Abstract
Background: In Clostridium difficile associated diarrhoea (CDAD), histological changes in the colonic mucosa range from minimal inflammation to pseudomembranous colitis (PMC). The disease also recurs in a considerable proportion of patients.
Aim: To investigate mucosal immune system cells in colonic biopsies of patients with CDAD.
Methods: Colonic biopsies were obtained from 12 control patients with diarrhoea, six patients with CDAD and minimal inflammation, and 10 patients with CDAD with pseudomembranous colitis (samples obtained from areas with and without inflammatory exudate). Immunohistochemical studies were performed using antibodies to T cells (CD3), macrophages (CD68), B/plasma cells (CD79α), and to IgA, IgM, and IgG. Labelled cells in lamina propria were quantified.
Results: In contrast to T cells, there were significant reductions in B/plasma cell and macrophage counts in all biopsies from patients with CDAD compared with controls (p<0.001). Studies using anti-immunoglobulin antibodies showed significant reductions in IgA producing cells in CDAD biopsies (p<0.05), with the greatest reduction in samples from patients with PMC. In contrast, there was a significant increase (p<0.05) in IgG producing cells in CDAD biopsies. Only patients with PMC relapsed. In these patients, B/plasma cell and IgA producing cell counts (in biopsies with and without inflammatory exudates) were significantly lower (p<0.01) in mucosal samples from those who subsequently relapsed (five) than those who did not.
Conclusions: A selective reduction in mucosal IgA producing cells and macrophages is associated with colonic disease in C difficile infected patients. Severe reduction in colonic IgA producing cells may predispose to recurrence of CDAD.
Keywords: B cells, Clostridium difficile toxins, plasma cells, mucosal immunology, pseudomembranous colitis
The bacterium Clostridium difficile is the leading infectious cause of nosocomial diarrhoea in developed countries.1–3 The disease is mediated by two secreted toxins,4 and its presentation ranges from asymptomatic carriage to life threatening and sometimes fatal pseudomembranous colitis (PMC).5–7 Despite initial adequate treatment, a considerable proportion of patients relapse, with some having multiple relapses.8–11 Factors reported to be associated with recurrence include previous episodes of C difficile associated diarrhoea (CDAD), increasing age, chronic renal insufficiency, high white blood counts, and impaired antibody responses to toxin A. 8,10–12
At sigmoidoscopy, PMC can be readily identified by the presence of characteristic yellow/white plaques (pseudomembranes), which are often separated from each other by mucosa that may macroscopically appear normal or erythematous.13 Histologically, the yellow/white plaques are exudates of inflammatory cells, fibrin, mucin, and cellular debris, arising from distinct areas of epithelial ulceration (“volcano” lesions). The lamina propria under the area of ulceration consists of a large number of inflammatory cells, of which neutrophils are prominent by routine haematoxylin and eosin staining.13,14 However, there is little information on the characterisation of other mucosal cell types in CDAD. There are a large number of T cells,15 B/plasma cells,16 and macrophages17,18 in the normal colonic lamina propria. A major function of these cells of the mucosal immune system is to facilitate the production of secretory IgA, which is transported by epithelial cells to the lumen, to provide protection against pathogenic microorganisms.19 Impaired mucosal protection via alterations in the number or function of cells in the lamina propria may lead to increased susceptibility to CDAD and/or its recurrence.
“Despite initial adequate treatment, a considerable proportion of patients relapse, with some having multiple relapses”
In our study, we have investigated mucosal populations of T cells, B/plasma cells, immunoglobulin producing cells, and macrophages in colonic biopsies of (1) patients with CDAD and PMC, (2) patients with CDAD and either absent or minimal colonic inflammation, and (3) controls. We show that the numbers of mucosal macrophages, B/plasma cells, and IgA producing cells are significantly reduced in patients with CDAD, with the greatest reduction in those with PMC. The numbers of lamina propria B/plasma cells and IgA producing cells were also significantly lower in biopsies of patients in whom the disease recurred, compared with those with a single episode.
