Abstract
Aims: To identify how many particles should be examined to enable a confident assessment of the presence or absence of iron stores and the quantity of iron in a bone marrow aspirate to be made.
Methods: One hundred and ninety consecutive bone marrow aspirate samples were stained with Perls’ stain and the iron content of 10 consecutive particles was recorded. The first particle found to be positive and the particle that was most positive were also noted.
Results: A minimum of seven particles must be examined to establish the absence of stainable iron. A minimum of nine particles must be reviewed to see the maximum iron stores in 100% of samples and therefore make a valid judgment of whether iron stores are reduced, normal, or increased. By these criteria, 46% of the samples tested here could not be optimally assessed for absence of iron or maximum iron stores.
Conclusions: The sensitivity of examination of bone marrow aspirates for iron stores can be optimised by increasing the number of particles reviewed to seven or more. This may require the staining of additional slides.
Keywords: iron deficiency, bone marrow, bone marrow aspirate, bone marrow particle
Iron deficiency is relatively common in the community as a result of both physiological and pathological losses.1 Usually, iron status is assessed initially by the use of surrogate markers, such as ferritin or combinations of more specialised haematinic markers—for example, serum transferrin receptor and the total iron binding capacity.2,3 However, although a low ferritin concentration is indicative of iron deficiency, the diagnosis may be missed when ferritin is raised in the context of inflammation.4 Similarly, anaemia of chronic disease may complicate the interpretation of haematinic markers when iron deficiency coexists.5 Therefore, the gold standard for the diagnosis of iron deficiency has been considered to be the assessment of a bone marrow aspirate stained with Perls’ stain. Historical papers comment on appropriate grading of iron stores on bone marrow aspirate preparations stained in this manner, but give no guidance as to how extensive an examination should be performed to distinguish between no visible iron (grade 0) or very slight stainable iron (grade 1) and normal or increased iron stores.6 Indeed, one study shows that up to 50% of specimens that were said to be deficient in iron did not correlate with the clinical iron status of the patient.7 This suggests that either the specimens or their examination were inadequate to identify the low amounts of iron present. It has been suggested that a minimum of two bone marrow films should be examined,8 but the number of particles that should be examined has not been assessed, and it seems likely that this would be of greater relevance. We aimed to answer the question of how many particles should be examined so that a confident assessment of the presence or absence of iron stores can be made. In addition, we determined the number of particles that must be reviewed to detect the maximum stained iron in a particle and therefore make a valid overall assessment of iron stores.
“We aimed to answer the question of how many particles should be examined so that a confident assessment of the presence or absence of iron stores can be made”
METHODS
Selection of samples
One hundred and ninety consecutive bone marrow aspirates obtained over a period of 12 months in the department of haematology, St Mary’s Hospital, London, were selected for the assessment of iron stores. Bone marrow aspirates were obtained in the course of investigation of a range of haematological conditions, including anaemia and haematological malignancy.
Perls’ stain for iron
Bone marrow was aspirated from the posterior iliac crest or, occasionally, from the sternum. The aspirate was spread on to glass slides, air dried, and fixed with methanol. Slides were stained in 10 g/litre potassium ferrocyanide for 10 minutes before being washed in tap water for 20 minutes and counterstained in 0.1% aqueous safranin.9
Assessment of iron stores
The number of bone marrow particles available for each marrow sample was assessed. Where fewer than 10 particles were present on a single slide, further slides were stained to optimise the number of particles, wherever possible, to 10 or more. Each particle was assessed in turn and the iron content in 10 consecutive particles was recorded. The first iron particle noted to be positive and the particle with the highest iron content were noted.
Iron stores were graded according to the method of Rath and Finch6 (table 1).
Table 1.
Grading of bone marrow haemosiderin
RESULTS
Evaluation of bone marrow aspirate specimens for suitability for assessment of iron status
Of 190 consecutive bone marrow aspirate specimens, 87 (45.7%) contained 10 or more particles and 43 (22.6%) were aparticulate.
