Abstract
Non-operative diagnosis has become the norm in breast disease assessment and, until relatively recently, fine needle aspiration cytology has been the sampling method of choice. The introduction of automated core biopsy guns in the mid 1990s led to the additional introduction of core biopsy in assessment units. This paper presents a summary of the guidance on handling and routine reporting of breast needle core biopsy specimens in the context of breast disease multidisciplinary assessment. This guidance has been produced by the UK National Coordinating Committee for Breast Screening Pathology and is endorsed by the European Commission working group on breast screening pathology.
Keywords: breast cancer, guidelines, needle biopsy, pathology reporting
Since 1993, the UK National Coordinating Committee for Breast Cancer Screening Pathology (NCCBSP) has been responsible for pathology quality assurance in the UK National Health Service Breast Screening Programme (NHSBSP). The committee has published guidelines for fine needle aspiration cytology (FNAC) procedures and reporting in breast cancer screening,1 which have been adopted with minor modifications by the European Union, and form the basis of the European guidelines.2 Non-operative diagnosis has become the norm in breast screening assessment and, until relatively recently, FNAC has been the sampling method of choice. The introduction of automated core biopsy guns in the mid 1990s led to the additional introduction of core biopsy in assessment units. Updated guidelines published in 2001 by the NCCBSP3 were endorsed by the European Commission working group on breast screening pathology, and include recommendations for specimen handling and reporting of needle core biopsy (WBN), in addition to FNAC samples, in breast screening assessment. We present here a summary of those guidelines.
“The highest levels of diagnostic accuracy in the non-operative diagnosis of breast disease are achieved by means of a triple approach, combining the results of imaging and clinical examination with fine needle aspiration cytology and/or needle core biopsy”
The role of non-operative diagnosis in the assessment of breast lesions is to provide, whenever possible, a definitive diagnosis, allowing rapid referral for treatment in malignant cases, ideally in one operative procedure. Definitive non-operative diagnosis of benign conditions is also useful, leading to prompt reassurance and discharge of the patient from the clinic and return to routine recall. The highest levels of diagnostic accuracy in the non-operative diagnosis of breast disease are achieved by means of a triple approach,4 combining the results of imaging and clinical examination with FNAC and/or WBN. When all three modalities agree, the degree of diagnostic accuracy exceeds 99%.5 Similar levels of accuracy have been obtained for impalpable lesions where clinical examination was non-contributory.6 Review of published series of image guided breast WBN shows that higher sensitivity and specificity are obtained using core biopsies rather than FNAC,7 but inevitably inadequate and false negative results from FNAC and WBN sampling are more frequent for difficult impalpable lesions, and these must not be interpreted in isolation. If imaging findings are thought to be strongly suspicious of malignancy and core biopsy is normal or benign, then management should be based on the imaging findings. Review at a multidisciplinary meeting is essential. Recently, detailed clinical guidance for the management of the assessment process has been published by the NHSBSP.8
SPECIMEN HANDLING
The proper interpretation of core biopsies requires detailed knowledge of both the clinical and mammographic findings, and this information should be provided on the request form. The completed request form should include clinical details, specifying the radiographic sign and the site of biopsies.
Biopsies performed for microcalcifications should be x rayed to determine the presence of calcium. The specimen radiograph should identify calcifications representative of the mammogram examination. Whenever possible, a radiological comment regarding the presence of representative microcalcification of the mammographic lesion in the sample should be provided along with the specimen x ray. The particular cores in which microcalcification is detected may be marked with a vital dye or sent to the laboratory in a separate pot, allowing targeted examination to deeper levels if the calcification is not apparent in the initial levels. Any calcifications identified histologically should be of a size detectable within the resolution of mammography, usually considered to be 100 μm or greater, and if access to the specimen radiograph is available a correlation between the position of the radiographic calcification and the histological calcification can be made.