MATERIALS AND METHODS
Study population
Colonic biopsies were obtained from patients with diarrhoea (⩾ 3 liquid motions for more than 24 hours), as part of a prospective study to investigate the role of flexible sigmoidoscopy in the management of hospitalised patients suspected to have CDAD.20 The biopsies were divided into four groups (A–D). Group A (n = 12) comprised mucosal samples from control patients with self limiting diarrhoea whose stool tests were negative for conventional enteric pathogens (Salmonella spp, Campylobacter spp, Shigella sp, and Escherichia coli O157) and C difficile cytotoxin, and whose sigmoidoscopy was normal, as was histological examination of colonic biopsies. Group B (n = 6) comprised patients with CDAD (positive stool test for C difficile cytotoxin) with absent or minimal inflammation macroscopically at sigmoidoscopy (no pseudomembranes) and on histological examination. Groups C and D (n = 10) comprised patients with CDAD (confirmed by positive stool test for C difficile cytotoxin) who had PMC at sigmoidoscopy, which was confirmed on histological examination. For group C, the colonic biopsies were taken from areas of mucosa without overlying pseudomembranes, and in which histologically there was often only mild inflammation without epithelial ulceration. Mucosal samples in group D were obtained from the same patients as for group C but the biopsies were taken from colonic mucosa with overlying pseudomembranes, and all of the mucosal sections contained volcano lesions (focal epithelial ulceration and associated inflammatory exudate and inflammation in the underlying lamina propria) on histological examination. Table 1 gives the age, sex, frequency, and duration of diarrhoea for the three patient groups. All biopsies, collected before the initiation of medical treatment in patients with CDAD, were fixed in 0.9% saline containing 10% formalin and subsequently embedded in paraffin wax before immunohistochemistry.
Table 1.
Details of patients studied
| Age (years) | Sex | Diarrhoea duration(days) | Diarrhoea frequency (stool/day) | Temp (°C) | WBC(×109/l) | CRP(mg/l) | Serum IgA | Serum IgM | Serum IgG | |
| Group A | 76 (2.7) | 6M 6F | 6 (2) | 5.7 (1) | 36 (0.2) | 12 (1.7) | 68 (29) | 1.59 (0.35) | 0.89 (0.39) | 7.01(1) |
| Group B | 64 (10) | 4M 2F | 6.4 (1.7) | 6.7 (1) | 37 (0.2) | 14 (4) | 81 (34) | 3.01 (1) | 0.73 (0.21) | 10.5 (2.6) |
| Groups C/D | 82 (2.5) | 6M 4F | 16.6 (5)** | 6.3 (1) | 37 (0.2) | 21 (2)* | 129 (24)* | 2.41 (0.51) | 0.70 (22) | 7.97 (1.24) |
Group A, controls; group B, CDAD; groups C and D, PMC. Patients with PMC had significantly higher WBC and CRP than controls (group A; *p<0.05) but not the CDAD group with no or minimal inflammation (group B). The duration of diarrhoea was longer in patients with PMC than both controls (group A) and the CDAD group with absent or minimal inflammation (group B) (**p<0.05). There was no significant difference in age, sex, diarrhoea frequency, or temperature between the groups. Results are expressed as mean (SEM).
CDAD, Clostridium difficile associated diarrhoea; CRP, C reactive protein; PMC, pseudomembranous colitis; WBC, white blood cell count.
Patients with CDAD were followed up to identify those with relapsing disease after successful treatment of the initial episode. Recurrence of CDAD was confirmed by the identification of C difficile cytotoxin in stool sample(s) and/or the presence of PMC at sigmoidoscopy (and response to metronidazole or vancomycin).
Our study was approved by the ethics committee of the Nottingham University Hospital NHS trust and informed consent was obtained from all the patients.
Immunohistochemistry for lamina propria cell populations
Sections (5 μm thick) were treated with antigen unmasking solution (1mM EDTA, pH 8; Sigma Chemical Co, St Louis, Missouri, USA) in a microwave oven for four minutes and were subsequently washed (at room temperature) in distilled water, followed by phosphate buffered saline. Endogenous peroxidase activity was blocked by incubation in methanol (Fisher Chemicals, Fisher Scientific UK Ltd, Loughborough, UK) containing 1% hydrogen peroxide (Sigma Chemicals Co) for 30 minutes. Immunohistochemistry was performed using a Vectastain Universal elite ABC peroxidase kit (Vector Laboratories Inc, Burlingame, California, USA). In brief, after the application of normal horse serum, the sections were incubated with specific antibodies at 4°C. The antibodies were specific for T cells (rabbit polyclonal anti-CD3; Dako, Ely, Cambridgeshire, UK), B/plasma cells (monoclonal anti-CD79α; anti-mb-1;21,22 Dako), macrophages (monoclonal anti-CD68; Dako), IgA producing cells (rabbit polyclonal anti-IgA; Serotec, Kiddington, Oxfordshire, UK), IgG producing cells (monoclonal anti-IgG; Serotec), and IgM producing cells (monoclonal anti-IgM; Dako). Bound primary antibodies were detected using an avidin–biotin peroxidase technique (Vectastain ABC peroxidase kit; Vector Laboratories Inc). For negative controls, the specific (primary) antibody was replaced with phosphate buffered saline, and for studies in which the primary antibodies were to immunoglobulin producing cells, sections from samples with ulcerative colitis were used as positive controls.23
Cell counting technique
Cell counts were carried out using a SM-LUX light microscope (Leitz, Wetzlar, Germany) fitted with a stage and 10 × 10 simple grid graticule in the eyepiece. The grid was placed over the lamina propria on the far left of the section at ×250 magnification and a random number chart24 was used to generate a grid reference. Labelled cells in the selected area were counted at ×400 magnification, and then the field of view was moved 0.5 mm at a time such that positive cells in the lamina propria, extending from the epithelium to the muscularis mucosa, were counted. The stage was subsequently moved 0.5 mm to the right of the section and the cell counting procedure was repeated. The positively labelled cells within the grid frame were counted, and any intersecting the lower and right borders disregarded. Any cells or intersections that were not within the lamina propria were disregarded. At least 12 fields were examined for each slide—the number of fields needed to provide a consistent mean cell count within 5% of the true mean in our study.25 For sections containing volcano lesions, cells in the lamina propria adjacent to the area of epithelial ulceration and inflammatory exudate were counted.
