Abstract
Aims: The perforin mediated pathway is the major pathway of cytotoxicity induced by activated T cells and natural killer cells, and may be involved in the development of pulmonary fibrosis.
Methods: Perforin and granzyme B expression were examined in idiopathic pulmonary fibrosis by means of immunohistochemistry, and perforin knockout mice were used to examine whether or not perforin mediated cytotoxicity participates in the pathophysiology of bleomycin induced pneumopathy.
Results: Perforin and granzyme B expression were upregulated in infiltrating lymphocytes in lung tissue from patients with idiopathic pulmonary fibrosis compared with normal lung parenchyma. Perforin and granzyme B expression were upregulated predominantly in infiltrating mononuclear cells after bleomycin instillation in wild-type mice. Although the development of bleomycin induced pneumopathy was not completely prevented, the pathological grade of inflammation and fibrosis, and the number of apoptotic cells in lung tissue, were significantly decreased in perforin knockout mice compared with wild-type mice.
Conclusions: These results suggest that perforin mediated apoptosis may be associated with the pathophysiology of lung injury and fibrosis.
Keywords: perforin, granzyme B, lung injury, fibrosis, apoptosis
Apoptosis may play a role in human diseases in two different ways. First, diseases may be caused by a malfunction of the apoptosis mechanism. Repair after acute lung injury requires the elimination of proliferating mesenchymal and inflammatory cells from the alveolar air space or alveolar walls.1 Failure to clear unwanted cells by apoptosis will prolong the inflammation because of the release of their toxic contents. Second, excessive apoptosis may cause various diseases. Intratracheal instillation of agonistic anti-Fas antibody or recombinant Fas ligand induces acute alveolar epithelial injury and lung inflammation.2,3 Repeated inhalation of agonistic anti-Fas antibody induces epithelial cell apoptosis and lung inflammation, which subsequently leads to pulmonary fibrosis in mice.4
“Although granzyme B mediated cell death is dependent upon perforin, these two cytotoxic granules together can produce the features of T cell mediated apoptosis”
There are two major mechanisms by which cytotoxic T cells (CTLs) and natural killer (NK) cells induce apoptosis in target cells.5 One is the crosslinking of the Fas expressed on the target cell membrane by the Fas ligand expressed on the surface of effector cells. The other is the release of two cytotoxic granules, perforin and granzyme B, from the effector cells. Perforin is the cytotoxic pore forming protein, which mediates target cell lysis by CTLs and NK cells,6,7 whereas granzyme B is a serine protease released by CTLs and NK cells, which triggers a series of biochemical events that lead to apoptosis.8,9 The membrane damage caused by purified perforin is not sufficient to induce DNA fragmentation.10 Although granzyme B has the capacity to enter the cell cytoplasm autonomously, it remains inactive without the signal from perforin to initiate apoptosis.11 Although granzyme B mediated cell death is dependent upon perforin, these two cytotoxic granules together can produce the features of T cell mediated apoptosis.12,13
Perforin mediated cytotoxicity has been reported to be involved in various disorders. In patients with primary Sjogren’s syndrome, salivary gland epithelial cells are apoptotic compared with control subjects, and perforin and granzyme B are expressed in lymphocytic infiltrates.14 Perforin and the Fas ligand may participate in β cell elimination by cytotoxic T cells in autoimmune diabetes,15 and in graft versus host disease in mice.16 Perforin and Fas ligand are also reported to be associated with human diseases such as acute respiratory distress syndrome,17 and with muscle fibre injury in polymyositis and dermatomyositis.18
Although the aetiology of idiopathic pulmonary fibrosis (IPF) is still uncertain, it is felt that the initial lesion before the formation of fibrosis is probably alveolitis, which is characterised by the loss of type I epithelial cells, and type II pneumocyte hyperplasia. Although there are various initiating factors or causes, the terminal stages are characterised by proliferation and progressive accumulation of connective tissue replacing normal functional parenchyma. We previously showed that there was DNA damage or apoptosis in lung epithelial cells, and that Fas mediated apoptosis may be involved in IPF and in animal models of pulmonary fibrosis.19–21
Bleomycin induced pneumopathy in mice is an animal model of acute lung injury and fibrosis. Epithelial cell apoptosis is involved in the pathophysiology of this model.22–24 Accordingly, to investigate the involvement of perforin mediated apoptosis in the development of lung injury and fibrosis, we examined whether or not perforin knockout (PKO) mice are resistant to the development of bleomycin induced pneumopathy. We also performed immunohistochemistry for perforin and granzyme B proteins in lung tissue from patients with IPF to verify the association between these cytotoxic granules and this devastating lung disease.