Optimal fixation is paramount. Biopsies should be placed in fixative solution immediately and sent promptly to the laboratory. Ideally, biopsies should be fixed routinely for a minimum of six hours, although specimens may be fixed rapidly with the aid of microwave techniques.
After processing, haematoxylin and eosin stained sections from one level are usually sufficient for core biopsies from mass lesions, but core biopsies taken for the investigation of microcalcification should have a minimum of three levels examined. In practice, most laboratories choose to examine all core biopsies from screen detected lesions at a minimum of three levels initially. In problematic cases further levels and immunohistochemical studies may be helpful. Examination of further levels should be performed if the calcification is not immediately apparent on histological examination. Step sectioning should take account of the need to identify relevant calcifications of sufficient size (100 μm or greater). It should also be remembered that calcium oxalate crystals are often indistinct on routine haematoxylin and eosin stained sections, but are readily visible with polarised light.
REPORTING CATEGORIES
The histological examination of core biopsy samples is performed to fulfil the assessment process role, by giving a pathology category classification (B1–5), and is not designed to give a definitive diagnosis, although this is possible in most cases. Thus, although most core biopsy samples can be readily categorised as normal, benign, or malignant, it must be recognised that a small proportion (probably less than 10%) of samples cannot. The reporting guidelines have been devised in recognition of this, and should be used for all screen detected lesions (microcalcification, architectural deformities, and mass lesions). This approach is also recommended for symptomatic practice.
“Although most core biopsy samples can be readily categorised as normal, benign, or malignant, it must be recognised that a small proportion (probably less than 10%) of samples cannot”
The reporting categories take into account purely the histological nature of the specimen and not the clinical or imaging characteristics. It is not feasible for pathological interpretation to judge independently whether a sample is adequate and from the mammographic lesion. This requires multidisciplinary discussion. For these reasons there is no inadequate biopsy category for core biopsy specimens.
B1 NORMAL TISSUE
This indicates a core of normal tissue, whether or not breast parenchymal structures are present; thus, this category is equally appropriate for a core including normal breast ducts and lobules or mature adipose tissue or stroma only. A B1 report should include a description of the components present and comment should be made regarding the presence of breast epithelial structures.
Normal histology may indicate that the lesion has not been sampled, although this is not necessarily so. In the case of certain benign lesions, such as hamartomas or lipomas, apparently normal histological features would be expected on core biopsy. Minor mammographic architectural distortions may also result in minimal changes, such as a slight increase in stromal fibrosis on biopsy. Cores with B1 diagnoses may contain microcalcification—for example, within involutional lobules. In these cases it is important that discussion between pathology and radiology colleagues is undertaken to confirm the appropriateness of the microcalcification in the histological specimen. Small foci of calcification within involuted lobules are common and frequently too small to be visible mammographically; thus, a report that merely records the presence of this calcification without additional comments on its nature, size, and site may be misleading and lead to false reassurance. It is evident that microcalcification, either singly or in clusters, less than 100 μm in diameter is not visible radiologically.9
B2 BENIGN LESIONS
A core is classified as B2 benign when it contains a benign abnormality. This category is appropriate for a range of benign lesions including fibroadenomas, fibrocystic changes, sclerosing adenosis, and duct ectasia and extends to include other non-parenchymal lesions, such as abscesses and fat necrosis. In some cases, it may be difficult to determine whether a specific lesion is present—for example, if minor fibrocystic changes are seen. The multidisciplinary approach is once again vital in these cases to determine whether the histopathological features are in keeping with the radiological and clinical findings. It may be appropriate and prudent to classify the lesion as B1, rather than B2, if only very minor changes are present; such histopathological features would clearly be insufficient to explain a well defined mass lesion, and classification as B2 would be inappropriate.
B3 UNCERTAIN MALIGNANT POTENTIAL
This category mainly consists of lesions that may provide benign histology on core biopsy, but either are known to show heterogeneity or to have an increased risk (albeit low) of associated malignancy.