All the cell counts were performed by two investigators (SSJ and CPL), who were blinded to the clinical details of the patients. The mean counts by the two investigators for macrophages and B/plasma cells were within 5% for all groups. Mean counts of T cells were also within 5% for all groups except controls (group A), which were within 14%.
Serum immunoglobulin concentrations
Venous blood samples were collected at the time of flexible sigmoidoscopy to determine the immunoglobulin concentrations in the serum. Serum IgA, IgM, and IgG were measured by nephelometry and compared between the patient groups under study.
Statistical tests
Analyses were performed using Kruskal-Wallis one way ANOVA, unpaired Mann Whitney U test, and Wilcoxon signed ranks test. Significance was accepted as p < 0.05. Data are expressed as mean (SEM).
RESULTS
Patients
Patients with PMC (mucosal tissue groups C and D) had significantly higher white blood counts and C reactive protein concentrations than did controls (group A; p < 0.05; table 1), but when compared with the CDAD group with absent or minimal inflammation (group B), the difference was not significant. The duration of diarrhoea was significantly longer in patients with PMC than in the other two groups (groups A and B; p < 0.05). The resolution of diarrhoea was defined as a return to normal bowel habit (formed stool < 3 times daily). There was no significant difference in age, sex, diarrhoea frequency, or temperature between the groups. Of the control patients, 11 had self limiting diarrhoea attributed in seven to antibiotics prescribed for co-morbid conditions (diarrhoea stopped on discontinuation of antibiotics), constipation with overflow diarrhoea in three, and suspected viral gastroenteritis in one (the remaining patient had carcinoid syndrome).
Mucosal lamina propria cell populations
Compared with controls (group A), there were significantly fewer macrophages in all groups of biopsies from patients with CDAD (fig 1). Of the biopsies from patients with CDAD, there was a trend towards fewer macrophages in sections containing volcano lesions (group D), but this difference was not significant. There were no significant differences in lamina propria T cell counts between the groups of biopsies studied. In contrast, compared with the control group (group A), B/plasma cell counts were significantly lower in all groups of biopsies from patients with CDAD (figs 1, 2). Moreover, there were significantly fewer B/plasma cells (p < 0.01) in sections with volcano lesions (group D) compared with the other CDAD affected sections (groups B and C).
Figure 1.
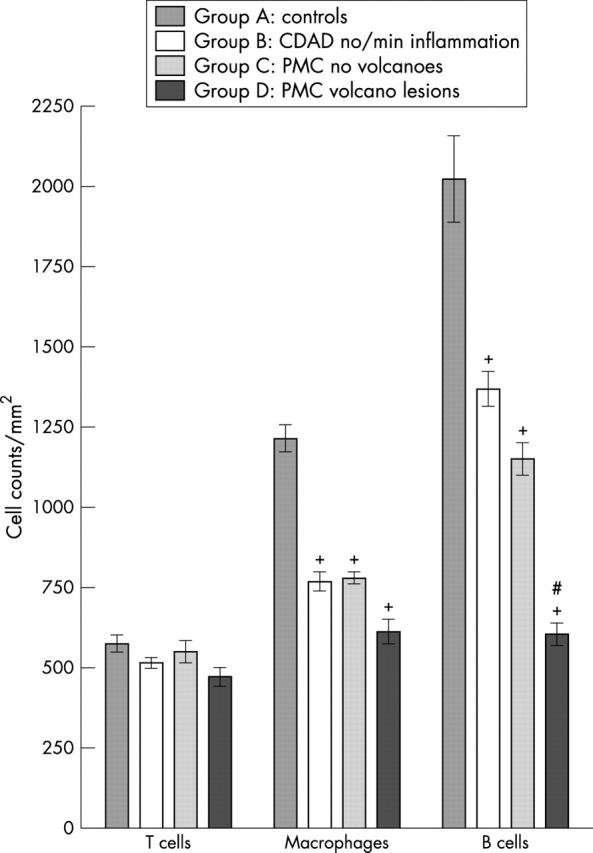
Lamina propria T cell, macrophage, and B/plasma cell counts (mean ±SEM) in colonic biopsies from control patients (group A), patients with Clostridium difficile associated diarrhoea (CDAD) with absent/minimal inflammation (group B), and patients with pseudomembranous colitis (PMC). Biopsies from the PMC group were obtained from (1) mucosa adjacent to pseudomembrane (group C) and (2) mucosa with overlying pseudomembrane and with a “volcano” lesion (group D). Compared with controls (group A), macrophage and B/plasma cell counts (but not T cell counts) were significantly reduced in all groups of biopsies from patients with CDAD (groups B, C, and D). B/plasma cell counts were also significantly lower in group D biopsies, compared with those in groups B (+p < 0.001) and C (#p < 0.01).