METHODS
Model of bleomycin induced pulmonary fibrosis in mice
Our experiments were approved by the committee on ethics regarding animal experiments of Kyushu University Faculty of Medicine, Japan, and were performed according to the guidelines of the American Physiological Society. PKO mice with a C57BL/6 background were kindly provided from Dr H Hengartner (Institute of Experimental Immunology, University of Zürich, Zürich, Switzerland). C57BL/6 wild-type (WT) mice were purchased from the Laboratory for Animal Experiments (Kyushu University, Fukuoka, Japan). The animals were anaesthetised with an intraperitoneal injection of sodium pentobarbital (Dinabot, Osaka, Japan). The anaesthetised animals received an intratracheal instillation of 50 μl of bleomycin hydrochloride (Nippon Kayaku, Tokyo, Japan) solution containing 1.0 U of bleomycin/kg of body weight dissolved in sterile saline. After treatment, the mice were returned to their cages and allowed food and water ad libitum. Mice were anaesthetised at 14 days after the bleomycin instillation and then sacrificed.
Histopathology of mouse lung tissues
After thoracotomy, the pulmonary circulation was flushed with saline, and the lungs were explored. The lung samples were fixed in 10% formalin overnight, and then embedded in paraffin wax. A 5 μm paraffin wax section was adhered to slides and stained with haematoxylin and eosin. The pathological grade of inflammation and fibrosis in a midsagittal section from the lung was evaluated under ×40 magnification. The pathological grade was determined according to the following criteria by three investigators (KK, NH, and KY): 0, no lung abnormality; 1, presence of inflammation and fibrosis involving < 25% of the lung parenchyma; 2, lesions involving 25–50% of the lung; 3, lesions involving > 50% of the lung. All investigators agreed on the decisions regarding pathological grade for each section.
Apoptosis analysis in mouse lung tissues
Apoptosis was detected by the terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL) method utilising the Dead End Colorimetric Apoptosis Detection system (Promega, Madison, Wisconsin, USA), as described previously.21 The number of TUNEL positive cells in the whole area of the midsagital section was counted under a microscope with ×200 magnification.
RNA preparation and analysis
Total RNA was prepared from lung tissues by the ISOGEN RNA extraction kit (Nippon Gene, Tokyo, Japan). cDNA was prepared by reverse transcription (RT). The polymerase chain reaction (PCR) amplifications were performed in a 50 µl reaction volume containing 5 µl of each type of cDNA, plus 10mM Tris HCl (pH 8.3), 50mM KCl, 1.5mM MgCl2, 0.1% Triton X-100, 0.2mM dNTPs, and 1.25 U of Taq polymerase (Takara Biochemicals, Tokyo, Japan). The following primers were used. Mouse β actin: sense, 5′-TCCTGTGGCATCCATGAAACT-3′, antisense, 5′-CTTCGTGAACGCCACGTGCTA-3′; mouse perforin: sense, 5′-TCTGAGACAGGCTATCAGCC-3′, antisense, 5′-CATCAGTAGCTGTGGTGTAG-3′. The conditions for amplification were as follows: one cycle of 93°C for three minutes, followed by 30 cycles of 93°C for one minute, 55°C for one minute, and 72°C for two minutes, with a final extension at 72°C for seven minutes (one cycle only). Cycle curve studies confirmed that for none of the primer pairs had the reactions reached the plateau of the amplification curve at 30 cycles.
Immunohistochemistry for mouse and human lung tissues
This study of IPF was performed on eight lung samples obtained by thoracoscopic lung biopsy. The diagnosis of IPF was established according to the previously described criteria.25 The histological findings of lung biopsy specimens were compatible with those of usual interstitial pneumonia (UIP). The patients with UIP comprised eight men, whose ages ranged from 42 to 67 years (median, 58). Seven were smokers and one was a non-smoker. The results from the UIP specimens were compared with those from five normal lung parenchyma specimens obtained by lobectomy for lung cancer. The patients with lung cancer comprised four men and one woman, whose ages ranged from 56 to 76 years (median, 65), and all were smokers.
The sections were dewaxed in xylene, and then rehydrated in ethanol, before finally being rinsed with distilled water. Immunohistochemistry was performed using a modified streptavidin–biotinylated peroxidase technique using a Histofine SAB-PO kit from Nichirei Corporation (Tokyo, Japan). The sections were incubated with goat anti-perforin polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA), goat anti-granzyme B polyclonal antibody (Santa Cruz Biotechnology), or a control non-specific goat IgG at 4°C overnight. The sections were lightly counterstained with haematoxylin and mounted. The results of immunohistochemistry in lung tissues from patients with IPF were graded semiquantitatively by three investigators (KK, NH, and KY) as follows: grade 1, positive cells comprise less than 10%; grade 2, positive cells comprise 10–50%; and grade 3, positive cells comprise more than 50% in each cell type. All investigators agreed on the decisions regarding staining grade for each kind of cell in each specimen.