Atypical intraductal epithelial proliferations
Atypical epithelial hyperplastic lesions—where a uniform population of cells arranged in an appropriate manner involves one duct space or partially involves two or more duct spaces—are included under the B3 classification. These appearances should be sufficiently structured to raise the possibility of low grade ductal carcinoma in situ (DCIS), but insufficient in the tissue available to fulfil the diagnostic criteria.10 There is a range of severity, from those lesions that are insufficient for a definite diagnosis of DCIS, but highly suspicious, to those that only show a minor degree of atypia, normally architectural, which requires further assessment, and judgement of appropriate categorisation B3 or B4 is required.
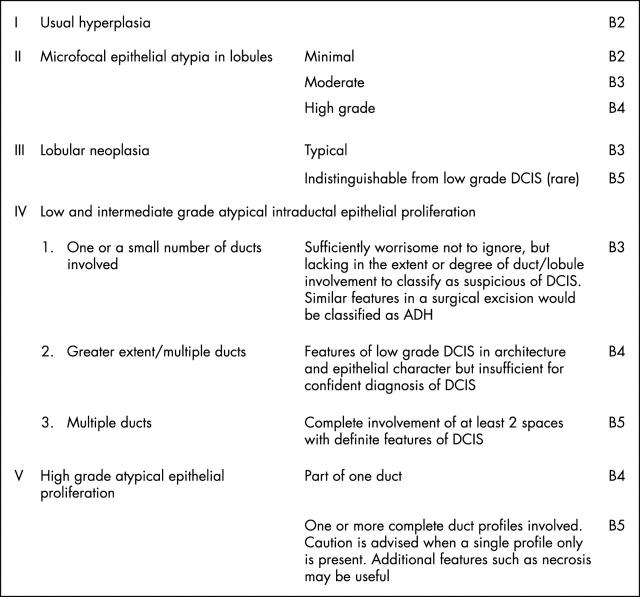
The definition of atypical ductal hyperplasia (ADH) is derived from surgical resection specimens and relies on a combination of histological, morphological, and size extent criteria. For this reason, accurate diagnosis of ADH is not possible on core biopsy. However, it has been shown that, on subsequent surgical resection, core biopsy samples that include atypical intraductal epithelial proliferative foci of insufficient extent for classification as DCIS may form part of an established in situ neoplastic lesion, with or without associated invasion. This view is based on several studies detailing the subsequent surgical diagnoses in cases described as ADH on non-operative core biopsy. In over 50% of cores, surgical excision biopsy has shown either in situ or invasive carcinoma.11 This is not surprising because ADH is basically defined as an intraductal epithelial proliferation showing the features of low grade DCIS, but in less than two duct spaces, or less than 2 mm in diameter. Thus, the limited tissue sampling that can be achieved using core biopsy guns (often by stereotactic methods for foci of microcalcification) may provide insufficient material for the definitive diagnosis of low grade DCIS. In these cases, a diagnosis of atypical intraductal epithelial proliferation and a classification of B3 of uncertain malignant potential or B4 suspicious of malignancy should be made, dependant on the severity and extent of the lesion (fig 1).
Figure 1.
Epithelial proliferative lesion classification. ADH, atypical ductal hyperplasia; DCIS, ductal carcinoma in situ.
Lobular neoplasia
A small cell regular epithelial proliferation within lobules, which is considered to represent intralobular neoplasia (atypical lobular hyperplasia/lobular carcinoma in situ), should be classified as B3. This process does not have the same management implications as a diagnosis of DCIS and does not require therapeutic excision per se. Lobular neoplasia is most often a coincidental finding in a core biopsy from a screen detected lesion, and multidisciplinary discussion is essential because the abnormality identified radiologically may not be represented. These cases must be managed cautiously. On occasion, it may be impossible to classify a small cell epithelial proliferation in lobules and/or ducts as either lobular neoplasia or low grade DCIS, and in these circumstances a numerically higher category (B4 or B5) is prudent and should be considered.
