 10% phosphate buffered formalin were routinely processed in paraffin wax, and sectioned at 4 μm. Haematoxylin and eosin staining, phosphotungstic acid-haematoxylin, silver impregnation, and periodic acid Schiff were performed on these 4 μm sections. Blood group types H (O) and A were identified with the use of peroxidase conjugated UEA-1 (Ulex europaeus 1).
For the immunohistochemical staining, dewaxed sections were pretreated with 3% H2O2 for 20 minutes, washed, and blocked with a non-immune goat serum for 30 minutes. Table 1 lists the antibodies used. To clarify the histological characteristics of the morules, we used a variety of antibodies, categorised as follows: antibodies related to epithelial antigens, including squamous cell markers, cytokeratins, and involucrin; nerve cell and neuropeptide related antigens; oncofetal protein and proliferative cell marker related antigens; a few intermediate filaments other than cytokeratin; others. All antibodies were used according to the manufacturers’ instructions. Slides were incubated with primary antibodies for 20 minutes, washed three times, and incubated with biotinylated second antibody with avidin and biotinylated horseradish peroxidase complex (Dako, Kyoto, Japan). 3, 3′-Diaminobenzidine (Dako) was used as a chromogen.
Table 1.
Antibodies used for immunohistochemistry
| Antibody/antigen | Category | Source | M/P |
| CK AE1/AE3 | A | Dako, Glostrup, Denmark | M |
| CK5/6 | A | Dako, Denmark | M |
| CK7 | A | Dako, Carpinteria, California, USA | M |
| CK10 | A | Dako, Denmark | M |
| CK14 | A | YLEM, Avezzano, Italy | M |
| CK17 | A | Dako, Denmark | M |
| CK19 | A | Dako, Denmark | M |
| CK LP 34 | A | Dako, Denmark | M |
| Involucrin | A | Sigma, St Louis, Missouri, USA | M |
| EMA | A | Dako, USA | M |
| NSE | B | Dako, Denmark | M |
| Acetylcholine esterase | B | Chemicon International, Temecula, California, USA | M |
| S-100A | B | Dako, Denmark | P |
| GFAP | B | Dako, USA | P |
| Chromogranin A | B | Dako, Denmark | M |
| Synaptophysin | B | Dako, Denmark | P |
| Somatostatin | B | Dako, USA | P |
| Enkephalin | B | Wako, Japan | P |
| Serotonin | B | Dako, Denmark | M |
| Calcitonin | B | Dako, Denmark | M |
| VIP | B | Biomeda, Foster City, Califonia, USA | P |
| Insulin | B | Dako, USA | P |
| Glucagon | B | Dako, USA | P |
| CEA | C | Dako, Denmark | P |
| p53 | C | Dako, Denmark | M |
| PCNA | C | Dako, Denmark | M |
| Ki-67 | C | Dako, Denmark | P |
| c-kit | C | Dako, Kyoto, Japan | P |
| CD34 class I | C | Dako, Denmark | M |
| Apo-A | C | Dako, Denmark | M |
| Blood group A | C | Dako, USA | M |
| Blood group B | C | Dako, USA | M |
| Blood group AB | C | Dako, USA | M |
| Desmin | D | Dako, Denmark | M |
| Vimentin | D | Dako, Denmark | M |
| α-SMA | D | Dako, Denmark | M |
| ER | E | Dako, USA | M |
| PR | E | Dako, USA | M |
| β Catenin | E | Santa Cruz Biotech, Santa Cruz, California, USA | P |
Categories: A, epithelial antigens; B, nerve and neuropeptide related antigens; C, oncofetal and proliferative cell related antigens; D, intermediate filaments; E, others.
Apo-A, apolipoprotein A; CEA, carcinoembryonic antigen; EMA, epithelial membrane antigen; ER, oestrogen receptor; GFAP, glial fibrillary acidic protein; NSE, neurone specific antigen; M, monoclonal antibody; P, polyclonal antibody; PCNA, proliferating cell nuclear antigen; PR, progesterone receptor; SMA, smooth muscle antigen; VIP, vasoactive intestinal polypeptide.
For the detection of HPV, in situ hybridisation kits for HPV types 6, 11, 16, 18, 30, 31, 33, 35, 45, 51, and 52 (Dako, Carpinteria, California, USA) was used. In situ hybridisation was carried out according to the manufacturer’s instructions.
Detection of involucrin and transglutaminase I mRNA by RT-PCR
The expression of squamous cell markers—involucrin and transglutaminase I—was investigated by the use of reverse transcription PCR.
Total RNA was extracted from the samples according to the standard acid/guanidium/phenol/chloroform method. The RNA was then digested with DNase (Takara Co, Otsu, Japan). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene was amplified using the primer set reported previously.21 The primers were designed around introns to produce fragments of 2086 bp from DNA and 234 bp from RNA. Takara Ex Taq DNA polymerase (Takara Co) was used for the PCR. The genomic 2086 bp GAPDH DNA was not amplified. The RNA was reverse transcribed at 42°C for 60 minutes in a 20 μl reaction volume using a First Strand cDNA synthesis kit (Clontech Laboratories, Palo Alto, California, USA), according to the manufacturer’s instructions. cDNA was incubated at 95°C for five minutes to inactivate the reverse transcriptase, and was then used as template DNA in the PCR amplification of involucrin and transglutaminase I genes using the primer sets shown in table 2.
Table 2.