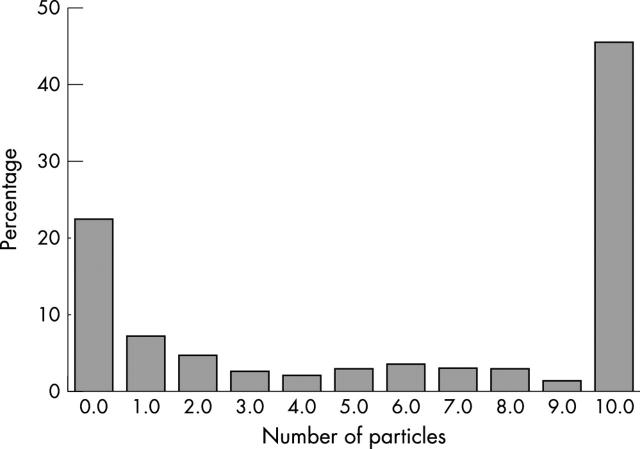
Figure 1 shows the distribution of the number of particles in each specimen. This distribution is polarised, with most preparations found to be completely aparticulate or to have an adequate number of particles. For the initial assessment, only samples with 10 or more particles were reviewed.
Figure 1.
Distribution of particle numbers in 190 consecutive bone marrow aspirates examined. Eighty seven (45.7%) had 10 or more particles, 43 (22.6%) had no particles, and 60 (31.5%) had between one and 10 particles.
Minimum numbers of aspirate particles required to establish a diagnosis of iron deficiency
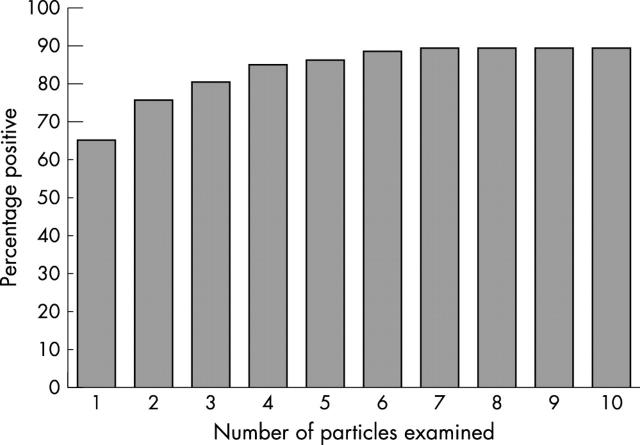
Eighty seven bone marrow aspirates with 10 or more particles were stained by Perls’ method for iron and the iron content of each consecutive particle was assessed. The first particle to show an iron grading of 1 or more was recorded. Figure 2 shows the cumulative distribution of samples becoming positive with each consecutive particle. The distribution plateaus at seven particles, when 90% of the sample shows at least one particle positive for iron. No further samples became positive even when 10 particles were reviewed. This indicates that seven particles must be assessed before a confident report of zero iron stores can be made.
Figure 2.
Percentage of patients with one or more particles positive for iron after examining one to 10 particles.
Of our original 190 specimens, 43 had no particles (22.6%) and 12 (6.3%) had one to six particles without stainable iron and, therefore, using these new criteria should be considered not assessable.
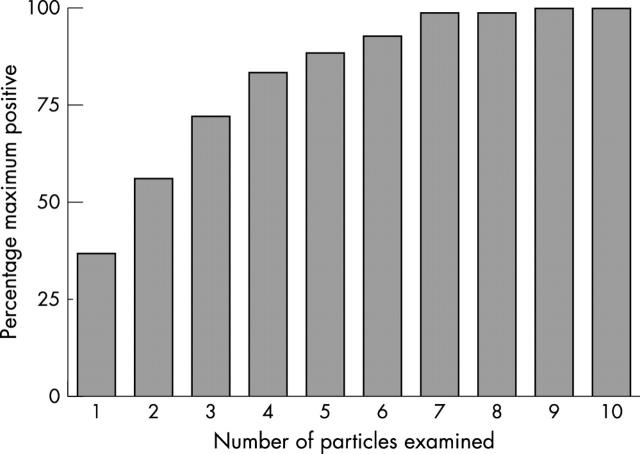
Minimum number of aspirate particles required to establish the maximum iron status of a sample
The 87 bone marrow aspirate samples with 10 or more particles were then assessed to determine the particle exhibiting the maximum iron staining (fig 3). This distribution plateaus at nine particles, with no further increase in the grading of iron stores achievable with the 10th particle. The mean (SD) variation in iron content between particles in individual patients was 1.33 (0.9) units. Most (98.7%) samples had reached their maximum positivity by the seventh sample. Of the original 190 samples, 33 (17.3%) had visible iron but had only one to six particles and could not therefore be optimally graded for maximum positivity.
Figure 3.
Percentage of patients with one or more particles showing maximal positivity for iron after examining one to 10 particles; maximal positivity indicates the maximal positivity seen in a particular patient.
