Abstract
Background: Because of their suggested link with microsatellite instability high colorectal cancers, right sided hyperplastic polyps (HPs) may differ from their distally located counterparts. This is highlighted by the recognition of a variant HP, termed sessile serrated adenoma (SSA), which predominates in the proximal colon. HPs displaying the morphological features now associated with SSAs have been shown to have altered expression of “cancer associated” markers, but no studies have investigated whether this is dependent on anatomical location of the polyps.
Aims: To evaluate morphological and functional features in right versus left sided HPs from patients without colorectal cancer with the aim of identifying distinguishing characteristics.
Methods: HPs originating in the proximal and distal colorectum were histochemically and immunohistochemically stained to evaluate a panel of markers related to proliferation and differentiation. In addition, a series of morphological features was evaluated for each polyp.
Results: Crypt serration, crypt dilatation, and horizontal crypt growth were more common among HPs from the right side, whereas histochemical factors including mucin changes, global methylation status, and expression of carcinoembryonic antigen were not significantly different. An age disparity was also seen between patients with right versus left sided lesions, with patients with right sided lesions being an average of more than 10 years younger than those with left sided lesions.
Conclusions: These findings suggest that right and left sided HPs differ mainly in terms of growth regulation rather than cellular differentiation, implying that these lesions belong to a continuous spectrum of serrated polyps that differ quantitatively rather than qualitatively.
Keywords: colon, differentiation, hyperplastic, polyp, serration
Although most instances of sporadic colorectal cancer (CRC) seem to arise through the “adenoma–carcinoma” sequence,1 a subset of cases that includes microsatellite instability high (MSI-H) CRCs cannot be accounted for by this model.2–4 Instead, most MSI-H carcinomas appear to progress along a “serrated pathway”,5–9 implicating the inactivation of the DNA mismatch repair gene hMLH1,4,10 as a result of methylation of its promoter region.7,11–13
“There is a need to identify markers for high risk hyperplastic polyps, in addition to mechanisms that would explain the suggested rapid evolution of the serrated pathway”
Although the nature of the antecedent lesion for sporadic MSI-H cancers is still the subject of debate, there is increasing evidence for the precursor being either a hyperplastic polyp (HP) or a closely related lesion. MSI-H CRCs are thought to arise within a subset of HPs that tend to be large and proximally located. Because MSI-H cancers are predominantly proximal lesions, whereas HPs are uncommon in this location, it appears that right sided HPs have a higher potential for progression to malignancy compared with their left sided counterparts.9,14–17 However, it is clear that only a small proportion of even these HPs will undergo malignant transformation.14 There is a need to identify markers for high risk HPs, in addition to mechanisms that would explain the suggested rapid evolution of the serrated pathway.15
Recent studies have highlighted a variant HP termed sessile serrated adenoma (SSA), which occurs more frequently in the proximal colon.2,18 An earlier study showed that HPs with the morphological features now associated with SSAs were also characterised by altered expression of “cancer associated” markers, including carcinoembryonic antigen (CEA) and sialic acid variants.19 However, it was not shown whether these features were dependent upon the anatomical location of polyps. Our present study aimed to investigate whether there are particular morphological or functional characteristics that differentiate right from left sided HPs in patients without CRC. The present findings indicate that left and right sided HPs are distinguishable on the basis of morphological features that are identifiable through the examination of routine diagnostic slides but do not differ with respect to functional markers.
MATERIALS AND METHODS
Tissues
HPs were obtained from a total of 23 patients (16 men and seven women) who underwent full colonoscopy at the Royal Victoria Hospital (Montreal, Canada) during a six month period. The patients had either right sided or left sided HPs, but not both. A randomly selected subset of the more numerous left sided HPs was included for study. Polyps were considered right sided if they occurred proximal to the splenic flexure and left sided if they occurred distal to the splenic flexure. Fourteen of the patients had a single HP, five had two HPs, three had three HPs, and a single patient had five HPs. In all, 19 right and 19 left sided polyps were obtained from 11 and 12 patients, respectively. Our study received approval from the institutional review board of the faculty of medicine of McGill University, Canada.