Phyllodes tumour
Fibroepithelial lesions with cellular stroma, stromal overgrowth, and possibly some mitotic activity suggesting a phyllodes tumour should be designated B3. Thus, the presence of cellular stroma within such a lesion should prompt a search for other features that may aid in discrimination from a fibroadenoma. In rare cases, it is not possible to distinguish the two lesions and the sample should be reported as a “fibroepithelial lesion” and classified as B3, to avoid underdiagnosis of a phyllodes tumour. In practice, careful appraisal of the entire clinical picture will usually allow appropriate management to be undertaken, and these cases should be discussed at a multidisciplinary meeting.
Papillary lesions
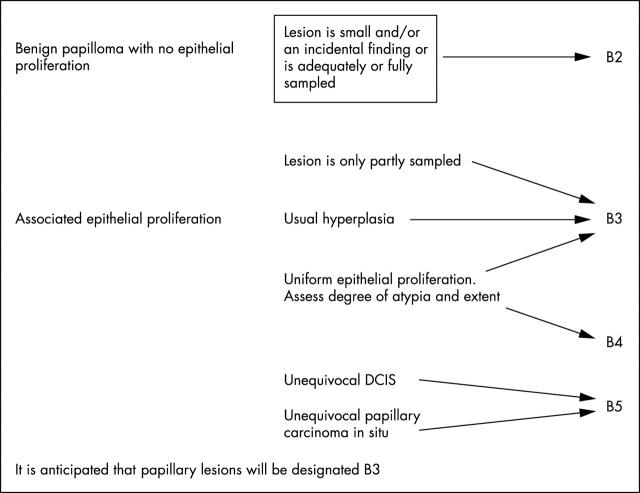
Papillary lesions may show considerable intralesional heterogeneity and the limited sampling achieved with core biopsy may miss areas of in situ cancer. Most of these lesions should be designated B3 of uncertain malignant potential. On rare occasions, when a small lesion has been very widely sampled and submitted for pathological examination, a benign B2 classification may be considered. Conversely, when a sample of a papillary lesion in a core biopsy shows atypia and is strongly suspicious of papillary carcinoma in situ, a B4 designation may occasionally be more appropriate (fig 2).
Figure 2.
Papillary lesion classification. DCIS, ductal carcinoma in situ.
Radial scar/complex sclerosing lesion
Biopsies showing features of a radial scar/complex sclerosing lesion such as areas of hyalinisation, elastosis, or tubular entrapment with epithelial proliferation should be categorised as B3. Although still a matter of debate, many authorities believe that some of these lesions are associated with malignancy. Thus, unless the sclerosing lesion is very widely sampled, the process should be designated as B3, because the presence of an associated area of DCIS or an invasive carcinoma cannot be excluded.
B4 SUSPICIOUS
Technical problems such as crushed or poorly fixed cores that contain probable carcinoma, but on which a definitive diagnosis cannot be made, are best included as B4. Similarly, apparently neoplastic cells contained within blood clot or adherent to the outer aspect of the sample should be classified as B4 suspicious. Very small foci of invasive carcinoma in which there is insufficient material to allow immunocytochemical studies may also reasonably be assigned to this category.
“Definitive therapeutic surgery should not be undertaken as a result of a B3 or B4 core biopsy diagnosis”
A complete single duct space bearing an unequivocal high grade atypical epithelial proliferative process can be classified as B5 malignant. Care must be taken if one or only part of a duct space is seen to contain a highly atypical epithelial process (particularly if no necrosis is present); this may be regarded as suspicious rather than definitely malignant. In particular, great care should be taken if the epithelial cells show any features of an apocrine phenotype, which may represent an atypical apocrine proliferation rather than DCIS.
Another lesion that can be allocated to this category is a non-high grade intraductal proliferation with a considerable degree of atypia, probably representing intermediate or low grade DCIS, where relatively few involved duct spaces are represented in the biopsy. A pragmatic approach is usually required by reporting an atypical intraductal proliferation and qualifying this according to the degree of suspicion; that is, “at least ADH, probably low grade DCIS”, and on the basis of extent or severity of atypia, allocating the case either to the B3 or to B4 category.