DISCUSSION
Our study shows that for the optimum assessment of a bone marrow aspirate for the absence of iron or for maximum iron stores a minimum of seven particles is required. Any sample that has fewer than seven particles and that contains no particles with stainable iron cannot be confidently said to have absent iron stores. This may explain why other studies have reported discrepancies between the bone marrow aspirate iron and the clinical status of the patient. A total of 46% of the samples in our study would have been considered not optimally assessable: 22.6% were aparticulate, 6.3% had only one to six particles and were negative, and 17.3% had only one to six particles but were positive. We were surprised that nearly a quarter of the samples were aparticulate. We suspect that this is an increasing phenomenon related to the demands for samples to be available for immunophenotyping, cytogenetic and molecular genetic analysis, and other specialised tests. We would always advocate the use of a small syringe to obtain a sample for the preparation of films before other diagnostic samples are taken; 0.2–0.3 ml is generally sufficient for this purpose. If it is clear that particles are not numerous, excess blood should be drained off before spreading further films. If the initial slide stained does not contain storage iron, further slides should be selected to give a minimum of seven fragments. Although the clinical importance of discriminating between grades 5 and 6 iron storage may be limited, it is essential to be able to assess marrow specimens for absent iron stores when investigating anaemia. An attempt should be made to optimise the number of particles to seven; if necessary, more than one slide should be stained and reviewed. If this cannot be achieved and no iron is seen on the existing particles, we suggest that the sample should be reported as lacking stainable iron, but it should be stated that the assessment is unreliable. However, if a small initial sample was obtained for film preparation, it is likely that a definitive report could be issued on a single film much more often.
Take home messages.
When examining bone marrow aspirates for iron stores, a minimum of seven particles must be examined to establish the absence of stainable iron
At least nine particles must be reviewed to see the maximum iron stores in 100% of samples and therefore make a valid judgment of whether iron stores are reduced, normal, or increased
To achieve this, additional slides may need to be stained
If less than seven particles are present and no iron is seen, the sample should be reported as lacking stainable iron, but the assessment should be stated to be unreliable
“We were surprised that nearly a quarter of the samples were aparticulate”
If fewer than seven particles are available for assessment in a thus far negative bone marrow, the examination of bone marrow trephine biopsy sections for haemosiderin might be helpful. This will be the subject of a further study.
REFERENCES
- 1.Cook JD, Skikne BS, Baynes RD. Iron deficiency: the global perspective. Adv Exp Med Biol 1994;356:219–28. [DOI] [PubMed] [Google Scholar]
- 2.Mazza J , Barr RM, McDonald JW, et al. Usefulness of the serum ferritin concentration in the detection of iron deficiency in a general hospital. Can Med Assoc J 1978;119:884–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Means RT Jr, Allen J, Sears DA, et al. Serum soluble transferrin receptor and the prediction of marrow aspirate iron results in a heterogeneous group of patients. Clin Lab Haematol 1999;21:161–7. [DOI] [PubMed] [Google Scholar]
- 4.Beerenhout C , Bekers O, Kooman JP, et al. A comparison between the soluble transferrin receptor, transferrin saturation and serum ferritin as markers of iron state in hemodialysis patients. Nephron 2002;92:32–5. [DOI] [PubMed] [Google Scholar]
- 5.Junca J , Fernandez-Aviles F, Oriol A, et al. The usefulness of the serum transferrin receptor in detecting iron deficiency in the anemia of chronic disorders. Haematologica 1998;83:676–80. [PubMed] [Google Scholar]
- 6.Rath CE, Finch CA. Sternal marrow haemosiderin: a method for the determination of available iron stores in man. J Lab Clin Med 1948;33:81–6. [PubMed] [Google Scholar]
- 7.Barron BA, Hoyer JD, Tefferi A. A bone marrow report of absent stainable iron is not diagnostic of iron deficiency. Ann Hematol 2001;80:166–9. [DOI] [PubMed] [Google Scholar]
- 8.Hansen H , Weinfeld A. Hemosiderin estimations and sideroblast counts in the differential diagnosis of iron deficiency and other anaemias. Acta Med Scand 1959;165:333–56. [DOI] [PubMed] [Google Scholar]
- 9.Swirsky D , Bain BJ. Erythrocyte and leucocyte cytochemistry and leukaemia classification. In: Lewis SM, Bain BJ, Bates I, eds. Practical haematology. London: Churchill Livingstone, 2001:269–95.