Primers used for involucrin and transglutaminase I mRNA
| Primers | Product (bp) | |
| Involucrin | ||
| Sense | 5′-ACTCCTAGAAGCCTTCTACTT-3′ | 408 |
| Antisense | 5′-AGGATTCTGGTAGCTCAACTA-3′ | |
| Transglutaminase I | ||
| Sense | 5′-AAGGAGACCAAGAAGG-3′ | 315 |
| Antisense | 5′-CTGTAACCCAGAGCCTTC-3′ |
Detection of HPV DNA
DNA samples were prepared according to the methods described by Impraim et al.27 The 110 bp β globin gene was detected in all DNA samples using the method and primers (PCO3 and PCO4) used by Saiki et al.28 Table 3 lists the primers and probes used for HPV types 6, 11, 16, 18, 31, 33, 39, 45, 52, and 58. The PCR reaction mixture contained 10mM Tris/HCl, pH 8.3, 50mM KCl, 1.5mM MgCl2, 0.01% gelatine, 0.4mM each of four dNTPs, 0.32 μg of each primer, and 2.4 units of Taq DNA polymerase (Cetus-Takara, Otsu, Japan). Reaction conditions were the same as reported previously,21,22 namely: annealing at 62°C for three minutes, denaturation at 94°C for two minutes, and extension at 74°C for four minutes for 40 cycles. The amplified DNA was transferred on to a nylon membrane (Hybond-N+; Amersham Life Science, Little Chalfont, Buckinghamshire, UK) after routine polyacrylamide gel (10%) electrophoresis, and Southern blot analysis was carried out.
Table 3.
Primers and probes used for the detection of human papillomavirus (HPV) DNA by the polymerase chain reaction
| Primer/Probe | Nucleotide position | Product (bp) | |
| HPV-6 E6 | |||
| Sense | 5′-GCTGGATATGCAACAACAGTTG-3′ | 348–369 | 189 |
| Antisense | 5′-CATGCATGTTGTCCAGCAGTG-3′ | 516–536 | |
| Probe | 5′-GCTACCTGTGTCACAAACCG-3′ | 412–431 | |
| HPV-11 E6 | |||
| Sense | 5′-GCAGCGTGTGCCTGTTGCTTA -3′ | 285–305 | 230 |
| Antisense | 5′-AAGCAACGACCCTTCCACTGG-3′ | 494–514 | |
| Probe | 5′-CCTGTGTCACAAGCCGTTGTG-3′ | 416–436 | |
| HPV-16 E6 | |||
| Sense | 5′-GATGGGAATCCATATGCTGTA-3′ | 269–289 | 240 |
| Antisense | 5′-TCGACCGGTCCACCGACCCCT-3′ | 488–508 | |
| Probe | 5′-GCCACTGTGTCCTGAAGAAAAGC-3′ | 427–449 | |
| HPV-16 E7 | |||
| Sense | 5′-CCAGAGACAACTGATCTCTAC-3′ | 610–630 | 171 |
| Antisense | 5′-GTGTGTGCTTTGTACGCACAAC-3′ | 759–780 | |
| Probe | 5′-TAACCTTTTGTTGCAAGTGTGACTCTACGCTTCG-3′ | 725–758 | |
| HPV-18 E6 | |||
| Sense | 5′-CAGTATACCCCATGCTGCATGCC-3′ | 278–300 | 159 |
| Antisense | 5′-GGTTTCTGGCACCGCAGGCA-3′ | 417–436 | |
| Probe | 5′-CAGACTCTGTGTATGGAGACAC-3′ | 349–370 | |
| HPV-18 E7 | |||
| Sense | 5′-GAGCCGAACCACAACGTCAC-3′ | 747–766 | 152 |
| Antisense | 5′-GGATGCACACCACGGACACA-3′ | 879–898 | |
| Probe | 5′-TCCAGCAGCTGTTTCTGAACACCCTG-3′ | 846–871 | |
| HPV-31 E7 | |||
| Sense | 5′-GGGCTCATTTGGAATCGTGTG-3′ | 811–831 | 100 |
| Antisense | 5′-AACCATTGCATCCCGTCCCC-3′ | 910–891 | |
| Probe | 5′-TACCTGCTGGATCAGCCATTGTAGTTACAG-3′ | ||
| HPV-33 E7 | |||
| Sense | 5′-TGAGGATGAAGGCTTGGACC-3′ | 671–690 | 110 |
| Antisense | 5′-TGACACATAAACGAACTGTG-3′ | 780–761 | |
| Probe | 5′-TGTGACAACAGGTTACAATGTAGTAATCAG-3′ | ||
| HPV-39 E7 | |||
| Sense | 5′-CCAAAGCCCACCTTGCAGGA-3′ | 601–620 | 123 |
| Antisense | 5′-ATGGTCGGGTTCATCTATTTC-3′ | 723–703 | |
| Probe | 5′-TCCTAATTGCTCGTGACATACAAGGTCAAC-3′ | ||
| HPV-45 E7 | |||
| Sense | 5′-CCCGACGAGCCGAACCACAG -3′ | 741–760 | 101 |
| Antisense | 5′-TCTAAGGTCCTCTGCCGAGC-3′ | 841–822 | |
| Probe | 5′-AGCTCAATTCTGCCGTCACACTTACAACAT-3′ | ||
| HPV-52 E7 | |||
| Sense | 5′-GCAGAACAAGCCACAAGCAA-3′ | 691–710 | 105 |
| Antisense | 5′-TAGAGTACGAAGGTCCGTCG-3′ | 795–776 | |
| Probe | 5′-ATAGCCGTAGTGTGCTATCACAACTGTGAC-3′ | ||
| HPV-58 E7 | |||
| Sense | 5′- CGAGGATGAAATAGGCTTGG-3′ | 672–691 | 109 |
| Antisense | 5′-ACACAAACGAACCGTGGTGC-3′ | 780–761 | |
| Probe | 5′-TGTTGTTCAATGTTACATCATTAATCGACA-3′ | ||
Detection of β catenin gene mutation
The β catenin gene was amplified using the same DNA samples mentioned above. The PCR primer set (sense 5′-ATGGAACCAGACAGAAAAGCG-3′, and antisense 5′-CAGGATTGCCTTTACCACTCA-3′) was used according to the method of Paracios and colleagues31 and Kajino et al.32 The PCR products were analysed by use of the L1-COR DNA sequencer, model 4200 (L1-COR Inc, Lincoln, Nebraska, USA).