The management of cases classified as B4 will usually be either diagnostic excision biopsy of the area or repeat core biopsy sampling to obtain a definitive diagnosis. Definitive therapeutic surgery should not be undertaken as a result of a B3 or B4 core biopsy diagnosis.
B5 MALIGNANT
This category is appropriate for cases of unequivocal malignancy on core biopsy. Further categorisation into in situ and invasive malignancy should be undertaken whenever possible. One of the benefits of core biopsy is that it can allow distinction between in situ and invasive carcinoma. However, it should be borne in mind that, owing to sampling error, the exclusive presence of DCIS in the core does not exclude the possibility of an invasive focus being present. In approximately 20% of cases sampled by standard methods, coexisting invasive carcinoma will be identified in the subsequent surgical excision specimen.12 The nuclear grade, architecture, and the presence of necrosis of the DCIS can be indicated on the core biopsy report. In particular, the presence of associated calcification should be recorded.
PROGNOSTIC INFORMATION
Grading on core biopsy can be performed and is reasonably accurate. Current evidence suggests that concordance between grade on core biopsy and that in the definitive excision specimen can be achieved in approximately 75% of cases. However, it should be made clear to the clinicians that the grade may differ (almost invariably by only one level) from that in the subsequent resection specimen. The mitotic count in particular may be lower in the core biopsy than in the excision specimen, therefore leading to underscoring on the core.
Tumours may also often be typed according to the most common categories, such as ductal/no specific type or classic lobular carcinoma. However, invasive carcinoma of special type cannot be accurately predicted, although this may be suggested with some degree of accuracy in the core biopsy report.
Oestrogen receptor assessment
The assessment of predictive factors, such as the oestrogen receptor (ER), on core biopsies correlates with subsequent surgical excision specimens,13 and ER determination has been shown to predict response to hormone treatment.14 As with determination on excision biopsy samples, a standard protocol and method of assessment should be used.
PITFALLS IN DIAGNOSIS
Some lesions may present particular diagnostic problems in core samples.
B1 pitfalls
A potential pitfall includes focal lactational change, which may be seen in women who are neither lactating, nor pregnant, and indeed are nulliparous and/or postmenopausal. The involved acini are usually lured by plump vacuolated cells with a “hobnail” architecture but, less frequently, may appear atypical with irregular, large, or pyknotic nuclei. The epithelial cells may appear degenerative, and rarely the benign nature of the process may be mistaken for cancerisation of lobules by DCIS. The recognition of the vacuolation of the cytoplasm and the typical hobnail architecture will enable the correct diagnosis to be established.
B2 pitfalls
Mild atypia of the epithelium within lobular units is one of the most common problems encountered in core biopsy samples. Care must be taken not to over diagnose such minimal degrees of atypia, which may represent usual epithelial hyperplasia, apocrine change, or reactive changes (for example, adjacent to a previous sampling procedure). Usual epithelial hyperplasia and other forms of benign hyperplasia, such as that of the gynaecomastoid type, are commonly seen in cores from benign fibroadenomas. This often shows apparent discohesion as a result of the trauma of the core biopsy sampling process, and “telescoping” of the epithelium is seen within the duct spaces, thus resembling a more sinister epithelial proliferative process. As with usual epithelial hyperplasia in surgical excision specimens, the lack of uniformity and distribution/streaming of the epithelial cells with bland nuclear features and paucity of mitoses is of assistance in reaching a diagnosis. Usual epithelial hyperplasia of the gynaecomastoid type with a micropapillary architecture should not be mistaken for micropapillary ADH/DCIS.