Scoring of morphological and patient features
Using the haematoxylin and eosin stained slide, the following morphological features2,18 were evaluated in up to 20 appropriately oriented crypts for each polyp: (1) ratio of intraepithelial lymphocytes (IELs) to the total number of epithelial cell nuclei counted, (2) number of horizontal crypts (extending parallel to the muscularis mucosae) of the total number of crypts counted, (3) number of branched crypts of the total number of crypts counted, (4) number of dilated crypts of the total number of longitudinally oriented crypts counted, (5) number of dilated crypts of the total number of transversely oriented crypts counted, (6) longitudinal serration index (number of “teeth” of the total number of longitudinally oriented crypts counted), (7) transverse serration index (number of teeth of the total number of transversely oriented crypts counted), (8) number of crypts showing serration in the lower third of the total number of longitudinally oriented crypts counted, and (9) number of crypts showing serration in the lower third of the total number of transversely oriented crypts counted. Data from transversely and longitudinally oriented crypts were added together to give a global assessment of polyps. Patient age, sex, and site of lesion (right versus left colon) were recorded for each HP.
Antibodies and staining
Sections (4 μm) of formalin fixed, paraffin wax embedded tissues were cut, mounted on to coated slides, and dried overnight at 37°C. Immunohistochemical staining was performed using recommended dilutions of mouse monoclonal antibodies against Ki-67 (clone MIB-1; Dako, Carpinteria, California, USA), sialyl Lewisx (SLex; clone 2Q539; US Biological, Sampscott, Massachusetts, USA), CEA (clone C6G9; Sigma, St Louis, Missouri, USA), and 5-methylcytosine (5-MeC; clone 162 33 D3; Oncogene, San Diego, California, USA). For all antibodies except anti-5-MeC, sections were dewaxed by routine techniques and heat induced antigen retrieval was performed in 0.001M EDTA buffer (pH 8.0) in a microwave oven for 15 minutes. Sections were subsequently incubated at room temperature for one hour with primary antibodies and then with a peroxidase labelled polymer (EnVision + System/HRP, K4005; Dako) for 30 minutes. Sections were subsequently incubated with the chromogen 3-amino-9-ethylcarbazole, counterstained with haematoxylin, and mounted using an aqueous medium. For staining with anti-5-MeC, antigen retrieval and staining were carried out as described previously.20 Detection was performed using a Histostain Plus kit (85–9643; Zymed, San Francisco, California, USA) with 3,3-diaminobenzidine as the chromogen. Appropriate positive and negative controls were carried out for each stain. For each patient, one slide was also stained using a routine haematoxylin and eosin stain and a mild periodic acid Schiff (mPAS) stain, as described previously.19
Scoring of immunohistochemical features
Scoring was carried out without knowledge of the site from which each polyp had been excised. For staining by mPAS, anti-CEA monoclonal antibody, and anti-SLex monoclonal antibody, lesions were scored for both intensity (0 for no staining, 1 for pale staining, 2 for moderate staining, 3 for dark staining) and distribution (0 for no staining, 1 for heterogeneous/patchy staining, 2 for diffuse staining). Ki-67 staining was evaluated by counting the number of stained cells/total number of epithelial cells in the lower, middle, and upper third of three well oriented, longitudinal crypts from each polyp. 5-MeC staining was evaluated by counting the number of unstained nuclei/total number of epithelial nuclei in the lower, middle, and upper third of five well oriented, longitudinal crypts from each polyp. Overall intensity of 5-MeC staining was also evaluated through comparison with the intensity of staining of stromal nuclei in the same tissue section (0 for identical intensity as stromal nuclei, 1 for greater intensity than stromal nuclei, 2 for lower intensity than stromal nuclei).
Analysis
HPs were divided into two groups based upon site of origin and the differences between these two groups were subsequently analysed. Age, sex, size of largest fragment, Ki-67 staining, 5-MeC staining, and all morphological features were analysed using the Student’s t test. mPAS staining, in addition to anti-SLex, anti-CEA, and anti-5MeC intensity were analysed by the Wilcoxon test. In all cases, p values < 0.05 were considered significant.
RESULTS
Patient features
No difference was detected in the sex distribution between patients with left and right sided polyps. In contrast, age difference proved to be significant (p = 0.004), with patients having right sided lesions being, on average, more than 10 years younger (mean age, 50.37 years) that those with left sided lesions (mean age, 61.84 years).