Statistical analysis
When comparing the number of TUNEL positive cells, ANOVA followed by Scheffe’s F test was used. The comparison of pathological grade was analysed by the Kruskal-Wallis test followed by the Mann-Whitney U test. p Values of less than 0.05 were considered significant. Statistical analysis was performed with Stat View J-4.5 (Abacus Concepts Inc, Berkeley, California, USA).
RESULTS
Mouse perforin mRNA expression in lung tissues
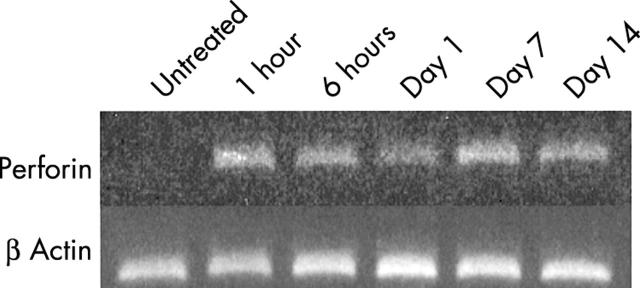
Figure 1 shows representative results of RT-PCR for mouse perforin mRNA in lung tissue from mice after bleomycin instillation. Mouse perforin mRNA expression was significantly increased after bleomycin instillation. RT-PCR was repeated twice using mRNA extracted from different mice and the results were consistent.
Figure 1.
Time course of perforin mRNA expression in lung homogenate after bleomycin instillation.
Immunohistochemistry for perforin and granzyme B in mouse lung tissues
Figure 2 shows representative results of immunohistochemistry for perforin and granzyme B proteins in bleomycin induced pulmonary fibrosis in mice. Positive signals for perforin and granzyme B were predominantly detected in infiltrating mononuclear cells in lung tissues of WT mice after bleomycin instillation. Positive signals for perforin and granzyme B were also found in some epithelial cells, and in a few mononuclear cells in saline instilled mice. Because perforin mRNA was upregulated as early as one hour after bleomycin instillation, we tried to assess the expression of perforin protein over time. We performed immunohistochemistry for perforin using lung tissues obtained from three mice at the following time