Figure 2.
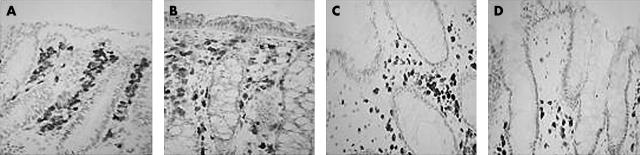
Representative photomicrographs showing B/plasma cells in biopsies from (A) control patients (group A), (B) patients with Clostridium difficile associated diarrhoea (CDAD) and absent/minimal inflammation (group B) and samples from patients with pseudomembranous colitis obtained from (C) mucosa adjacent to pseudomembrane (group C) and (D) mucosa with overlying pseudomembrane and with a “volcano” lesion in the same section (group D). Immunohistochemistry was performed using an anti-CD79α monoclonal antibody.
To characterise the changes in B/plasma cells further, immunoglobulin producing cells were studied using antibodies to IgA, IgG, and IgM. In biopsies from the diarrhoea control patients (group A), IgA producing cells were predominant (mean, 89%; SEM, 2%), followed by IgM producing cells (mean, 9%; SEM, 2%), and IgG producing cells (mean, 2%; SEM, 1%). Compared with the controls (group A), there were significantly fewer IgA producing cells in all groups of biopsies from patients with CDAD, with the greatest reduction in sections containing volcano lesions. Indeed, the number of IgA producing cells in the sections from group D and sections from biopsies taken adjacent to pseudomembranes (group C), were significantly lower than in mucosal samples obtained from patients with CDAD and absent or minimal inflammation (group B; p < 0.01 for both comparisons; fig 3).
Figure 3.
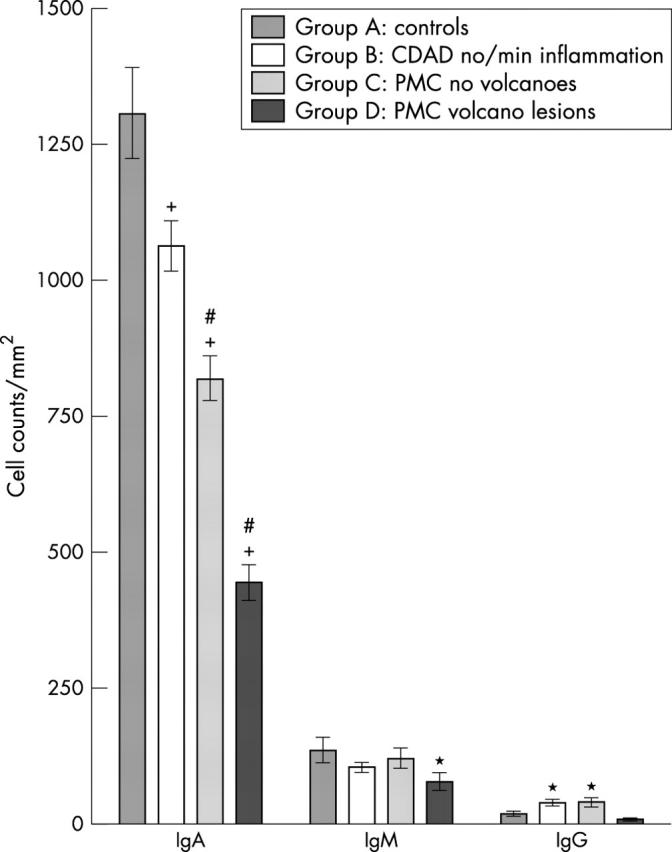
Lamina propria counts of IgA, IgM, and IgG producing cells (mean ±SEM) in colonic biopsies from control patients (group A), patients with Clostridium difficile associated diarrhoea (CDAD) and absent/minimal inflammation (group B), and patients with pseudomembranous colitis (PMC). Biopsies from patients with PMC were obtained from mucosa adjacent to pseudomembrane (group C) and mucosa with overlying pseudomembrane and with a “volcano” lesion (group D). Compared with controls (group A), IgA producing cells were reduced in biopsies from patients with CDAD and absent/minimal inflammation (group B) (*p < 0.05), and those obtained from patients with PMC (groups C and D; +p < 0.001 for both). IgA producing cells were also significantly lower in group C and D biopsies, when compared with those in group B (#p < 0.01 for both comparisons). Compared with the other groups, there was a significant reduction in the number of IgM producing cells in group D biopsies (*p < 0.05). In contrast, IgG producing cells were significantly increased in group B and C biopsies (both *p < 0.05).