Morphological features
Crypt branching (p = 1.000) showed no significant predilection for either side, and neither did the number of IELs (p = 0.970). Significant differences between proximal and distal HPs were noted for global crypt dilatation (p = 0.010), global serration index (p = 0.007), and global basal serration (p = 0.002)(fig 1). p Values indicated greater significance for well oriented longitudinal crypts, but significance was maintained even after factoring in non-significant transversely cut crypts to obtain a global score. However, it should be noted that perfectly oriented crypts were poorly represented in larger polyps because of the convoluted crypt orientation in larger lesions. The presence of horizontal crypts also proved to be different (p = 0.020) between polyps from each side of the colorectum.
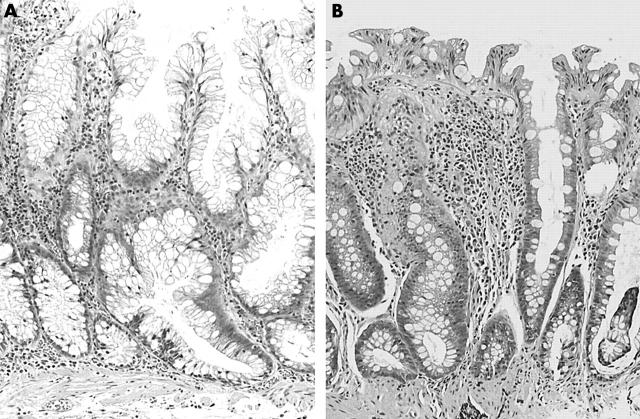
Figure 1.
(A) Right sided hyperplastic polyp displaying crypt dilatation, increased serration, and crypt elongation parallel to the muscularis mucosae. (B) Left sided hyperplastic polyp displaying crypts with only superficial serration and no horizontal elongation. This has been described by others as a “goblet cell type” hyperplastic polyp.18 Haematoxylin and eosin stain; original magnification, ×100.
Monoclonal antibodies and mPAS staining
No differences emerged between left and right sided polyps for global SLex staining (p = 0.270), although the value for SLex intensity did approach significance (p = 0.087) (fig 2). Results for global mPAS staining fell just short of significance (p = 0.059), as did those for mPAS intensity (p = 0.057). Although both of these stains detect changes in acetylation patterns of colorectal sialic acid, a lack of correlation between these staining results was noted for many individual polyps (data not shown). No differences were noted for either intensity or distribution when staining for CEA. Staining for Ki-67 and 5-MeC was not significantly different between left and right sided HPs in the three crypt regions evaluated (fig 3).
Figure 2.
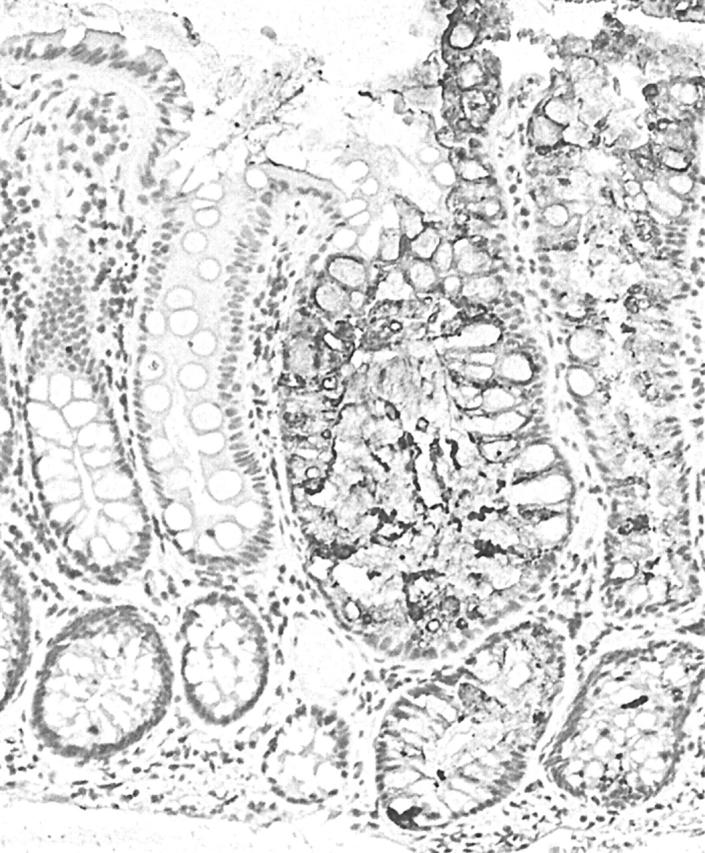
Sialyl Lewisx (SLex) staining of secretory mucous in a right sided hyperplastic polyp. Staining is almost totally confined to the abnormal hyperplastic crypts, indicating a switch from the production of O-acetylated sialic acid to non-O-acetylated sialic acid. This alters the antigenicity of the terminal blood group substances, such as SLex, of the mucin oligosaccharide chains. Avidin–biotin complex technique; original magnification, ×100.