point: one hour, six hours, one day, three days, five days, seven days, 10 days, and 14 days after bleomycin instillation. Positive signals for perforin were increased in inflammatory lesions along with the development of pneumopathy, and were maximal at seven to 14 days.
Figure 2.
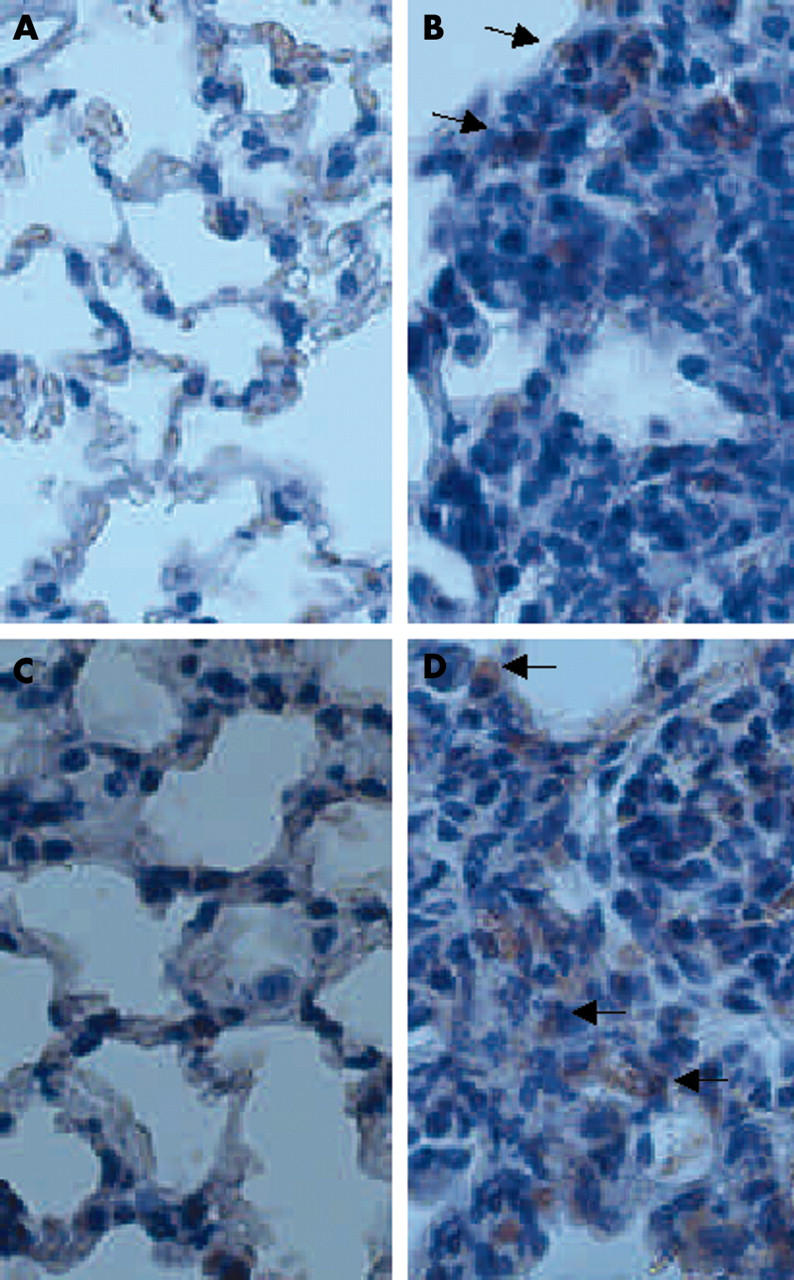
The results of immunohistochemistry for perforin and granzyme B proteins in lung tissue of mice. (B) Perforin and (D) granzyme B were detected predominantly in infiltrating mononuclear cells of lung tissues obtained 14 days after bleomycin instillation (arrows), but not in saline instilled mice (A and C, respectively). Original magnification, ×250.
Histopathological evaluation of bleomycin induced pneumopathy
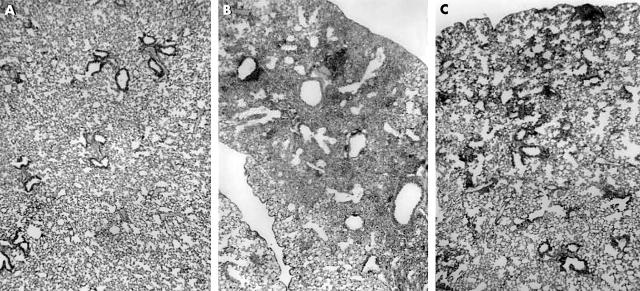
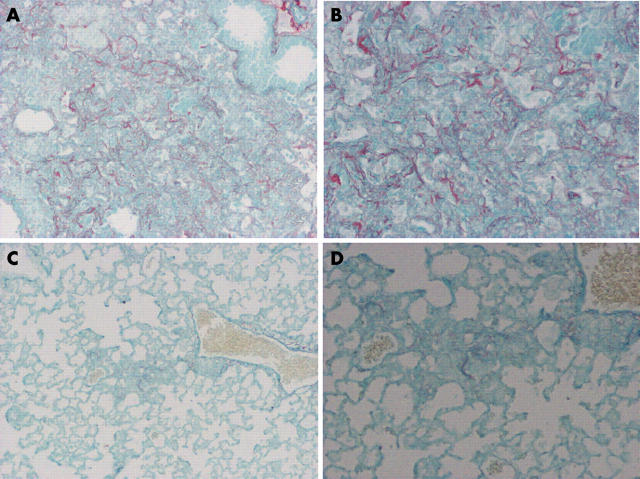
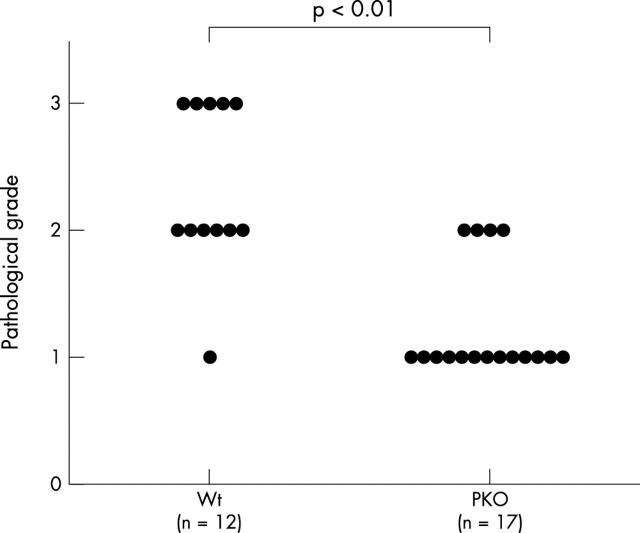
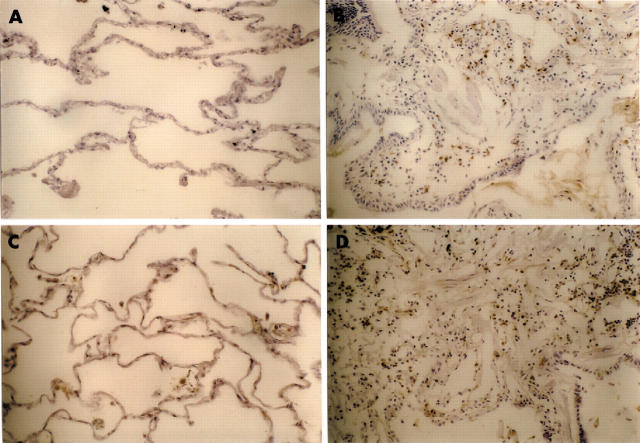
Figure 3 shows representative histological findings of this model in WT and PKO mice after bleomycin instillation. After 14 days, a large number of lymphocytes infiltrated the lung interstitium, and thickening of the alveolar septa, collapse of alveolar spaces, and proliferation of fibroblasts were observed. In PKO mice, inflammatory cell infiltration and fibrosis in lung tissue were attenuated compared with WT mice. Figure 4 shows representative results of Sirius red staining. The positive staining for collagen fibres was consistent with affected lesions. Strong staining was seen in WT mice compared with PKO mice after bleomycin instillation. Figure 5 shows the semiquantitative results of histological evaluation. The pathological grade at day 14 was significantly decreased in PKO mice (n = 17) compared with WT mice (n = 12) (p < 0.01).