RESULTS
Morphological findings
Morules were noted in association with endometrioid carcinomas and also non-cancerous endometriums. They filled the lumen of the glandular structures of the cancer or of the non-cancerous endometrial glands, and seemed to be formed from the glandular epithelium (fig 1A,B), although occasionally they were found outside the glandular lumen also. They seemed to be directly formed from stromal tissue. In the non-cancerous endometrium, they were frequently seen in cases of complex type, atypical endometrial hyperplasia. The morules were rounded, solid epithelial cell collections consisting of uniform squamoid, usually spindle shaped cells, with a faint to moderately stained eosinophilic cytoplasm and round or oval clear nuclei. The chromatin formed a thin peripheral rim. The morphology of the morules was found to be the same whether they were associated with carcinoma or non-cancerous lesions. In carcinomas, the cancer cells neighbouring the morules were columnar, often well differentiated, and had clear cytoplasm forming the glandular structure. In contrast, in adenocarcinomas with squamous differentiation, the squamous elements were usually more atypical, and often resembled the morules. The squamous cells frequently showed prickle cell differentiation and keratinisation. Squamous differentiation occurred in close association with the glandular epithelium and was not found outside the glandular structures of the adenocarcinoma (fig 1C). The adenocarcinoma cells adjacent to the squamous cell carcinoma element were enlarged with a large amount of cytoplasm. However, there were four cases of carcinoma that had morules and foci of true squamous differentiation (fig 1D).
Figure 1.
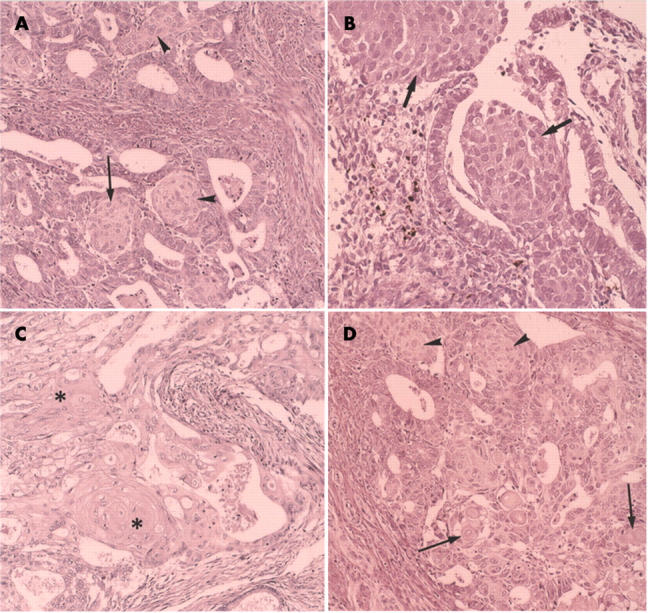
(A) Morules in endometrioid carcinoma. The cells of the morules have a large amount of cytoplasm, which is faintly eosinophilic. Arrows indicate the morules that seem to be linked to glandular epithelial cells. Arrowheads indicate morules outside the glandular structures. The nuclei are round or oval with the chromatin forming a thin peripheral rim. Haematoxylin and eosin (H&E) staining; original magnification, ×100. (B) Morules in a benign endometrial lesion (atypical endometrial hyperplasia). Arrows indicate the morules, one of which is found outside the glands. The morphology of the morules is the same as that seen in the carcinoma cases. H&E staining; original magnification, ×150. (C) Squamous differentiation in an endometrioid carcinoma. The asterisks indicate squamous differentiation. Note the prickle cell differentiation and keratinisation. H&E staining; original magnification, ×100. (D) Squamous differentiation and morules simultaneously found in the endometrioid carcinoma. Arrows indicate squamous differentiation. Arrowheads indicate morules. H&E staining; original magnification, ×100.