Figure 3.
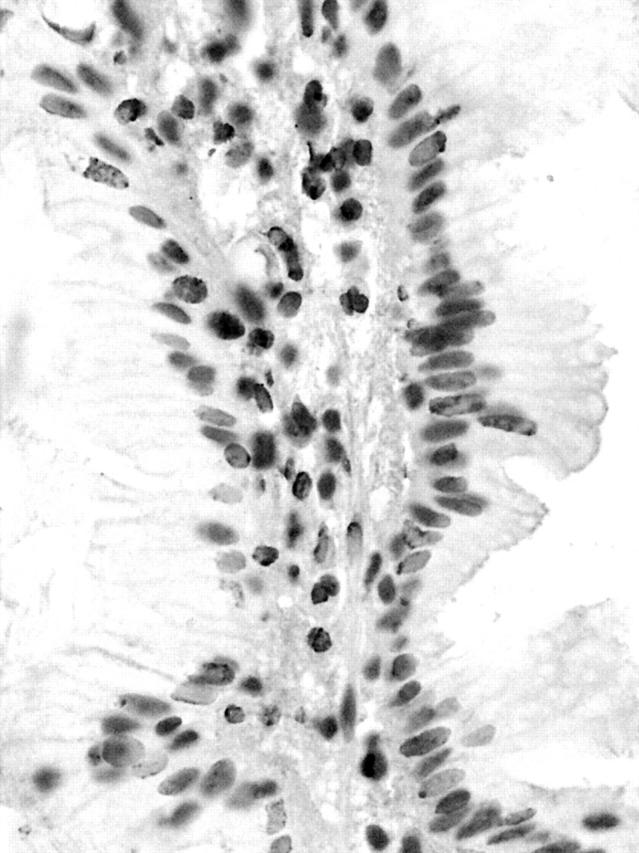
5-Methylcytosine staining of a right sided hyperplastic polyp. Heavily methylated nuclei appear dark grey, whereas undermethylated nuclei appear a much paler shade of grey. Compared with the nuclei of the stromal cells, stained crypt epithelial nuclei are a lighter shade of grey, indicating that they may be relatively undermethylated. Avidin–biotin complex technique; original magnification, ×400.
DISCUSSION
In our study, morphological characteristics were found to be distributed differently among right and left sided HPs. In particular, the presence of crypt dilatation, horizontal crypts, serration in the lower third of crypts, and a high serration index were associated with a proximal HP location. Recent studies have identified an HP variant that is distinguished from classic HPs on the basis of multiple morphological features. These lesions, termed sessile serrated adenomas (SSAs), show a predilection for the proximal colon and have been linked with MSI-H CRC.2,18 A high proportion of right sided HPs show the features of SSAs.2,18 However, it is unclear whether SSAs and HPs are fundamentally different lesions, or represent a continuous spectrum of “serrated polyps”. In an earlier study, increased expression of CEA and altered sialic acid composition co-segregated with certain morphological features that have now been associated with SSAs.19 The aim of our present study was to determine whether functional markers expressed by right and left sided HPs highlight biological differences that are not only more diagnostically discriminating than the subtle morphological changes described by others, but may also serve as evidence that proximal and distal HPs are biologically distinct lesions.
Early studies indicated that HPs are age related lesions, but these dealt only with HPs in the rectum.21 The finding of a full decade of difference in age between patients with proximally versus distally located HPs was unexpected, given the predominance of right sided bowel cancers in older patients.22 It has been estimated that only one in 25 right sided HPs will progress to an MSI-H CRC.6 The relatively young age of subjects with right sided HPs in our study suggests that the lesions may remain unchanged for many years before malignant transformation.