IgM producing cells were significantly reduced only in sections with volcano lesions (group D) (fig 3). In contrast, IgG expressing cells were significantly increased in biopsies from patients with CDAD and absent/minimal inflammation (group B) and those obtained adjacent to pseudomembranes (group C).
Mucosal cell populations and subsequent recurrence of CDAD
Over a mean (SEM) follow up period of 16 (1.7) months, five of the 10 (three male and two female) patients with PMC relapsed. The mean (SEM) age of the patients who went on to have recurrent CDAD was 76 (4) years, compared with 88 (1) years for those who only had one episode. None of the patients with CDAD but absent/minimal inflammation (group B) relapsed (mean follow up, 20 months; SEM, 1.5).
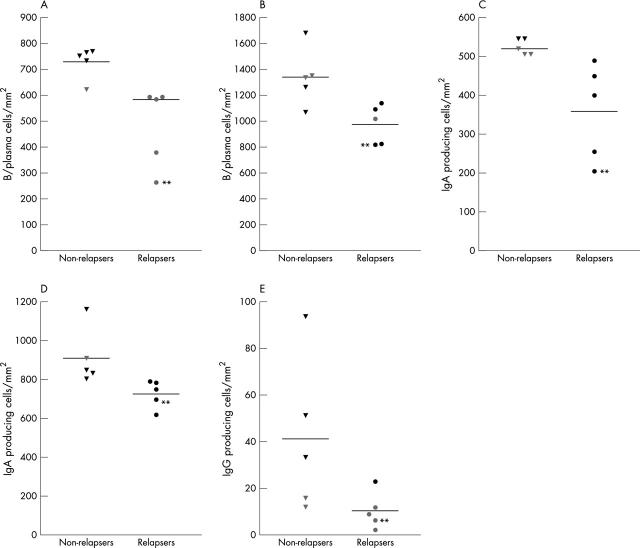
In patients with PMC, the colonic lamina propria cells in the five patients who subsequently relapsed were compared with the remaining five who did not have a recurrence. There were significantly fewer B/plasma cells and IgA producing cells in sections of biopsies taken adjacent to pseudomembranes (group C) and in sections containing volcano lesions (group D) in patients with PMC who relapsed than in those who did not (fig 4A–D) (p < 0.01). Counts of B/plasma and IgA producing cells in one patient who relapsed twice were among the lowest in the group.
Figure 4.
Lamina propria counts of (A, B) B/plasma cells, (C, D) IgA producing cells, and (E) IgG producing cells in colonic biopsies of patients with pseudomembranous colitis who subsequently either relapsed or had no recurrence of the disease (non-relapsers). Colonic biopsies were obtained from mucosa with overlying pseudomembrane (and containing “volcano” lesion in the section (A) and (C)) and from mucosa adjacent to pseudomembrane ((B), (D), and (E)). There were significant differences in cell counts in all the comparisons ((A–D), p < 0.01; (E), p < 0.05). Cell counts in the one patient who relapsed twice are also shown (**).
There were significantly fewer IgG producing cells in the lamina propria of mucosal samples adjacent to pseudomembranes (group C) in patients who had a recurrence of CDAD, compared with those who did not (fig 4E). There were also fewer IgG producing cells in sections of relapsers containing volcano lesions (group D), but this difference did not reach significance (mean, 7.8 (SEM, 4.4) in relapsers v 10.3 (3.5) in non-relapsers). Lamina propria counts of T cells, macrophages, and IgM producing cells did not differ between patients with PMC who relapsed and those who did not (mean, 437 (SEM, 80), 634 (91), 77 (17) v 504 (54), 588 (103), 107 (30), respectively; refers to group D biopsies).
Serum immunoglobulin values
There were no significant differences between the three groups of patients (groups A, B, and C/D) in serum concentrations of IgA, IgM, or IgG (table 1). There were also no significant differences in serum concentrations of these immunoglobulins between patients with PMC who relapsed and those who did not (data not shown).