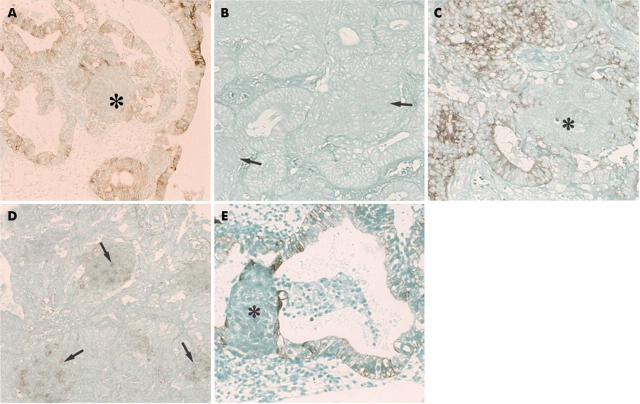
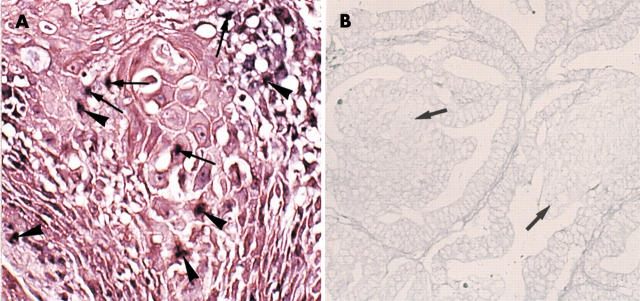
The morules were immunonegative with all the anticytokeratin antibodies (fig 2A,B). They were also negative for involucrin and epithelial membrane antigen (EMA) (fig 2C), but showed weak to strong, diffuse staining for neurone specific enolase (NSE) (fig 2D). Although a few EMA or cytokeratin (AE1/AE3 antibody) positive cells were found in morules in rare instances, serial sections revealed that these cells were intermingled glandular epithelial cells (fig 2E), and that the morules were not stained. Furthermore, staining for high molecular weight cytokeratin (Moll’s number 10) was negative in such cases. Three morule samples showed a sporadic, positive reaction with the S-100 and anti-acetylcholine esterase antibodies, and in one case diffuse staining for somatostatin was demonstrated, although staining for the other neuropeptides, the oestrogen receptor, and the progesterone receptor was negative. In contrast, in the cases of squamous differentiation, even when they resembled the morules, all of the cells with squamous differentiation showed a diffuse positive reaction for the cytokeratins (fig 3A), involucrin (fig 3B), and EMA. In adenocarcinomas with squamous differentiation, the adenocarcinoma cells neighbouring the squamous element were often enlarged, with a large amount of cytoplasm, and were immunopositive for involucrin and cytokeratins.
Figure 2.
(A) Immunohistochemistry for cytokeratins (AE1/AE3 antibodies) in morules. The asterisk indicates the morules. No immunoreactivity was seen in the morules, although the glandular epithelial cells (atypical endometrial hyperplasia) were positive. Original magnification, ×100. (B) Immunohistochemistry for cytokeratin (antibody to cytokeratin 10) in morules. The morules and the adenocarcinoma components (atypical endometrial hyperplasia) were immunonegative. The arrows indicate the morules. Original magnification, ×150. (C) Immunohistochemistry for epithelial membrane antigen (EMA) in morules. The morules were negative for EMA (asterisk), but the glandular epithelial cells (endometrioid carcinoma) were positive. Original magnification, ×150. (D) Immunohistochemical demonstration of neurone specific enolase (NSE) in morules. Staining for NSE was strong in the morules (arrowheads) (endometrioid carcinoma). Original magnification, ×150. (E) Immunohistochemical demonstration of cytokeratin in morules. Cytokeratin (AE1/AE3 antibodies) was found in a few cells (asterisk) in morules, but serial sections revealed that they were glandular epithelial cells. Glandular epithelial cells were positively stained (atypical endometrial hyperplasia case). Original magnification, ×150.
Figure 3.
(A) Immunohistochemical demonstration of cytokeratin (AE1/AE3 antibodies) in cells with squamous differentiation. The asterisk indicates the keratin positive cells with squamous differentiation. All cells with squamous differentiation stained positively. The adenocarcinoma cells neighbouring the squamous differentiation tissue (arrowheads) were also immunopositive (endometrioid carcinoma). Original magnification, ×150. (B) Immunohistochemical demonstration of involucrin in tissue with squamous differentiation. The asterisk indicates involucrin positive cells (squamous differentiation tissue). Original magnification, ×150. Arrowheads indicate morules that are negative for involucrin (same case as that shown in fig 1D). (C) Immunohistochemical demonstration of proliferating cell nuclear antigen (PCNA) in morules. The arrow indicates morules. PCNA was detected in the nuclei of the cancer cell, but not in the morules (endometrioid carcinoma). Original magnification, ×100.
Five cases of endometrioid carcinoma with squamous differentiation and two cases of adenosquamous carcinoma were positive for p53 protein, but four cases of endometrioid carcinoma with squamous differentiation and three adenosquamous carcinomas were negative (data not shown). Immunoreactivity was seen in both the adenocarcinoma and squamous cell carcinoma components. In contrast, seven cases of morules associated with endometrioid carcinoma were negative for p53 protein. Two cases with adenocarcinoma elements in endometrioid carcinoma with morules were positive for p53, although the morules themselves were negative. PCNA and Ki-67 were negative in all morules (fig 3C), although the adenocarcinoma cell elements were strongly positive. PCNA and Ki-67 were also positive in all cases of adenocarcinoma with squamous differentiation and adenosquamous carcinomas, although the cells in the centre of the squamous cell nests were only weakly stained. Sporadic immunoreactivity for β catenin was found in the nuclei and cell membrane of the morules in three cases (two endometrioid carcinomas with morules and one case of benign lesions). In the glandular components of carcinomas, sporadic nuclear and cell membrane immunopositivity for β catenin was seen around the morules or squamous differentiation areas (data not shown). A few cells with clear nuclei in the morules associated with endometrioid carcinoma and benign lesions were strongly positive for biotin immunohistochemically (data not shown). Small numbers of cancer cells were also positive for biotin. All morules were negative for the blood group antigens, whereas four cases of endometrioid carcinoma were positive for blood group B antibody and three cases were positive for AB antibody; in these cases, intermingled glandular epithelial cells in the morules and the lumens of vessels were also positive for these antibodies, although the morule cells themselves were negative. In contrast, a few morules in benign lesions were stained by UEA-1 (data not shown).