No significant differences were found between left and right sided polyps in terms of staining with either mPAS or antibodies against SLex. It is known that changes occur in the structure of sialic acid (loss of O-acetylation) secreted by malignant colorectal epithelium,19,23–27 and that these changes may be revealed by mPAS staining. SLex is detectable in cancerous and precancerous lesions but not in normal colorectal mucosa. The basis for this observation is the same change in sialic acid structure (loss of O-acetylation) that renders SLex antigenic.23 Because no significant difference was detected between the right and left sided HPs, which nevertheless differed from the normal colorectal mucosa (results not shown), it appears that these mucin histochemical alterations are not specific for the anatomical site of origin of HPs. The trend towards increased sialic acid abnormality in right sided HPs might assume significance in a larger series. However, the findings suggest that proximal and distal HPs are related lesions that differ quantitatively rather than qualitatively. It would be of interest to examine the mucin core proteins present in right versus left sided HPs because a previous study by Biemer-Hüttmann et al found that serrated polyps were associated with upregulation of intestinal mucin MUC2 and gastric mucin MUC5AC, both of which are known to be upregulated in mucinous MSI-H cancers.5
“The increased serration seen in right sided hyperplastic polyps in our present study may be explained by a greater antiapoptotic effect in this subgroup”
Staining with anti-CEA antibody yielded no significantly different results for either intensity or distribution between the two groups of polyps. Previous studies have found increased CEA expression in HPs,16,19 a finding that parallels the commonly observed increase in CRCs.19,28,29 This increased expression of CEA, a GPI linked transmembrane protein implicated in cell signalling,28 could conceivably serve as an indicator of potentially neoplastic progression, even though it does not seem to be differentially expressed in right versus left sided HPs. The lack of a difference in numbers of IELs present in polyps from opposite sides of the colon suggests that lymphocytes are not recruited in abnormally large numbers to areas of hyperplasia, but only with the advent of dysplasia.
The lack of a significant difference in proliferation zone length between distal and proximal HPs, as indicated by staining for the proliferation inducible Ki-67 antigen, implies that expansion of this zone may be common to all subsets of HPs. An increase in cell proliferation, without an accompanying increase in the rate of anoikis (exfoliation induced apoptosis at mucosal surfaces), would represent a possible mechanism for the development of glandular serration.6 The increased serration seen in right sided HPs in our present study may be explained by a greater antiapoptotic effect in this subgroup. It is possible that the observed morphological disparity results from different underlying mechanisms for inhibiting apoptosis. This is supported by the fact that apoptosis inhibition by mutated K-ras is linked to the Akt/protein kinase B mediated inhibition of BAD and caspase 9, whereas BRAF mutation blocks caspase activation downstream of BAD mediated cytochrome c release.30,31 BRAF mutation occurs more frequently in large, right sided HPs occurring in the condition hyperplastic polyposis.32
Take home messages.
There was a significant association between proximal hyperplastic polyps (HPs) and the presence of horizontal crypts, crypt dilatation, serration in lower crypt thirds, and a high serration index
These findings suggest that right and left sided HPs differ mainly in terms of growth regulation rather than cellular differentiation, implying that these lesions belong to a continuous spectrum of serrated polyps that differ quantitatively rather than qualitatively
The structural differences found in the right sided polyps may be attributable to pressure from increased cell numbers within individual crypts, in turn reflecting a more profound impairment of apoptosis
With respect to staining for 5-MeC, the lack of a significant difference in the upper, middle, or lower areas of the crypts from right versus left sided polyps indicates a more or less common DNA methylation status in polyps from either side of the colorectum. This particular feature was evaluated because, on the one hand, MSI-H colorectal cancers are associated with hypermethylation in the promoters of certain genes33–37 whereas, on the other hand, global hypomethylation has been associated with many cancers.20,34,38–43 Although no consistent methylation differences were apparent, it is notable that only epithelial nuclei from HPs located in the distal colorectum were found to be globally methylated to a greater extent than those of their stromal counterparts. All right sided HPs contained either equally or less methylated epithelial nuclei. A possible explanation for this could be the previous suggestion that global hypermethylation may result from increased methylation at sites of DNA damage, which decreases the general availability of methyltransferases.44
In conclusion, this investigation has shown a significant association between proximal HPs and the presence of horizontal crypts, crypt dilatation, serration in lower crypt thirds, and a high serration index. This is consistent with findings by others.2,14,18 Because the main differences in our study proved to be morphological, rather than functional, it appears that proximal HPs show an exaggerated imbalance of cellular proliferation rather than a dysregulation of cellular differentiation compared with their distal counterparts. The structural differences found in the right sided polyps may be attributable to pressure from increased cell numbers within individual crypts, in turn reflecting a more profound impairment of apoptosis.
Acknowledgments
The authors would like to thank Dr B Case from the Department of Pathology, McGill University (Montreal, QC, Canada) for his advice on statistical analysis.