DISCUSSION
In its severe form, C difficile infection leads to pronounced colonic inflammation, characterised by infiltration of the mucosa by polymorphonuclear cells and focal areas of epithelial ulceration, from which pseudomembranes arise.14 In milder forms of the disease, mucosal inflammation may be minimal or absent, and the diarrhoea may resolve soon after discontinuation of the offending antibiotics. In our study, we have investigated cells of the mucosal immune system in colonic biopsies from control patients with self limiting diarrhoea (with no inflammation on histological examination of colonic biopsies) and those with mild and severe forms of C difficile associated disease. Our initial studies had shown that mucosal lamina propria cell counts of macrophages, T cells, B/plasma cells, and immunoglobulin producing cells in our control group did not differ significantly from those in normal colonic mucosal samples obtained from operation resection specimens (distant from tumour; data not shown). In the normal colonic lamina propria, there are more T cells than macrophages, and the higher macrophage counts seen in our study probably reflect their larger size, which makes them more likely than T cells to be sectioned,26 together with their strong CD68 cytoplasmic immunoreactivity. The anti-CD79α monoclonal antibody used in our study is known to label B cells and plasma cells,21,22 and our findings using anti-Ig antibodies confirmed the presence of a large number of immunoglobulin producing cells,27 with IgA expressing cells predominating, as reported previously.28,29
There were significantly fewer mucosal macrophages and B/plasma cells in colonic biopsies from patients with CDAD compared with control samples. The reduction in B/plasma cells was greatest in biopsies with pseudomembranes. In contrast, there were no significant differences between the groups in the numbers of T cells present in the lamina propria.
Studies using anti-immunoglobulin specific antibodies showed that the low B/plasma cell counts in colonic biopsies of patients with CDAD resulted mainly from a reduction in IgA producing cells. It is interesting to note that previous studies have shown an increase in all three types of immunoglobulin producing cells in mucosal samples with active inflammatory bowel disease and coeliac disease, with the greatest increase being in IgG producing cells in these two diseases.23 In contrast, we found a selective reduction in IgA producing cells in colonic biopsies of patients with CDAD, with only a small, but significant increase in the number of IgG producing cells (except in biopsies containing volcano lesions).
It is conceivable that the reduction in the number of lamina propria macrophages and IgA producing cells results from the direct effects of the secreted toxins. In vitro studies have shown that purified toxins A and B are capable of inducing rapid cell death in not only epithelial cells,30–32 but also macrophages and monocytes.33,34 To mediate their cytotoxic effects in intestinal macrophages, adequate amounts of the toxins would have to gain access to the lamina propria. This could occur at sites of epithelial ulceration, but not where the epithelium is intact. Moreover, T cells and B cells are more resistant to toxin A induced cell death than are monocytes and macrophages.34,35 Therefore, the reduction in the number of macrophages and IgA expressing cells in the lamina propria of CDAD biopsies distant from areas of epithelial ulceration (group C) and with minimal/absent inflammation (group B) may not be the result of the direct effects of the C difficile toxins.
An alternative explanation is that the reduction in mucosal macrophages and IgA producing cells in patients with CDAD was present before C difficile infection. Indeed, the pre-existing reduction in the number of these cells could have predisposed these individuals to acquiring the initial infection and could also have influenced the severity of mucosal inflammation. This could explain the finding that the reduction in IgA expressing cells was greatest in biopsies (with and without volcano lesions) from patients with PMC. It is also of interest that only patients with PMC subsequently relapsed. Moreover, colonic biopsies of patients who subsequently relapsed had a significantly lower number of IgA producing cells than those who did not have a recurrence. Indeed, the patient who had two recurrences had the lowest IgA producing cell counts. However, because only biopsies obtained during the index episode of C difficile infection were investigated, further work is required to support the above hypothesis. Such further work would include studies on biopsies obtained at intervals after the resolution of CDAD.
“Our findings raise the possibility that the severity of a pre-existing impairment of the mucosal immune response is related to the degree of mucosal inflammation and the risk of subsequent recurrence”
Previous studies have demonstrated systemic and mucosal immune responses to C difficile toxins.36–41 In non-immunocompromised patients, serum immunoglobulin (IgG) and faecal IgA antitoxin A antibody titres were found to be significantly higher in patients who suffered a single episode than in those with relapsing CDAD.38 Several other studies, but not all,39 have reported similar findings with reference to serum immunoglobulin concentrations.8,40,42 Increased serum concentrations of immunoglobulins against C difficile37 and of IgG antitoxin A antibodies 43 have also been reported to be associated with asymptomatic carriage of C difficile.
Take home messages.