Table 4 summarises the results of the immunohistochemical staining. Histologically, seven of 20 cases of endometrial adenocarcinoma had morules only, four cases had both morules and foci of true squamous differentiation, and nine cases had areas of squamous differentiation but no morules. In the cases with both morules and squamous differentiation, the histology of these elements was liable to be confused, although the morules were immunonegative for EMA, involucrin, and all cytokeratin molecules. None of the adenosquamous carcinomas had morules, but all had squamous cell carcinoma and adenocarcinoma components. Eight non-cancerous samples had morules, but had no squamous differentiation tissue/squamous metaplasia (table 5).
Table 4.
Immunohistochemistry results
| Antibody | Category | Morules | SD |
| CK AE1/AE3 | A | – | + |
| CK5/6 | A | – | −/+ |
| CK7 | A | – | −/+ |
| CK10 | A | – | −/+ |
| CK14 | A | – | −/+ |
| CK17 | A | – | – |
| CK19 | A | – | −/+ |
| CK LP 34 | A | – | – |
| Involucrin | A | – | + |
| EMA | A | – | + |
| NSE | B | + | – |
| Acetylcholine esterase* | B | −/+ | −/+ |
| S-100* | B | −/+ | – |
| GFAP | B | – | – |
| Chromogranin A | B | – | – |
| Synaptophysin† | B | – | – |
| Somatostatin | B | −/+ | – |
| Enkephalin | B | – | – |
| Serotonin | B | – | – |
| Calcitonin | B | – | – |
| VIP | B | – | – |
| Insulin | B | – | – |
| Glucagon | B | – | – |
| CEA | C | – | −/+ |
| p53 | C | – | −/+ |
| PCNA | C | – | + |
| Ki-67 | C | – | −/+ |
| c-kit | C | – | – |
| CD34 | C | – | – |
| Apo-A | C | – | – |
| Blood group A | C | – | – |
| Blood group B | C | – | −/+ |
| Blood group AB | C | – | −/+ |
| Desmin | D | – | – |
| Vimentin | D | – | – |
| α Smooth muscle actin | D | – | – |
| Oestrogen receptor | E | – | – |
| Progesterone receptor | E | – | + |
| β Catenin | E | −/+ | −/+ |
Categories: A, epithelial antigens; B, nerve and neuropeptide related antigens; C, oncofetal and proliferative cell related antigens; D, intermediate filaments; E, others.
–, Immunohistochemical staining negative in all cases; −/+, both negative and positive cases were seen; +, all cases positively stained; *, a sporadic positive reaction was found in 3 cases; †, a sporadic positive reaction was seen in one case.
Apo-A, apolipoprotein A; CEA, carcinoembryonic antigen; CK, cytokeratin; EMA, epithelial membrane antigen; GFAP, glial fibrillary acidic protein; NSE, neurone specific antigen; PCNA, proliferating cell nuclear antigen; SD, squamous differentiation; VIP, vasoactive intestinal polypeptide
Table 5.
Cases with morules and squamous differentiation
| Number of cases | ||||
| Morules | Morules+SD | SD | Total | |
| Endometrioid carcinoma | 7 | 4 | 9 | 20 |
| Adenosquamous | ||||
| Carcinoma | 0 | 0 | 5 | 5 |
| Benign endometrial lesions | 8 | 0 | 0 | 8 |
| Atypical hyperplasia | 6 | |||
| Others (IUD and submucosal leiomyoma) | 2 | |||
| Total | 15 | 4 | 14 | 33 |
SD, squamous differentiation; IUD, intrauterine contraceptive device.
Detection of involucrin and transglutaminase I mRNA
Involucrin and transglutaminase I mRNA were not detected in the cases with morules (data not shown), but were found in all the cases with squamous differentiation tissue. These results are compatible with the immunohistochemical observations.
Detection of HPV DNA
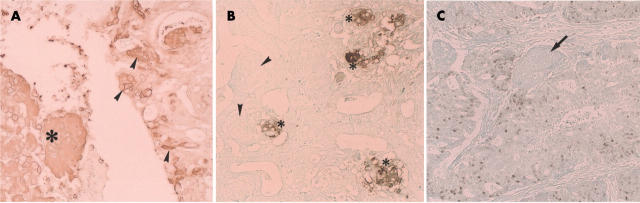
Using in situ hybridisation, integrated and episomal forms (Cooper’s type 333) of HPV DNA were demonstrated in four cases with foci of squamous differentiation in endometrioid carcinoma (fig 4A). One of these cases was one of the four that exhibited both morules and squamous differentiation tissue, and the other three were from the nine cases showing only squamous differentiation. HPV was also demonstrated in the squamous cell carcinoma component in two of five adenosquamous carcinoma cases. A positive reaction was found in the nuclei and showed type 3 signals (integrated and episomal forms) according to Cooper’s criteria.33 In contrast, none of the morules show a positive reaction for HPV (fig 4B). No morules, whether associated with carcinoma or with non-cancerous lesions, were infected with HPV.