Figure 3.
The results of histopathological analysis. (A) Normal lung parenchyma from untreated mice. (B) Pronounced thickening of the alveolar septa, collapse of alveolar spaces, large numbers of lymphocytes accumulated in the alveolar walls, and proliferation of fibroblasts at day 14 after the bleomycin instillation in wild-type mice, and (C) attenuated lung inflammation and fibrosis in perforin knockout mice 14 days after bleomycin instillation.
Figure 4.
Representative results of Sirius red staining for collagen. (A, B) Strong staining was seen in affected lesions in lung tissues 14 days after the bleomycin instillation in wild-type mice compared with (C, D) perforin knockout mice. Original magnification: (A, C), ×125; (B, D) ×250.
Figure 5.
The effect of perforin deficiency (PKO) on pathological grade in bleomycin induced pneumopathy in mice. Each circle corresponds to the data from one mouse. The results were compared with wild-type (Wt) mice treated with bleomycin (p < 0.01).
TUNEL staining
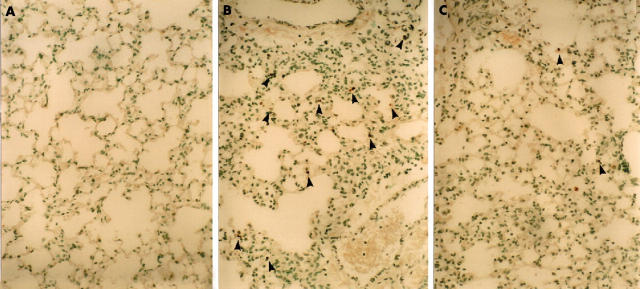
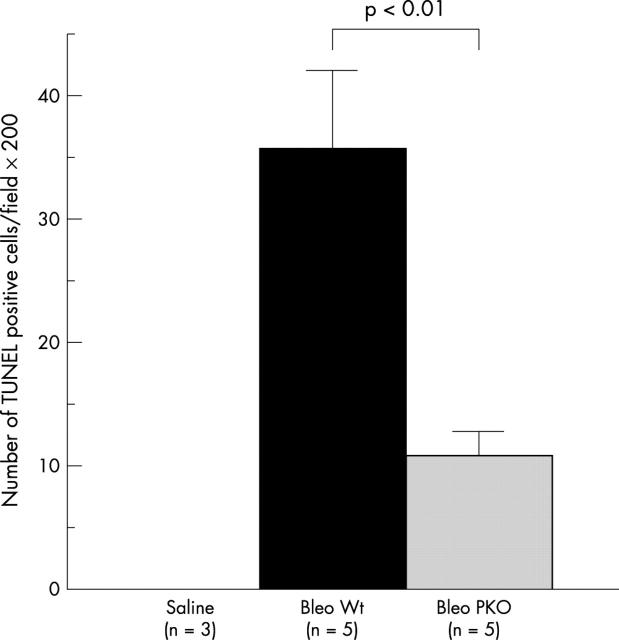
Figure 6 shows representative results of TUNEL staining. Although the types of apoptotic cells were not clearly identified, some of the bronchiolar and alveolar epithelial cells or inflammatory cells in inflammatory lesions showed evidence of apoptosis as estimated by the TUNEL method 14 days after bleomycin instillation in WT mice, but not in saline instilled mice. Figure 7 shows that the number of positive signals for TUNEL at 14 days was significantly decreased in lung tissues from PKO mice (n = 5) compared with those from WT mice (n = 5).