There were significantly fewer B/plasma cells and macrophages (but not T cells) in all biopsies from patients with Clostridium difficile associated diarrhoea (CDAD) compared with controls
The reduction in B/plasma cells was the result of a selective reduction in IgA (not IgG) producing cells
The greatest reductions in IgA producing cells were in samples from patients with pseudomembranous colitis, particularly those who relapsed
Severe reduction in colonic IgA producing cells may predispose to recurrence of CDAD
The above studies and the results of our study support the concept of the important role of the immune response in determining, not only the development of clinical disease after colonisation by toxigenic C difficile, but also the risk of recurrence. The results of our study imply that the secretory IgA response is impaired, as a result of a reduction in IgA producing cells, in patients with CDAD. Although there are alternative explanations for our findings, they do raise the possibility that the severity of a pre-existing impairment of the mucosal immune response is related to the degree of mucosal inflammation and the risk of subsequent recurrence. The impaired mucosal antibody response, caused by a reduction in immunoglobulin producing cells, could be compounded by a reduction in the number of intestinal macrophages because they have been shown to be capable of enhancing spontaneous immunoglobulin production by isolated human intestinal lamina propria cells.44
Acknowledgments
This work was supported by the Community Fund (formerly National Lottery Charities Board) via the Digestive Disorders Foundation and the Medical Research Council.
Abbreviations
CDAD, Clostridiumdifficile associated diarrhoea
PMC, pseudomembranous colitis
REFERENCES
- 1.McFarland LV. Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am J Infect Control 1995;23:295–305. [DOI] [PubMed] [Google Scholar]
- 2.Guerrant RL, Hughes JM, Lima NL, et al. Diarrhea in developed and developing countries: magnitude, special settings, and etiologies. Rev Infect Dis 1990;12 (suppl 1) :S41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yannelli B, Gurevich I, Schoch PE, et al. Yield of stool cultures, ova and parasite tests, and Clostridium difficile determinations in nosocomial diarrheas. Am J Infect Control 1988;16:246–9. [DOI] [PubMed] [Google Scholar]
- 4.Borriello SP. Pathogenesis of Clostridium difficile infection. J Antimicrob Chemother 1998;41 (suppl C) :13–19. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med 1978;298:531–4. [DOI] [PubMed] [Google Scholar]
- 6.George RH, Youngs DJ, Johnson EM, et al. Anion-exchange resins in pseudomembranous colitis. Lancet 1978;2:624. [DOI] [PubMed] [Google Scholar]
- 7.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med 1994;330:257–62. [DOI] [PubMed] [Google Scholar]
- 8.Kyne L, Warny M, Qamar A, et al. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 2001;357:189–93. [DOI] [PubMed] [Google Scholar]
- 9.McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease [published erratum appears in JAMA 1994 Aug 17;272:518]. JAMA 1994;271:1913–18. [PubMed] [Google Scholar]
- 10.Fekety R, McFarland LV, Surawicz CM, et al. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis 1997;24:324–33. [DOI] [PubMed] [Google Scholar]
- 11.Do AN, Fridkin SK, Yechouron A, et al. Risk factors for early recurrent Clostridium difficile-associated diarrhea. Clin Infect Dis 1998;26:954–9. [DOI] [PubMed] [Google Scholar]
- 12.McFarland LV, Surawicz CM, Rubin M, et al. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol 1999;20:43–50. [DOI] [PubMed] [Google Scholar]
- 13.Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 1997;92:739–50. [PubMed] [Google Scholar]
- 14.Price AB, Davies DR. Pseudomembranous colitis. J Clin Pathol 1977;30:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James SP, Kiyono H. Gastrointestinal lamina propria T cells. In: Ogra PL, Mestecky J, Lamm ME, eds. Mucosal immunology. San Diego: Academic Press, 1999:381–97.
- 16.Brandtzaeg P, Farstad IN. The Human mucosal B-cell system. In: Ogra PL, Mestecky J, Lamm ME, eds. Mucosal immunology. San Diego: Academic Press, 1999:439–68.
- 17.Mahida YR, Patel S, Gionchetti P, et al. Macrophage subpopulations in lamina propria of normal and inflamed colon and terminal ileum. Gut 1989;30:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavli P, Maxwell L, Van de Pol E, et al. Distribution of human colonic dendritic cells and macrophages. Clin Exp Immunol 1996;104:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGhee JR, Czerkinsky C, Mestecky J. Mucosal vaccines: an overview. In: Ogra PL, Mestecky J, Lamm ME, eds. Mucosal immunology. San Diego: Academic Press, 1999:741–57.