There is a risk of over diagnosis of invasive carcinoma when confronted by sclerosing adenosis in a core biopsy, because the normal lobular arrangement may be less apparent than on an excision biopsy specimen. Immunohistochemical staining for collagen IV, laminin, and/or smooth muscle actin to demonstrate the presence of a basement membrane and a dual epithelial/myoepithelial layer, respectively, can be extremely useful in this situation. The stromal lack of fibroplasia, generally seen in an invasive carcinoma, may be helpful in achieving a correct diagnosis. In difficult cases, immunohistochemistry for smooth muscle actin may be invaluable; the presence of the myoepithelial component present in sclerosing lesions and absent in tubular carcinomas can be confirmatory.
Radiotherapy induced changes to the breast may be difficult to differentiate from foci of recurrent or residual carcinoma, both in situ and invasive. Radiation induces a degree of atypia of the breast epithelium and also in the histiocyte population, which is often prominent after radiotherapy and recent surgery. The macrophages may also show degenerative features, so that carcinoma cells may conversely mimic macrophages. Immunocytochemistry can be helpful in difficult cases because irradiated neoplastic cells retain cytokeratin expression, whereas macrophages show a histiocytic phenotype—for example, CD68 expression.
B3/B4 pitfalls
Apocrine atypia, particularly in association with a sclerosing lesion such as sclerosing adenosis (so called “apocrine adenosis”), may be especially difficult to identify correctly in non-operative diagnostic samples. In core biopsy large nuclei, often with prominent nucleoli, may be mistaken for DCIS if pleomorphism is also present. The typical granular eosinophilic cytoplasmic appearance of apocrine cells should be sought. Pure apocrine DCIS is relatively rare and when an apocrine proliferation is seen within ducts in a core biopsy, additional features of malignancy such as significant atypia, intraluminal necrosis, the presence of mitoses, and multiple duct involvement should be sought for confirmatory evidence. Mild or moderate degrees of apocrine proliferation with atypical features in a duct space should be assessed with caution, and it may be prudent not to record a definite diagnosis, but to classify such a process as B3, of uncertain malignant potential. Conversely, papillary apocrine change should not be mistakenly classified as other than benign B2.
Stromal proliferations may cause considerable difficulties in diagnosis in core biopsy samples. Occasionally, a second biopsy sample will be taken from a patient containing a fibroblastic proliferation which may represent the target lesion, but which may reflect tissue reaction and repair at the previous biopsy site. If the lesion represents the core site, an associated histiocyte reaction or indeed fat necrosis may be present, and haemosiderin laden macrophages can be seen. Sometimes fibroblastic stroma may be identified in a sample from a patient who has not undergone previous FNAC or core biopsy, and which may represent a spindle cell proliferation such as fibromatosis or part of a spindle cell tumour, such as a nerve sheath tumour or myofibroblastoma. Immunohistochemistry may prove unhelpful and a multidisciplinary approach must be applied to the clinical, radiological, and histopathological features. When a definitive histological diagnosis cannot be made the abnormality should be reported as a spindle cell lesion of uncertain histogenesis or nature and classified as B3.
B5 pitfalls
Small foci of invasive lobular carcinoma can be missed in histological sections and be dismissed as chronic inflammation or stromal cells. The targetoid infiltrative pattern of classic lobular carcinoma may be of assistance, but a reactive lymphocyte process can also have a periductal or perilobular distribution. Cytokeratin immunohistochemistry, to demonstrate the neoplastic cells, is of value in difficult cases, but recognition of the abnormal cell proliferation requires vigilance because the features can be subtle.
“Small foci of invasive lobular carcinoma can be missed in histological sections and be dismissed as chronic inflammation or stromal cells”
Malignant lymphoma may rarely be identified in core biopsies and should be classified as B5 malignant. Most of these lesions are of high grade B cell morphology and may mimic epithelial malignancy. Low grade lymphomas may be more difficult to distinguish, mimicking a chronic inflammatory processes. To avoid misclassification, a panel of lymphoid markers may be necessary to demonstrate the immunophenotype of the cells present and to allow correct diagnosis.