Figure 4.
(A) Demonstration of human papillomavirus (HPV) by the use of in situ hybridisation. HPV DNA (arrows and arrowheads) is demonstrated in the nuclei of cells with squamous differentiation in an endometrioid carcinoma. According to Cooper’s criteria, HPV was present in the integrated (arrowheads) and episomal (arrows) forms (type 3, Cooper’s criteria). Original magnification, ×150. (B) Demonstration of HPV DNA by use of in situ hybridisation. Neither the neoplastic glandular cells nor the morules harbour HPV DNA (endometrioid carcinoma case). The arrows indicate morules. Original magnification, ×150.
Two of the six cases of adenosquamous carcinoma that were HPV positive by in situ hybridisation were also positive using PCR. They were positive for HPV-18 and HPV-16, respectively. However, in the other four cases the foci of squamous differentiation were very small, and HPV failed to be detected.
Detection of β catenin gene mutation
Two of the endometrioid carcinoma cases with morules and one case of adenosquamous carcinoma had a mutation of the β catenin gene. A heterozygous substitution mutation was found at the glycogen synthase kinase 3β phosphorylation site at Ser33Cys(C→G). These three cases showed a positive immunoreaction for β catenin in the nuclei and cytoplasm (data not shown).
DISCUSSION
No epithelial cell markers were detected immunohistochemically in the morules, although they were clearly demonstrated in the squamous differentiation tissue, even when it resembled morule tissue. In addition, cytokeratin 14, other high molecular weight cytokeratins, and involucrin, which are well known squamous cell markers,10,22,34 were all negative in the morules. Some foci of squamous differentiation resembled morules and could easily be confused with them, although detailed examination revealed certain morphological differences between morules and squamous differentiation tissue. The nuclei of tissue with squamous differentiation were more atypical and slightly larger than those found in the morules, and prickle cell differentiation was also found in some places. Diffuse staining (varying in strength) for NSE was found in the morules, but not in squamous differentiation tissue. Most of the neuropeptides tested negative in the morules, although somatostatin was positive, and in three cases sporadic staining for S-100 protein and acetylcholine esterase was seen. These findings suggest that morules are neuroectodermal-like cell clusters, as has been reported for the morules found in fetal adenocarcinoma of the lung.10–14 However, in pulmonary fetal adenocarcinoma, staining for several neuropeptides—such as enkephalin, serotonin, and somatostatin, and argyrophil staining was reported to be positive in the morules,7,11,12,14,33 which resembled fetal lung tissue.10,11,14 Other neuropeptides that were not investigated in our present study should be examined to clarify the characteristics of endometrial morules. The oestrogen receptor, progesterone receptor, and bcl-2 were not detected in the morules, although the progesterone receptor was found in the nuclei of adenocarcinoma components and benign glandular cells, and bcl-2 was positive in the foci of true squamous differentiation in the endometrioid carcinomas, as reported previously.35 Overexpression of the tumour suppressor gene p53 and proliferative cell antigens—PCNA and Ki-67—was not demonstrated in the morules. The oncofetal expression13,16,18,36 of blood group antigens has been reported in the morules of thyroid carcinoma,15 but the endometrial morules studied here were negative. Thus, endometrial morules are not thought to be neoplastic cells. Furthermore, in a separate study we investigated 13 endometriums from fetal, neonatal, and childhood necropsies, including 10 cases of infantile sudden death syndrome, but no morules were seen (data not shown). In our present study, morules were often found in benign endometrial lesions, such as endometrial hyperplasia and chronic endometritis. In carcinomas, squamous differentiation was frequently demonstrated, and morules were often seen.
It has been reported that biotin is strongly expressed in the nuclei of morule cells in lung cancer14 and colonic adenoma,17 and also in the endometrial gland during gestation.37 However, in the morules studied here, only a few cells were positive for biotin. β Catenin gene mutation has been reported in morules in the lung,19 hair follicle tumours,32 and in endometrioid carcinoma.29,38 In endometrioid carcinomas, mutations of the β catenin gene are the most common genetic abnormalities, and have been reported frequently in exon 3 (codons 32, 33, 37, and 41).29,39,40 Furthermore, β catenin is a submembranous molecule of the adherens junction, and β catenin mutation is always associated with morule formation.19,20 However, in our present study, such mutation at Asp32Glu was demonstrated in only a few cases. The mutated β catenin gene in the endometrial lesions was not clearly associated with the morules. Interestingly, HPV DNA was not detected in the morules, although it was present in a considerable number of foci of true squamous differentiation. In general, HPV infection is associated with squamous cell carcinoma and squamous differentiation, as reported previously.21,22
Take home messages.
Morules are histologically distinct from squamous metaplasia/squamous differentiation tissue
Morules are probably benign neuroectodermal-like cell clusters, and are not infected with human papillomavirus (HPV)
In contrast, some of the tissue with true squamous differentiation was associated with HPV infection
“Foci of squamous differentiation in some endometrioid carcinomas were positive for human papillomavirus, although the morules were not”
Up to now, morules have been thought of as squamous differentiation tissue found in endometrial carcinoma and benign endometrial lesions.1–8 Morphologically, the morules consisted of uniform cells, and often filled the lumens of the glands. They were also found outside the glands. Morules have been reported to be sporadically immunopositive for involucrin, cytokeratins, and EMA, but in our study the cells found positive for such antigens in the morules were in fact intermingled glandular epithelial cells, and the morules themselves were negative. In contrast, the neuroendocrine characteristics of morules have been reported11,12,14 in fetal adenocarcinoma of the lung.