Abbreviations
CEA, carcinoembryonic antigen
CRC, colorectal cancer
HP, hyperplastic polyp
IEL, intraepithelial lymphocyte
5-MeC, 5-methylcytosine
mPAS, mild periodic acid Schiff
MSI-H, microsatellite instability high
SLex, sialyl Lewisx
SSA, sessile serrated adenoma
REFERENCES
- 1.Vogelstein B , Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988;319:525–32. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein NS, Bhanot P, Odish E, et al. Hyperplastic-like colon polyps that preceded microsatellite unstable adenocarcinomas. Am J Clin Pathol 2003;119:778–96. [DOI] [PubMed] [Google Scholar]
- 3.Smyrk TC, Watson P, Kaul K, et al. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 2001;91:2417–22. [PubMed] [Google Scholar]
- 4.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 2002;20:1043–8. [DOI] [PubMed] [Google Scholar]
- 5.Biemer-Hüttmann A-E, Walsh MD, McGuckin MA, et al. Mucin core protein expression in colorectal cancers with high levels of microsatellite instability indicates a novel pathway of morphogenesis. Clin Cancer Res 2000;6:1909–16. [PubMed] [Google Scholar]
- 6.Higuchi T , Jass JR. My approach to serrated polyps of the colorectum. J Clin Pathol 2004;57:682–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitehall VLJ, Wynter CVA, Walsh MD, et al. Morphological and molecular heterogeneity within nonmicrosatellite instability-high colorectal cancer. Cancer Res 2002;62:6011–14. [PubMed] [Google Scholar]
- 8.Iino H , Jass JR, Simms LA, et al. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol 1999;52:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jass JR. Serrated route to colorectal cancer: back street or super highway? J Pathol 2001;193:283–5. [DOI] [PubMed] [Google Scholar]
- 10.Jass JR, Iino H, Ruszkiewicz A, et al. Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut 2000;47:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori Y , Selaru FM, Sato F, et al. The impact of microsatellite instability on the molecular phenotype of colorectal tumors. Cancer Res 2003;63:4577–82. [PubMed] [Google Scholar]
- 12.Calin GA, Gafa R, Tibiletti MG, et al. Genetic progression in microsatellite instability high (MSI-H) colon cancer correlates with clinico-pathological parameters: a study of the TGFbRII, BAX, HMSH3, HMSH6, IGFRII and BLM genes. Int J Cancer 2000;89:230–5. [PubMed] [Google Scholar]
- 13.Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res 1997;57:808–11. [PubMed] [Google Scholar]
- 14.Jass JR. Hyperplastic-like polyps as precursors of microsatellite-unstable colorectal cancer. Am J Clin Pathol 2003;119:773–5. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins NJ, Ward RL. Sporadic colorectal cancers with microsatellite instability and their possible origins in hyperplastic polyps and serrated adenomas. J Natl Cancer Inst 2001;93:1307–13. [DOI] [PubMed] [Google Scholar]
- 16.Jass JR. Hyperplastic polyps of the colorectum—innocent or guilty? Dis Colon Rectum 2001;44:163–6. [DOI] [PubMed] [Google Scholar]
- 17.Takayama T , Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med 1998;339:1277–84. [DOI] [PubMed] [Google Scholar]
- 18.Torlakovic E , Skovlund E, Snover DC, et al. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003;27:65–81. [DOI] [PubMed] [Google Scholar]
- 19.Jass JR, Filipe MI, Abbas S, et al. A morphologic and histochemical study of metaplastic polyps of the colorectum. Cancer 1984;53:510–15. [DOI] [PubMed] [Google Scholar]
- 20.Piyathilake CJ, Johanning GL, Frost AR, et al. Immunohistochemical evaluation of global DNA methylation: comparison with in vitro radiolabeled methyl incorporation assay. Biotech Histochem 2000;75:251–8. [DOI] [PubMed] [Google Scholar]
- 21.Arthur JF. Structure and significance of metaplastic nodules in the rectal mucosa. J Clin Pathol 1968;21:735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Togo G . Relationship between grade of microsatellite instability and target genes of mismatch repair pathways in sporadic colorectal carcinoma. Dig Dis Sci 2001;46:1615–22. [DOI] [PubMed] [Google Scholar]
- 23.Jass JR. Glycoprotein histochemistry and the histogenesis of colorectal cancer. In: Tahara E, ed. Molecular pathology of gastroenterological cancer: application to clinical practice. Tokyo: Springer-Verlag, 1997:129–46.