Metastasis to the breast from malignancies derived elsewhere is well recognised, although rarely biopsied. A full clinical history is essential to avoid the misdiagnosis of a metastatic carcinoma as a primary breast carcinoma. A panel of antibodies frequently allows identification of the likely site of a metastatic adenocarcinoma and enables appropriate clinical investigation/management. Breast carcinomas usually express cytokeratin 7 and 18 (and not cytokeratin 20), epithelial membrane antigen, and carcinoembryonic antigen/non-specific crossreacting antigen; in addition, approximately 80% will express ER.
Metaplastic carcinomas, or very rarely primary sarcomas, may mimic stromal proliferations, and a high index of suspicion may enable confirmatory diagnosis by immunohistochemical examination with a range of anticytokeratin antibodies (at least one broad spectrum and a high molecular weight cytokeratin, such as MNF116 and LP34). Primary breast sarcomas are rare. They most frequently originate in association with phyllodes tumours, but in core biopsy specimens an epithelial component is often not present. The most common phyllodes associated sarcomas seen are liposarcomas and fibrosarcomas, although osteosarcomas, chondrosarcomas, and rhabdomyosarcomas can be identified. Angiosarcomas may be a cause of a false negative diagnosis because they may be relatively subtle and bland and may be mistaken for radiotherapy induced changes, particularly when they occur in this situation in the treated breast. Primary leiomyosarcoma (and leiomyoma) may be found in the breast; leiomyomas are most often seen in a retroareolar site. All these lesions can be difficult or impossible to diagnose definitively in core samples. A high index of suspicion and the judicious use of immunohistochemistry can facilitate or support a diagnosis, but non-diagnostic classification as B3 or B4 is often prudent.
Acknowledgments
The authors of this manuscript are members of the non-operative diagnosis subgroup of the UK National Coordinating Committee for Breast Cancer Screening Pathology, which is responsible for pathology quality assurance in the UK National Health Service Breast Screening Program (NHSBSP). These guidelines have been endorsed by members of the European Commission Working Group on Breast Screening Pathology (EWGBSP). Membership of the UK National Coordinating Committee for Breast Cancer Screening Pathology: Dr Al-sam, Dr N Anderson, Professor TJ Anderson, Dr L Bobrow, Dr I Buley, Mr D Coleman, Dr E Connolly, Dr J Coyne, Dr NS Dallimore, Dr IO Ellis (Chairman), Professor CW Elston, Dr S Humphreys, Mrs S Kodikara, Dr DAS Lawrence, Dr J Lowe, Dr J Macartney, Dr S Moss, Dr D Parham, Mrs J Patnick, Dr SE Pinder, Dr C Quinn, Dr AJ Robertson, Dr J Shrimankar, Professor RA Walker, Dr CA Wells, Mr R Winder, Dr HD Zakhour. Membership of the European Commission Working Group on Breast Screening Pathology (by country): Austria: Dr A Reiner; Belgium: Dr M Drijkoningen, Dr D Faverly; Denmark: Dr F Rank; Finland: Dr P Heikkila; France: Professor JP Bellocq, Dr B Sigal-Zafrani, Dr J Jacquemier; Germany: Professor W Boecker; Greece: Dr N Apostolikas; Ireland: Professor P Dervan, Professor CE Connolly; Italy: Dr S Bianchi, Professor G Bussolati, Professor V Eusebi; The Netherlands: Dr R Holland, Dr JL Peterse; Portugal: Dr M Lacerda, Dr I Amendoeira; Spain: Dr C De Miguel, Dr J Martinez-Penuela; Sweden: Dr M Sylvan; UK: Dr IO Ellis, Professor CW Elston, Dr CA Wells (Chairman). Observers (by country), Israel: Professor V Kerner; Switzerland: Professor B Borisch; Hungary: Dr G Cserni, Dr J Kulka.