Figure 6.
The effect of perforin deficiency on terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL) staining in lung tissues after bleomycin instillation. (A) No positive signals for TUNEL in normal lung parenchyma, and (B) TUNEL positive cells in lung tissues at 14 days (arrowheads). (C) These positive signals for TUNEL (arrowheads) were decreased in PKO mice 14 days after bleomycin instillation. Original magnification, ×250.
Figure 7.
The effect of perforin deficiency on the number of terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL) positive cells in bleomycin (Bleo) induced pneumopathy in mice. Data are shown as the mean (SEM) obtained from five mice (*p < 0.01). PKO, perforin knockout; Wt, wild-type.
Immunohistochemistry for perforin and granzyme B in IPF
Figure 8 shows representative results of immunohistochemistry for perforin and granzyme B in lung tissue from patients with IPF and in normal lung parenchyma. Table 1 shows the semiquantitative results of immunohistochemistry for each type of cell. Positive signals for perforin and granzyme B were predominantly detected in infiltrating lymphocytes in lung tissues of IPF. Positive signals for perforin and granzyme B were also found in the cytoplasm of some epithelial cells, macrophages, and fibroblasts. No positive signal for perforin and few signals for granzyme were seen in the normal lung parenchyma.
Figure 8.
The results of immunohistochemistry for perforin and granzyme B proteins in lung tissues from patients with idiopathic pulmonary fibrosis. (B) Perforin and (D) granzyme B were detected predominantly in infiltrating lymphocytes, but not in normal lung parenchyma (A and C, respectively). Original magnification, ×125.
Table 1.
Semiquantitative results of immunohistochemistry for perforin and granzyme B in IPF
| Patient | Perforin | Granzyme B | ||||||
| Epi | Fib | Macro | Lymph | Epi | Fib | Macro | Lymph | |
| IPF | ||||||||
| 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 1 |
| 2 | 1 | 1 | 0 | 3 | 1 | 1 | 1 | 1 |
| 3 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| 4 | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 3 |
| 5 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| 6 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 2 |
| 7 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| 8 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Controls | ||||||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Grades of IPF: 0, no positive cells; 1, <10% of cells positive; 2, 10–49% of cells positive; 3, >50% of cells positive.
Epi, epithelium; Fib, fibroblast; IPF, idiopathic pulmonary fibrosis; Lymph, lymphocyte; Macro, macrophage.
DISCUSSION
Pulmonary fibrosis induced by intratracheal instillation of bleomycin is basically an acute lung injury model followed by rapid development of pulmonary fibrosis, which eventually resolves in five to six weeks. Therefore, this model seems to be an acute lung injury model rather than a model of IPF; however, it is still a good model to investigate the fibrosing process. We demonstrated here that perforin mRNA expression was upregulated in lung homogenates, and perforin protein was predominantly detected in infiltrating inflammatory cells of bleomycin induced pneumopathy in mice. The development of this model was partially prevented in PKO mice, in which positive signals for TUNEL staining were significantly decreased compared with control mice. Although the TUNEL assay is not specific for epithelial cells, we have previously determined by electron microscopy that most apoptotic cells in this model are epithelial cells.26 We also found that perforin and granzyme B expression were upregulated predominantly in infiltrating lymphocytes of lung tissue from patients with IPF. Epithelial cell apoptosis in IPF has been demonstrated previously using TUNEL assay and electron microscopy.27–29 Although there is no direct evidence to show the involvement of perforin mediated apoptosis in epithelial cell damage in both IPF and bleomycin induced pneumopathy, the affected lesions showed infiltration of mononuclear cells that were positively stained for perforin, and epithelial cells that were positively stained by the TUNEL method. These results suggest an association between the perforin mediated apoptosis pathway and epithelial cell damage in pulmonary fibrosis.