Separately, Czerwenka et al reported that there was no clear association between HPV infection and endometrioid carcinoma with squamous cells,26 although in some cases HPV had been postulated to be a possible cause of squamous differentiation of the endometrium. However, in our present study, foci of squamous differentiation in some endometrioid carcinomas were positive for HPV, although the morules were not. It is thought that HPV is associated with squamous differentiation.21 Endometrial morules are now thought to be similar to those found in the lung,10–14 thyroid,15 and carcinoma of the colon,16–18 which show neuroectodermal immunohistochemical characteristics. However, there are slight differences between endometrial morules and these other morules: the oncofetal expression of blood group antigens seen in the morules of the thyroid, lung, and colon13,15,16,31,36 was not demonstrated in endometrial morules, and the expression of neuropeptides is rare. Further studies of endometrial morules are needed; in particular, studies of the functional characteristics of the morules and the relation between morules and the prognosis of cancer.
Abbreviations
EMA, epithelial membrane antigen
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
HPV, human papillomavirus
NSE, neurone specific enolase
PCR, polymerase chain reaction
UEA-1, Ulex europaeus 1
REFERENCES
- 1.Anderson MC, Robby SJ, Russell P, et al. Endometritis, metaplasia, polyp and miscellaneous changes. In: Robby SJ, Anderson MC, Russell P, eds. Pathology of the female reproductive tract. London: Churchill Livingstone, 2002:285–303.
- 2.Buckley CH. Normal endometrium and non-proliferative conditions of the endometrium. In: Fox H, Wells M, eds. Haines and Taylor obstetrical and gynaecological pathology. 5th ed. London: Churchill Livingstone, 2003:391–441.
- 3.Novak ER, Woodruff JD. Squamous metaplasia or epidermization. In: Novak ER, Woodruff JD, eds. Novak’s gynecologic and obstetric pathology with clinical and endocrine relations. 8th ed. Philadelphia: WB Saunders, 1979:199–200.
- 4.Prat J. Female reproductive system. In: Damjanov I, Linder J, eds. Anderson’s pathology. 10th ed. St Louis: Mosby, 1996:2231–309.
- 5.Silverberg SG, Kurman RJ. Endometrial carcinoma. In: Silverberg SG, Kurman RJ, eds. Atlas of tumor pathology, 3rd Series, Fascicles 3. Tumors of the uterine corpus and gestational trophoblastic disease. Washington, DC: Armed Forces Institute of Pathology, 1991:47–89.
- 6.Parsons MA. Disorders of growth, differentiation and morphogenesis. In: Underwood JCE, ed. General and systemic pathology. 3rd ed. Edinburgh: Churchill Livingstone, 2000:73–99.
- 7.Dutra FR. Intraglandular morules of the endometrium. Am J Clin Pathol 1959;31:60–5. [DOI] [PubMed] [Google Scholar]
- 8.Hendrickson MR, Kempson RL. Endometrial epithelial metaplasias: proliferations frequently misdiagnosed as adenocarcinoma. Report of 89 cases and proposed classification. Am J Surg Pathol 1980;4:525–42. [PubMed] [Google Scholar]
- 9.Warhol MJ, Rice RH, Pinkus GS, et al. Evaluation of squamous epithelium in adenoacanthoma and adenosquamous carcinoma of the endometrium: immunoperioxidase analysis of involucrin and keratin localization. Int J Gynecol Pathol 1984;3:82–91. [DOI] [PubMed] [Google Scholar]
- 10.Kodama T, Koide T, Shimozato Y, et al. Six cases of well-differentiated adenocarcinoma simulating foetal lung tubules in pseudoglandular stage, comparison with pulmonary blastoma. Am J Surg Pathol 1984;8:735–44. [DOI] [PubMed] [Google Scholar]
- 11.Manning JT, Ordonez NG, Rosenberg HS, et al. Pulmonary endodermal tumor resembling foetal lung. Report of a case with immunohistochemical studies. Arch Pathol Lab Med 1985;109:48–50. [PubMed] [Google Scholar]
- 12.Mardini G, Pai U, Chavez AM, et al. Endobronchial adenocarcinoma with endometrioid features and prominent neuroendocrine differentiation. Cancer 1994;73:1383–9. [DOI] [PubMed] [Google Scholar]
- 13.Miyake M, Zenita K, Tanaka O, et al. Stage-specific expression of SSEA-1-related antigens in the developing lung of human embryos and its relation to the distribution of these antigens in lung cancers. Cancer Res 1988;48:7150–8. [PubMed] [Google Scholar]
- 14.Nakatani Y, Dickersin GR, Mark EJ. Pulmonary endodermal tumor resembling foetal lung: a clinicopathologic study of five cases with immunohistochemical and ultrastructural characterization. Hum Pathol 1990;21:1097–107. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto Y, Yokoyama S, Sasaki A, et al. Oncofoetal expression of blood group-related antigen on morules in thyroid carcinoma. Pathol Int 1996;46:867–73. [DOI] [PubMed] [Google Scholar]