Abbreviations
ADH, atypical ductal hyperplasia
DCIS, ductal carcinoma in situ
ER, oestrogen receptor
FNAC, fine needle aspiration cytology
NCCBSP, National Coordinating Committee for Breast Cancer Screening Pathology
NHSBSP, National Health Service Breast Screening Programme
WBN, needle core biopsy
The authors are members of the non-operative diagnosis subgroup of the UK National Coordinating Committee for Breast Screening Pathology UK. This manuscript has been prepared on behalf of members of the UK National Coordinating Committee for Breast Screening Pathology and the European Commission working group on breast screening pathology.
REFERENCES
- 1.Cytology sub-group of the National Coordinating Committee for Breast Cancer Screening Pathology. Guidelines for cytology procedures and reporting in breast cancer screening. NHSBSP publication number 22. Sheffield: NHS Breast Screening Programme, 1993.
- 2.European Commission. European guidelines for quality assurance in mammography screening. de Wolf CJM, Perry NM, eds, 2nd ed. Luxembourg: Office for Official Publications of the European Communities, 1996.
- 3.Non operative diagnosis subgroup of the National Coordinating Committee for Breast Screening Pathology. Guidelines for non-operative diagnostic procedures and reporting in breast cancer screening. NHSBSP publication number 50. Sheffield, NHS Breast Screening Programme Publications, 2001 (pdf available from http://www.cancerscreening.nhs.uk/breastscreen/publications/nhsbsp50.pdf).
- 4.Lamb J, Anderson TJ, Dixon MJ, et al. Role of fine needle aspiration cytology in breast cancer screening. J Clin Pathol 1987;40:705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zajdela A, Chossein NA, Pillerton JP. The value of aspiration cytology in the diagnosis of breast cancer. Cancer 1975;35:499–506. [DOI] [PubMed] [Google Scholar]
- 6.Azavedo E, Svane G, Auer G. Stereotactic fine needle biopsy in 2594 mammographically detected non palpable lesions. Lancet 1989;1:1033–5. [DOI] [PubMed] [Google Scholar]
- 7.Britton PD, McCann J. Needle biopsy in the NHS Breast Screening Programme: how much and how accurate? Breast 1999;8:5–11. [Google Scholar]
- 8.Wilson R, Asbury D, Cooke J, et al. Clinical guidelines for breast cancer screening assessment. NHSBSP publication number 49. Sheffield: NHS Breast Screening Programme Publications, 2001 (pdf available from, http://www.cancerscreening.nhs.uk/breastscreen/publications/nhsbsp49.pdf).
- 9.Dahlstrom JE, Sutton S, Jain S. Histologic–radiologic correlation of mammographically detected microcalcification in stereotactic core biopsies. Am J Surg Pathol 1998;22:256–9. [DOI] [PubMed] [Google Scholar]
- 10.Royal College of Pathologists Working Group for Breast Screening Pathology. Pathology reporting in breast cancer screening. 2nd ed. London: NHSBSP Publications No 3, 1997.
- 11.Liberman L, Cohen MA, Dershaw DD, et al. Atypical ductal hyperplasia diagnosed at stereotaxic core biopsy of breast lesions: an indication for surgical biopsy. Am J Roentgenol 1995;164:1111–13. [DOI] [PubMed] [Google Scholar]
- 12.Liberman L, Dershaw DD, Rosen PP, et al. Stereotaxic core biopsy of breast carcinoma: accuracy at predicting invasion. Radiology 1995;194:379–81. [DOI] [PubMed] [Google Scholar]
- 13.Zidan A, Brown JSC, Peston D, et al. Oestrogen and progesterone receptor assessment in core biopsy specimens of breast carcinoma. J Clin Pathol 1997;50:27–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulding H, Pinder S, Cannon P, et al. A new immunohistochemical antibody for the assessment of estrogen receptor status on routine formalin-fixed tissue samples. Hum Pathol 1995;26:291–4. [DOI] [PubMed] [Google Scholar]