The Fas–Fas ligand pathway is important for inducing epithelial cell apoptosis in bleomycin induced pneumopathy in mice.21 The angiotensin converting enzyme inhibitor captopril or the caspase inhibitor Z-Val-Ala-Asp-fluoromethylketone can block epithelial cell apoptosis and lung fibrosis.30 A specific inhibitor of p38 mitogen activated protein kinase inhibits the augmented expression of tumour necrosis factor α, and the apoptosis of lung epithelial cells.31 Heme oxygenase 1 overexpression using adenovirus prevents bleomycin induced pulmonary fibrosis by attenuating apoptotic cell death.32 These studies support the possibility that epithelial cell apoptosis and various types of apoptosis related signalling are involved in the pathophysiology of this model, in which perforin mediated apoptosis signalling may be included. The partial inhibition of both apoptosis and pneumopathy in PKO mice also suggests the involvement of other pathways that induce epithelial cell damage and apoptosis in this model.
“Our results suggest an association between the perforin mediated apoptosis pathway and epithelial cell damage in pulmonary fibrosis”
Take home messages.
Perforin and granzyme B expression was upregulated in infiltrating lymphocytes found in lung tissue from patients with idiopathic pulmonary fibrosis when compared with normal controls
Perforin and granzyme B expression was upregulated predominantly in infiltrating mononuclear cells after bleomycin instillation in wild-type mice and, although bleomycin induced pneumopathy was not completely prevented in perforin knockout mice, the pathological grade of inflammation and fibrosis, and the number of apoptotic cells in lung tissues were significantly decreased compared with wild-type mice
Thus, perforin mediated apoptosis may be associated with the pathophysiology of lung injury and fibrosis
Perforin facilitates the entry of granzyme B into the target cell, and is required for granzyme B to mediate cell death.13 Granzyme B can cleave caspase 3, which directly leads to apoptosis, and the activation of caspases 6, 7, 8, 9, and 10.33 Granzyme B can also induce mitochondria mediated cell death by means of cleaving Bid without caspase activation. Granzyme B truncated Bid translocates to mitochondria and facilitates the release of cytochrome c.33 Furthermore, granzyme B can induce mitochondrial depolarisation and cell death without caspase activation in Bid, Bax, or Bak knockout cells, and can also directly process apoptotic caspase substrates such as PARP, DNA-PK, NuMA, and CAD/ICAD.34 Accordingly, although caspase inhibitors or inhibitors of death receptor mediated apoptosis have been experimentally examined for their ability to protect parenchymal cells and tissues from injury and cell death, a strategy against the perforin or granzyme B mediated cell death pathway seems to be required to regulate diseases such as lung injury and fibrosis.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (13670604, 15590815) from the Ministry of Education, Science and Culture of Japan. The English used in this manuscript was revised by Miss K Miller (Royal English Language Centre, Fukuoka, Japan).
Abbreviations
CTL, cytotoxic T cell
IPF, idiopathic pulmonary fibrosis
NK, natural killer
PCR, polymerase chain reaction
PKO, perforin knockout
RT, reverse transcription
TUNEL, terminal deoxynucleotidyl transferase mediated dUTP nick end labelling
UIP, usual interstitial pneumonia
WT, wild-type
REFERENCES
- 1.Polnovsky VA, Chen B, Henke C, et al. Role of mesenchymal cell death in lung remodeling after injury. J Clin Invest 1993;92:388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matute-Bello G, Winn RK, Jonas M, et al. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol 2001;158:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matute-Bello G, Liles WC, Frevert CW, et al. Recombinant human Fas ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am J Physiol Lung Cell Mol Physiol 2001;281:L328–35. [DOI] [PubMed] [Google Scholar]
- 4.Hagimoto N, Kuwano K, Miyazaki H, et al. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol 1997;17:272–8. [DOI] [PubMed] [Google Scholar]
- 5.Kägi D, Vignaux F, Ledermann B, et al. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 1994;265:528–30. [DOI] [PubMed] [Google Scholar]
- 6.Young JD, Hengartner H, Podack ER, et al. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer activity. Cell 1986;44:849–59. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenheld MG, Olsen KJ, Lu P. Structure and function of human perforin. Nature 1988;335:448–51. [DOI] [PubMed] [Google Scholar]
- 8.Masson D, Tschopp J. A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell 1987;49:679–85. [DOI] [PubMed] [Google Scholar]