- 20.Johal SS, Hammond J, Solomon K, et al. C difficile-associated diarrhoea in hospitalised patients: onset in the community and hospital and role of flexible sigmoidoscopy. Gut 2004;53:673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason DY, Cordell JL, Tse AG, et al. The IgM-associated protein mb-1 as a marker of normal and neoplastic B cells. J Immunol 1991;147:2474–82. [PubMed] [Google Scholar]
- 22.Mason DY, van Noesel CJ, Cordell JL, et al. The B29 and mb-1 polypeptides are differentially expressed during human B cell differentiation. Eur J Immunol 1992;22:2753–6. [DOI] [PubMed] [Google Scholar]
- 23.Brandtzaeg P, Halstensen TS, Kett K, et al. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology 1989;97:1562–84. [DOI] [PubMed] [Google Scholar]
- 24.Petrie A. Table A8: random numbers. In: Lecture notes in medical statistics. 2nd ed. London: Blackwell scientific publications, 1987, Appendix, 258.
- 25.Gray T. Quantitation in histopathology. In: Bancroft JD, Stevens A, eds. Theory and practice of histological technique. 4th ed. Edinburgh: Churchill Livingstone, 1996:655.
- 26.Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 1984;134 (Pt 2) :127–36. [DOI] [PubMed] [Google Scholar]
- 27.Lee E, Schiller LR, Fordtran JS. Quantification of colonic lamina propria cells by means of a morphometric point-counting method. Gastroenterology 1988;94:409–18. [DOI] [PubMed] [Google Scholar]
- 28.Gelzayd EA, Kraft SC, Fitch FW, et al. Distribution of immunoglobulins in human rectal mucosa. II. Ulcerative colitis and abnormal mucosal control subjects. Gastroenterology 1968;54:341–7. [PubMed] [Google Scholar]
- 29.Meuwissen SG, Feltkamp-Vroom TM, De La Riviere AB, et al. Analysis of the lympho-plasmacytic infiltrate in Crohn’s disease with special reference to identification of lymphocyte-subpopulations. Gut 1976;17:770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahida YR, Makh S, Hyde S, et al. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut 1996;38:337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiorentini C, Fabbri A, Falzano L, et al. Clostridium difficile toxin B induces apoptosis in intestinal cultured cells. Infect Immun 1998;66:2660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brito GA, Fujji J, Carneiro-Filho BA, et al. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J Infect Dis 2002;186:1438–47. [DOI] [PubMed] [Google Scholar]
- 33.Warny M, Kelly CP. Monocytic cell necrosis is mediated by potassium depletion and caspase-like proteases. Am J Physiol 1999;276 (3 Pt 1) :C717–24. [DOI] [PubMed] [Google Scholar]
- 34.Mahida YR, Galvin A, Makh S, et al. Effect of Clostridium difficile toxin A on human colonic lamina propria cells: early loss of macrophages followed by T-cell apoptosis. Infect Immun 1998;66:5462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon K, Robins RA, Mahida YR. C. difficile toxin A induces rapid and selective monocyte cell death [abstract]. Gut 2003;52 (suppl 1) :A54–55. [Google Scholar]
- 36.Kelly CP, Pothoulakis C, Orellana J, et al. Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A-receptor binding. Gastroenterology 1992;102:35–40. [DOI] [PubMed] [Google Scholar]
- 37.Mulligan ME, Miller SD, McFarland LV, et al. Elevated levels of serum immunoglobulins in asymptomatic carriers of Clostridium difficile. Clin Infect Dis 1993;16 (suppl 4) :S239–44. [DOI] [PubMed] [Google Scholar]
- 38.Warny M, Vaerman JP, Avesani V, et al. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect Immun 1994;62:384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson S, Gerding DN, Janoff EN. Systemic and mucosal antibody responses to toxin A in patients infected with Clostridium difficile. J Infect Dis 1992;166:1287–94. [DOI] [PubMed] [Google Scholar]
- 40.Bacon AE 3rd, Fekety R. Immunoglobulin G directed against toxins A and B of Clostridium difficile in the general population and patients with antibiotic-associated diarrhea. Diagn Microbiol Infect Dis 1994;18:205–9. [DOI] [PubMed] [Google Scholar]
- 41.Viscidi R, Laughon BE, Yolken R, et al. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis 1983;148:93–100. [DOI] [PubMed] [Google Scholar]
- 42.Aronsson B, Granstrom M, Mollby R, et al. Serum antibody response to Clostridium difficile toxins in patients with Clostridium difficile diarrhoea. Infection 1985;13:97–101. [DOI] [PubMed] [Google Scholar]
- 43.Kyne L, Warny M, Qamar A, et al. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 2000;342:390–7. [DOI] [PubMed] [Google Scholar]
- 44.Wu KC, Mahida YR, Priddle JD, et al. Effect of human intestinal macrophages on immunoglobulin production by human intestinal mononuclear cells isolated from patients with inflammatory bowel disease. Clin Exp Immunol 1990;79:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]