- 16.Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature 1985;314:53–7. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki A, Yokoyama S, Arita T, et al. Morules with biotin-containing optically clear nuclei in colonic tubular adenoma. Am J Surg Pathol 1999;23:336–41. [DOI] [PubMed] [Google Scholar]
- 18.Yuan M, Itzkowitz SH, Palekar A, et al. Distribution of blood group antigens A, B, H, Lewisa, and Lewisb in human normal, foetal, and malignant colonic tissue. Cancer Res 1985;45:4499–511. [PubMed] [Google Scholar]
- 19.Sekine S, Shibata T, Matsuno Y, et al. β-Catenin mutations in pulmonary blastomas: association with morule formation. J Pathol 2003;200:214–21. [DOI] [PubMed] [Google Scholar]
- 20.Nakatani Y, Masudo K, Miyagi Y, et al. Aberrant nuclear localization and gene mutation of β-catenin in low-grade adenocarcinoma of fetal lung type: up-regulation of the Wnt signalling pathway may be a common denominator for the development of tumors that form morules. Mod Pathol 2002;15:617–24. [DOI] [PubMed] [Google Scholar]
- 21.Kinjo T, Kamiyama K, Chinen K, et al. Squamous metaplasia induced by transfection of human papillomavirus DNA into cultured adenocarcinoma cells. Mol Pathol 2003;56:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuhako K, Nakazato I, Hirayasu T, et al. Human papillomavirus DNA in adenosquamous carcinoma of the lung. J Clin Pathol 1998;51:741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita M, Shroyer KR, Markham NE, et al. Association of human papillomavirus with malignant and premalignant lesions of the uterine endometrium. Hum Pathol 1995;26:650–8. [DOI] [PubMed] [Google Scholar]
- 24.Ip SM, Wong LC, Xu CM, et al. Detection of human papillomavirus DNA in malignant lesions from Chinese women with carcinomas of the upper genital tract. Gynecol Oncol 2002;87:104–11. [DOI] [PubMed] [Google Scholar]
- 25.O’Leary JJ, Landers RJ, Crowley M, et al. Human papillomavirus and mixed epithelial tumors of the endometrium. Hum Pathol 1998;29:383–9. [DOI] [PubMed] [Google Scholar]
- 26.Czerwenka K, Lu Y, Heuss F, et al. Human papillomavirus detection of endometrioid carcinoma with squamous differentiation of the uterine corpus. Gynecol Oncol 1996;61:210–14. [DOI] [PubMed] [Google Scholar]
- 27.Impraim CC, Saiki RK, Erlich HA, et al. Analysis of DNA extracted from formalin-fixed paraffin-embedded tissue sequenced-specific oligonucleotides. Biochem Biophys Res Commun 1987;142:710–16. [DOI] [PubMed] [Google Scholar]
- 28.Saiki RK, Scharf S, Faloona F. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985;230:1350–4. [DOI] [PubMed] [Google Scholar]
- 29.McNicol P, Paraskevas M, Gujion F. Variability of polymerase chain reaction-based detection of human papillomavirus DNA is associated with the composition of vaginal microbial flora. J Med Virol 1994;43:194–200. [DOI] [PubMed] [Google Scholar]
- 30.Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;198:12–19. [DOI] [PubMed] [Google Scholar]
- 31.Paracios J, Gamallo C. Mutations in the β-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res 1998;58:1344–7. [PubMed] [Google Scholar]
- 32.Kajino Y, Yamaguchi A, Hashimoto N, et al. β-Catenin gene mutation in human hair follicle-related tumors. Pathol Int 2001;51:543–8. [DOI] [PubMed] [Google Scholar]
- 33.Cooper K, Herrington CS, Stickland JE, et al. Episomal and integrated human papillomavirus in cervical neoplasia shown by non-isotopic in situ hybridisation. J Clin Pathol 1991;44:990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harnden P, Southgate J. Cytokeratin 14 as a marker of squamous differentiation in transitional cell carcinomas. J Clin Pathol 1997;50:1032–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saegusa M, Okayasu I. Down-regulation of bcl-2 expression is closely related to squamous differentiation and progesterone therapy in endometrial carcinomas. J Pathol 1997;182:429–36.9306964 [Google Scholar]
- 36.Hakomori S, Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst 1983;71:231–51. [PubMed] [Google Scholar]
- 37.Yokoyama S, Kashima K, Inoue D, et al. Biotin-containing intranuclear inclusions in endometrial glands during gestation and puerperium. Am J Clin Pathol 1993;99:13–17. [DOI] [PubMed] [Google Scholar]
- 38.Saegusa M, Okayasu I. Frequent nuclear β-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol 2001;194:59–67. [DOI] [PubMed] [Google Scholar]
- 39.Lee KR, Tavassoli FA, Prat J, et al. Surface epithelial-stromal tumours. In: Tavassoli FA, Devilee P, eds. WHO classification of tumours. Pathology and genetics. Tumours of the breast and female genital organs. Lyon: IARCP Press, 2003:117–45.
- 40.Moreno-Bueno G, Gamallo C, perez-Gallego L, et al. Beta-catenin expression pattern, beta-catenin gene mutations, and microsatellite instability in endometrioid ovarian carcinomas and synchronous endometrial carcinomas. Diagn Mol Pathol 2001;10:116–22. [DOI] [PubMed] [Google Scholar]