- 9.Trapani JA, Klein JL, White PC, et al. Molecular cloning of an inducible serine esterase gene from human cytotoxic lymphocytes. Proc Natl Acad Sci U S A 1988;85:6924–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duke RC, Persechini PM, Chang S, et al. Purified perforin induces target cell lysis but not DNA fragmentation. J Exp Med 1989;170:1451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi L, Mai S, Israels S, et al. Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization. J Exp Med 1997;185:855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kägi D, Ledermann B, Burki K, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 1994;369:31–7. [DOI] [PubMed] [Google Scholar]
- 13.Froelich CJ, Orth K, Turbov J, et al. New paradigm for lymphocyte granule-mediated cytotoxicity. Target cells bind and internalize granzyme B, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J Biol Chem 1996;271:29073–9. [DOI] [PubMed] [Google Scholar]
- 14.Polihronis M, Tapinos NI, Theocharis SE, et al. Modes of epithelial cell death and repair in Sjogren’s syndrome (SS). Clin Exp Immunol 1998;114:485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kägi D, Odermatt B, Seiler P, et al. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. J Exp Med 1997;186:989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun MY, Lowin B, French L, et al. Cytotoxic T cells deficient in both functional fas ligand and perforin show residual cytolytic activity yet lose their capacity to induce lethal acute graft-versus-host disease. J Exp Med 1996;183:657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto S, Kobayashi A, Kooguchi K, et al. Upregulation of two death pathways of perforin/granzyme and FasL/Fas in septic acute respiratory distress syndrome. Am J Respir Crit Care Med 2000;161:237–43. [DOI] [PubMed] [Google Scholar]
- 18.Goebels N, Michaelis D, Engelhardt M, et al. Differential expression of perforin in muscle-infiltrating T cells in polymyositis and dermatomyositis. J Clin Invest 1996;97:2905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwano K, Kunitake R, Kawasaki M, et al. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1996;154:477–83. [DOI] [PubMed] [Google Scholar]
- 20.Kuwano K, Miyazaki H, Hagimoto N, et al. The involvement of Fas–Fas ligand pathway in fibrosing lung diseases. Am J Respir Cell Mol Biol 1999;20:53–60. [DOI] [PubMed] [Google Scholar]
- 21.Kuwano K, Hagimoto N, Kawasaki M, et al. Essential roles of the Fas–Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest 1999;104:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh S, Mendoza T, Ortiz LA, et al. Bleomycin sensitivity of mice expressing dominant–negative p53 in the lung epithelium. Am J Respir Crit Care Med 2002;166:890–7. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Ibarra-Sunga O, Verlinski L, et al. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol 2000;279:L143–51. [DOI] [PubMed] [Google Scholar]
- 24.Hagimoto N, Kuwano K, Nomoto Y, et al. Apoptosis and expression of Fas/Fas ligand mRNA in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol 1997;16:91–101. [DOI] [PubMed] [Google Scholar]
- 25.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med 2000;16:646–64. [DOI] [PubMed] [Google Scholar]
- 26.Hagimoto N, Kuwano K, Nomoto Y, et al. Apoptosis and expression of Fas/Fas ligand mRNA in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Cell Mol Biol 1997;16:91–101. [DOI] [PubMed] [Google Scholar]
- 27.Uhal BD, Joshi I, Hughes WF, et al. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol 1998;275:L1192–9. [DOI] [PubMed] [Google Scholar]
- 28.Barbas-Filho JV, Ferreira MA, Sesso A, et al. Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IFP)/usual interstitial pneumonia (UIP). J Clin Pathol 2001;54:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuwano K, Nakashima N, Inoshima I, et al. Oxidative stress in lung epithelial cells from patients with idiopathic interstitial pneumonias. Eur Respir J 2003;21:232–40. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Ibarra-Sunga O, Verlinski L, et al. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol 2000;279:L143–51. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka H, Arai T, Mori M, et al. A p38 MAPK inhibitor, FR-167653, ameliorates murine bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2002;283:L103–12. [DOI] [PubMed] [Google Scholar]
- 32.Tsuburai T, Suzuki M, Nagashima Y, et al. Adenovirus-mediated transfer and overexpression of heme oxygen 1 cDNA in lung prevents bleomycin-induced pulmonary fibrosis via a Fas–Fas ligand-independent pathway. Hum Gene Ther 2002;13:1945–60. [DOI] [PubMed] [Google Scholar]
- 33.Heibein JA, Goping IS, Barry M, et al. Granzyme B-mediated cytochrome c release is regulated by the Bcl-2 family members bid and Bax. J Exp Med 2000;192:1391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas DA, Scorrano L, Putcha GV, et al. Granzyme B can cause mitochondrial depolarization and cell death in the absence of BID, BAX, and BAK. Proc Natl Acad Sci U S A 2001;98:14985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]