- 24.Jass JR, Allison LM, Edgar S. Monoclonal antibody TKH2 to the cancer-associated epitope sialosyl Tn shows cross reactivity with variants of normal colorectal goblet cell mucin. Pathology 1994;26:418–22. [DOI] [PubMed] [Google Scholar]
- 25.Jass JR, Allison LM, Edgar SG. Distribution of sialosyl Tn and Tn antigens within normal and malignant colorectal epithelium. J Pathol 1995;176:143–9. [DOI] [PubMed] [Google Scholar]
- 26.Jass JR, Smith M. Sialic acid and epithelial differentiation in colorectal polyps and cancer—a morphological, mucin and lectin histochemical study. Pathology 1992;24:233–42. [DOI] [PubMed] [Google Scholar]
- 27.Sugihara K , Jass JR. Colorectal goblet cell sialomucin heterogeneity: its relation to malignant disease. J Clin Pathol 1986;39:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schölzel S , Zimmerman W, Schwarzkopf G, et al. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol 2000;156:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salem RR, Wolf BC, Sears HF, et al. Expression of colorectal carcinoma-associated antigens in colonic polyps. J Surg Res 1993;55:249–55. [DOI] [PubMed] [Google Scholar]
- 30.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997;91:231–41. [DOI] [PubMed] [Google Scholar]
- 31.Erhardt P , Schremser EJ, Cooper GM. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol 1999;19:5308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kambara T , Simms LA, Whitehall VLJ, et al. BRAF mutation and CpG island methylation: an alternative pathway to colorectal cancer. Gut 2004;53:1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnold CN, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer 2003;106:66–73. [DOI] [PubMed] [Google Scholar]
- 34.Eads CA, Danenberg KD, Kawakami K, et al. CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res 1999;59:2302–6. [PubMed] [Google Scholar]
- 35.Kang Y-H, Bae SI, Kim WH. Comprehensive analysis of promoter methylation and altered expression of hMLH1 in gastric cancer cell lines with microsatellite instability. J Cancer Res Clin Oncol 2002;128:119–24. [DOI] [PubMed] [Google Scholar]
- 36.Kim HC, Kim CN, Yu CS, et al. Methylation of the hMLH1 and hMSH2 promoter in early-onset sporadic colorectal carcinomas with microsatellite instability. Int J Colorectal Dis 2003;18:196–202. [DOI] [PubMed] [Google Scholar]
- 37.Nagasaka T , Sharp GB, Notohara K, et al. Hypermethylation of O6-methylguanine-DNA methyltransferase promoter may predict nonrecurrence after chemotherapy in colorectal cancer cases. Clin Cancer Res 2003;9:5306–12. [PubMed] [Google Scholar]
- 38.Ballestar E , Esteller M. The impact of chromatin in human cancer: linking DNA methylation to gene silencing. Carcinogenesis 2002;23:1103–9. [DOI] [PubMed] [Google Scholar]
- 39.Bariol C , Suter C, Cheong K, et al. The relationship between hypomethylation and CpG island methylation in colorectal neoplasia. Am J Pathol 2003;162:1361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butler LM, Dobrovic A, Bianco T, et al. Promoter region methylation does not account for the frequent loss of expression of the Fas gene in colorectal carcinoma. Br J Cancer 2000;82:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costello JF, Frühwald MC, Smiraglia DJ, et al. Aberrant CpG-island methylation has non-random and tumor-type-specific patterns. Nat Genet 2000;25:132–8. [DOI] [PubMed] [Google Scholar]
- 42.Ehrlich M . DNA methylation in cancer: too much, but also too little. Oncogene 2002;21:5400–13. [DOI] [PubMed] [Google Scholar]
- 43.Piyathilake CJ, Frost AR, Bell WC, et al. Altered global methylation of DNA: an epigenetic difference in susceptibility for lung cancer is associated with its progression. Hum Pathol 2001;32:856–62. [DOI] [PubMed] [Google Scholar]
- 44.James SJ, Pogribney IP, Pogribna M, et al. Mechanisms of DNA damage, DNA hypomethylation, and tumor progression in the folate/methyl-deficient rat model of hepatocarcinogenesis. J Nutr 2003;133:3740S–47S. [DOI] [PubMed] [Google Scholar]